O/8RAR0 OF LAMPBLACK
advertisement

1WF.
27 MAR1939
CATA PHORES IS OF LAMPBLACK
O/8RAR0
by
Jean Irwin Wagner
Submitted in Partial Fulfillment of the
Requirements for the Degree of
MASTER OF SCIENCE
from the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
1938
Signature of Author .~...
..
............
,.......
Department of Chemical Engineering
Signature of Professor
in Charge of Research .............
.....
Signature of Chairman of Department
Committee on Graduate Students ....... ........
Harding Terrace
Centerport, New York
May 14, 1938.
Professor George W. Swett
Secretary of the Faculty
Massachusetts Institute of Technology
Cambridge, Massachusetts
Dear Sir:
I hereby submit a thesis entitled "Cataphoresis
of Lampblack" in partial fulfillment of the requirements
for the degree of Master of Science.
Respectfully yours,
V Jean Irwin Wagner
22771-1
AC KN OWLEDGL ENT
The author wishes to express his appreciation of
the very helpful advice and suggestions which were given
by Dr. Ernst A. Hauser under whom this investigation was
carried out.
He also wishes to thank the Dewey and Almy
Chemical Company for furnishing the dispersing agent
used in the experiments.
TABLE OF CONTENTS
I. SUMMARY ..... •................... ....... *..
II.
INTRODUCTION ...................
III. PROCEDURE .............
...........
..................
2
... 11
17
IV. RESULTS ...................................
SV.DISCUSSION
................................
VI. CONCLUSIONS ..............
22
..........
VII. RECOMMENDATIONS .....................
VIII.APPENDIX
1.
2.
3.
4.
* .*****
1
****....
..
27
......
28
....
....... ***.**
*.
Cell dimensions .............. .......
Calibration of cell ..................
Calculations .......................
Data .................................
5. Literature references ................
29
29
30
31
35
46
I.
SUMMARY
The following.properties of aqueous solutions of the dispersing agent disodiumdinaphthylmethane
disulphonate were measured; pH, surface tension, and
viscosity.
Dispersions of lampblack were prepared us-
ing these solutions.
The relative amount of lampblack
which was dispersed and the cataphoretic velocity of
the suspended particles were determined.
From the
cataphoretic velocity, the zeta potential and the surface density of charge were calculated.
The results indicate that the dispersing
agent behaves as though it were ionadsorbed on the
dispersed particles which results in increasing the
zeta potential and surface density of charge considerably above the values obtained in an ordinary
electrolyte like sodium sulphate.
The most important
factor governing the dispersing action is the surface
charge density to which the dispersing power is
directly proportional in dilute solutions of the agent.
The results also suggest that perhaps the
stability of the suspensoids is directly related to
the density of charge.
simple electrolyte and is. ion-adsorbed on the surface
of the susp ended particles.
The curves of dispersing power (see Figure 1)
are also suggestive of the action of simple
electrolytes on hydrophobic suspensions.
it
will be
noticed that as the concentration of the agent increases, the dispersing power increases until a maximnu, is reached
creases.
after which the dispersing action de-
This parallels
the fact that a hydrophobic
colloid which has been flocculated by the comolete
removal of adsorbed electrolytes will be repeptized
by the addition of an electrolyte,
and that when the
concentration of that electrolyte reaches a certain
value it will be flocculated.
Evidence that the dispersing action is related to the surface density of charge is g-iven by
t'he comarison of t1he dispersing power with. the surface density of charge (Figure 6).
It will be seen
that for the salt free agent the dispersing power is
very
to the surface
oa
po
-..
c _ proportional
n
jy directly
ver nea-rly-
density of cha.rge upo to the point of maximum dispersing power, after
hold, since
hich this relation does not
2 locculation
linear rIelation is
has begun.
A similar
found for the agent plus sodium
sulphate in very dilute solutions, but as the concentration inc-reases, the curve falls off somewhat.
-2-
II.
.
INTRODUCTION
It is well known that properties of sub-
stances in the colloidal state show a distinct change
as compared. with their properties in other states of
matter.
For this reason many substances which are
insoluble in water are used as finely divided aqueous
suspensions, the particles of which average 0.5 to 3
microns in diameter and hence are in the upper limit
of the colloidal range or just slightly above.
The
latter suspensions have been termed mechanical
turbidities or cloudy systems and are characterized
by an increased reactivity of the dispersed phase, by
a maximum strength of color, covering power etc.
Examples of particular applications of these systems
are found in ceramics, pigments, water insoluble dyestuffs (e.g. vat and acetate silk dyestuffs), compounding ingredients for rubber latex processes, and
horticultural insecticidal preparations.
An example
of the increased reactivity of a dispersed material
is afforded by dispersed sulphur.
If dried precipi-
tated sulphur is mixed with some plain water, it may
be kept in an ordinary clear glass container indefinitely without any sign of reaction.
If a sample of
the same sulphur is mixed with a small quantity of a
dispersing agent and is ground in a suitable mill
-3-
with some water, the resulting dispersion will react
with the small amount of iron in the glass and produce
a gray-black deposit on the walls of the container.
The method most commonly used in the preparation of such dispersions is mechanical disintegration
of the solid in the presence of water.
This process is
usually carried out in suitable grinding apparatus
(e.g. ball mills, pebble mills, colloid mills etc.)
and is known as wet grinding.
If grinding is prolonged,
it is observed that the reverse of the desired effect
is obtained, namely that mechanical coagulation of the
comminuted particles occurs, the result of which is a
coarser suspension than is obtained by shorter grinding.
It
has been found that the addition of cer-
tain water soluble substances tends to eliminate
mechanical coagulation and to produce more stable
dispersions.
For example, lactose, glucose, and urea
are known to aid the dispersion of indigo, sulphur,
selenium, and aniline black (10).
Recently, numerous
long chain high molecular weight organic compounds
have appeared under the term "dispersing agents."
A. Stewart and H.I;. Bunbury (9) state that these include the sulphonated oils, condensation products of
aldehydes with aromatic sulphonates (e.g. disodium-
-4-
dinaphthylmethane-disulphonate which is prepared by
condensing sulphonated napthalene with formaldehyde),
sulphated alcohols, sulphite cellulose lye and modified forms of this material.
They also mention that
soaps and proteins are often used but that these have
certain disadvantages when compared with the synthetic
products.
term
S.
G. Mark and W. F.
Russell (6)
limit the
dispersing agent to those substances which aid in
the production of fine dispersions and which are not
capillary active to any great extent.
This is in dis-
agreement with Stewart and Bunbury who include typical
surface-active substances such as the sulphated
alcohols and the sulphonated oils in their list of
dispersing agents.
It would seem logical therefore, to
classify dispersing agents as surface-active and non
surface-active.
Examples of surface active dispersing
agents have been given above, while disodiumdinaphthylmethane-disulphonate and salts of lignin sulphonic
acid are examples of dispersing agents with practically
negligible surface activity.
In addition to increasing the degree of
conmminution of the dispersed phase, these agents have
other marked effects on the physical properties of
suspensions.
For example, if sulphur is ground with
enough plain water to make a paste, the resulting
product will have a coarse,
flocculated appearance and
-5-
if
it
is
diluted with water the particles will form
flocs of a coarse net-like structure which will
settle out very rapidly; but if
is
a dispersing agent
used with the s ame - aount of sulphur and water,
the paste will have a smooth, even appearance and
when diluted,
the particles will not flocculate
but will slowly settle out to form a clay-like deposit.
It will also be noticed that the paste
made with the dispersing agent is more fluid than
the one prepared without it.
In other words, it is
possible through the use of dispersing agents to
make a paste of a given fluidity or consistency
using less water than one made without the addition
of such comnounds.
It also follows that the maxi-
mum amount of a solid which can be dispersed in a
given quantity of water is increased by the use of
a dispersi~n
agent.
As would be e:xpected, the addition of
electrolytes causes the flocculation of substances
dispersed with these agents.
The caiounts needed
to do this, however, are remarkably large.
A. Stevart and 1.
MV.Buunbury (9)
state that a dye-
stuff suspension in a 1/1000 molal solution of
dinaphthylmethane-disulphonate required a 1/125
-6-
molal concentration of sodium chloride just to
produce flocculation, and in a 1/500 molal solution of the agent,
the concentration of salt need-
ed was 1/50 molal.
This would indicate that the
agent has a definite protective effect which would
exclain to a certain extent the mechanism of the
dispersing action.
They also state that dispersing
agents probably owe their effects to being strongly
adsorbed at the particle surface with their
hydrophilic polar groups oriented toward the water
phase.
The hydrophobic nature of the surface is
thereby reduced with a corresponding decrease in
van der Waals forces and electrostatic effects
which cause
flocculation.
It is also their opinion
that these agents probably also increase the charge
on the particles which is normally negative and when
present in sufficient amount cause deflccculation.
J.
,. Mark and W.
opinion,
Russell
R.
(6)
are of the same
stating that the agents probably put
sufficiently large,
like charges on the individual
particles so that these repel each other and thereby
overcome the cohesive forces between primary particles,
thus breaking up 'flocs and forcing each particle to
.0
act as a separate entity.
s
The result is a true
dispersion containing particles of optimum fineness
with no appreciable aggregation remaining in the
system.
J. Reilly and W. N. Rae (7) state that the
purpose of dispersing agents is, by physical or
chemical means, to aid or accelerate the disintegration of the particles being subjected to a mechanical
dispersing action.
It is their opinion that theo-
retically the agents do this by precipitating or by
combining with the electrolytes which are present in
the material being disintegrated and which tend to
cause flocculation of the comminuted material.
They
also state that actually the agents appear to act as
protective colloids.
Since no experimental evidence was offered
in support of the above theories,
it
was decided that
this thesis should be a study of the deflocculating
power of a dispersing agent and its relation to the
charge on the dispersed particles and to other properties such as surface tension, and pH of aqueous solutions of the agent.
Disodiumdinaphthylmethane-
disulphonate was selected as the dispersing agent to
be used because it is commercially available and is
known to disperse lampblack (6).
The deflocculating or dispersing power of
I
%8-
the agent was determined.by an application of Stokes.
law of sedimentation which is
expressed by the
equation
V= 2 gr2
( d
- d
where V= velocity of sedimentation, r= radius of the
settling particle, dp a density of the particle,
dl = density of the suspending liquid, g = gravity
constant and 7?
= viscosity of the liquid.
From this,
it follows that if a heterogeneous dispersion of
particles of a given material in a given liquid is
allowed to settle under the influence of gravity, the
particles will settle out with a velocity proportional
to the square of their radius.
In order to obtain the
relative fineness of dispersions with the same concentration of dispersed phase it is only necessary to
allow equal volumes of each to stand in vessels of
equal dimensions for a definite length of time and at
the end of that time to determine the concentration of
the solid at a definite distance from the surface of
each dispersion.
The concentration is proportional to
the number of particles of equal radius which have
settled to the level at which the concentration is determined.
-9-
The zeta potential of a suspended spherical
particle has been expressed by D. C. Henry (4) in the
form of the equation
3
= v67'(2 AL * A)
D
3 AL
where f = zeta potential (in e.s.u.), v = cataphoretic
velocity of the particle per unit potential gradient,
I = coefficient of viscosity of the dispersing medium,
D = dielectric constant of the dispersing medium, A=
specific conductance of the dispersing medium and A
specific conductance of the suspended particle.
Assum-
ing that the suspended particles have a specific
conductance very nearly equal to zero, the expression
now becomes the familar
D
H. A. Abramson (2) has shown that for large particles
in dilute electrolytes the surface density of charge
on the particles may be calculated from the equation
aI
where
o
N
=
surface density of charge in e.s.u./cm.2
Avogadros number
j
-10-
D
= dielectric .constant of the dispersing medium
k
=
Boltzmanns constant
T
=
absolute temperature
cl(2)
- molal concentration of ions
Zl( 2 ) =
valence of the ions
E
=
elementary charge
S
=
zeta potential
For a uni-divalent electrolyte at 250C, z 2 = 2
and the equation reduces to
•0•
790
-
•/e77.61"
c
e
-
8.8 ,
- 3
+ 2e
7
(2.)
H. A. Abramson (3) has also shown that the course of
the - - c curve in dilute solutions of simple
electrolytes very closely follows a curve similar to
the Langmuir adsorption isotherm and which may be
represented by the equation
a where
,-
a-'
and
g'
(3.)
(3 c
= the limiting value of
-
= the slope of the a--c curve in dilute solution,
= a constant
-IIIII.
P4OCEDURE
ýolutions of the dispersing agent were prepared as
follows: 200 grams of commrercial disodium dinaphthylmethane-disulphonate (Daxad 11, Dewey and
Al-my Chemical Co.) were dissolved in 900 millilitres
of hot distilled water and the solution filtered and
evaporated to dryness.
The material was then powdered
and dried at 1100 C for one hour.
The dispersing agent was analyzed for
sulphate by the method of E. Hintz and H. Weber which
is described by F. Treadwell and W. Hall (11).
It was
found to have 13.31% by weight of sulphate calculated
as Na2 SO4.
No chloride was found present.
Solutions were made with concentrations of
the agent ranging from 0.0004 to 0.0922 molal (based
on the formula weight of 472) by weighing the required
amount of agent into a volumetric flask and adding
distilled water to the mark.
In order to obtain the dispersing agent
free from sodium sulphate, electrodialysis of a 30%
solution was tried.
It was found that the agent itself
dialyzed as well as the electrolyte.
by C. Robinson and H.A.T.
Mills
The method used
(8) for the purifica-
tion of benzopurpurin 2B was tried.
This consisted of
salting out the dye with sodium acetate and dissolving
the acetate adhering to it with ethyl alcohol.
In
I
-12-
-this case, however, the dispersing agent would not
salt out*
It
was finally found that the sulphate
could be removed by precipitating it as barium
sulphate with barium thiocyanate.
The barium
sulphate was centrifuged out in a tube type centrifuge and the solution was evaporated to dryness.
The thiocyanates left in the agent were removed by
extraction of the solid with ethyl alcohol in a
Soxhlet extractor until no test for thiocyanate could
be obtained by the addition of ferric chloride to a
sample of the alcohol extract coming from the solid.
The alcohol-wet solid dryed at 1050 C to a white
powder.
A water solution of it became slightly
opalescent on the addition of barium chloride and 6N
hydrochloric acid.
Analysis for sulphate failed to
give a weighable precipitate.
Solutions were prepared
from the dryed solid ranging from 0.0005 to 0.100 molal
in concentration.
These solutions had a turbidity which
was undoubtedly due to colloidal barium sulphate which
had not settled out in centrifuging.
Suspensions of lampblack were prepared by
shaking for one minute 0.100 grams of the solid with
exactly 100 millilitres of each of the solutions of
the dispersing agent.
They were allowed to stand
three days and at the end of that time the relative
degree of dispersion was determined by a Lange photo-
-13-
electric colorimeter.
Five millilitres of the
solution of the dispersing agent used in the preparation of the suspension which was being measured
was pipetted into each of the two glass cells and
diluted to fifty millilitres.
The zero reading was
adjusted and the scale reading for an absorption of
one hundred percent was set at eighty.
hand cell was then removed and cleaned.
The rightFive
millilitres of the dispersion was withdrawn at a
distance of one-half inch from the surface (this
distance being kept constant for each determination)
and diluted to fifty millilitres in the glass cell.
The absorption was then read from the microammeter
scale.
This reading was converted to an absorption
of one hundred percent by dividing by eight-tenths.
The absorbtion value is a relative measure of the
dispersing power of the agent at the particular
concentration used.
The cataphoretic velocity of the suspended particles was measured in
a glass cell of the
Northrup-Kunitz type, as manufactured by A.H.Thomas
Company, Philadelphia.
Calibration of the cell was carried out in
the following manner.
The zinc electrodes were fill-
ed with saturated zinc sulphate and the cell was
I
-14-
,filled with one-tenth normal potassium chloride.
Care was taken to exclude all air bubbles.
A direct
current voltmeter was connected across the zinc
electrodes and a Lindemann electrometer across the
sealed in platinum electrodes of the cell.
It is
necessary to use an electrometer of this type or one
with equally high resistance in order to avoid polarization of the platinum electrodes.
A potential was
then applied to the zinc electrodes, the voltmeter
reading recorded and the voltage drop through the
cell determined with the Lindemann electrometer.
Ten such determinations were made with applied
voltages ranging from 9.90 to 17.80.
The ratio of the
drop in the cell to the applied voltage was calculated
for each one and the arithmetic mean of them all was
taken.
This was found to be 0.22.
For any determina-
tion, the potential gradient in the cell may be
obtained by multiplying the applied voltage by this
ratio and dividing by the distance between the
platinum electrodes which was 35 nmm. in this case.
The depth of the cell was obtained by
direct measurement with a microscope micrometer.
Four determinations were made at each end of the
cell and in the middle.
The arithmetic mean of
each set was taken and the three figures obtained
were averaged in a similar manner.
This showed the
-15-
average depth of the cell to be 0.6460 mm.
width of the cell was 12nmm.
The
Substitution of these
values in the equation of Komagata (1) gave the distance of the stationary layer from the top or bottom
of the cell as 0.204 multiplied by the depth of the
cell.
To measure the cataphoretic velocity of the
carbon particles, the zinc electrode vessels were
filled with saturated zinc sulphate solution, the
cell was thoroughly washed with distilled water, then
with 10ml.
of the carbon suspension and finally was
filled with the suspension.
A Zeiss microscope fitted
with a 40x water immersion objective and a 15x
binocular eyepiece containing an eyepiece micrometer
was focussed at the depth of the stationary layer.
A potential was applied to the zinc electrodes and
the time was taken for a particle to move a distance
on the micrometer eyepiece scale equivalent to 45
microns in the cell.
The current was reversed and
the same procedure was again followed.
For each
suspension ten such determinations were made and the
arithmetic mean of them all was taken.
Only particles
with diameters ranging approximately from 2 to 5
microns were selected for observation in each case.
Large particles were selected for observation because
-16-
of better visability and-because particle size does
not enter into the equation for cataphoretic
velocity.
The viscosity of solutions of the dispersing agent was measured with an Ostwald viscosimeter
at 250 ± 0.10 C.
The viscosity of the solutions of
sodium sulphate was obtained from the International
Critical Tables (5).
The hydrogen ion concentration in each of
the solutions and in each of the dispersions made
with them was measured with a Beckmann pH meter.
A du Nouy interfacial tensiometer was used
to determine the surface tension of solutions of the
salt-free dispersing agent.
taken.
Two sets of data were
In one, the surface tension was measured at
intervals of about one minute from the time immediately after pouring the solution into the vessel until
constant readings were obtained.
These data have been
plotted as "readings taken to constant value" in
Figure 5.
In the other, readings were taken at defi-
nite time intervals up to 31 hours after pouring into
the vessel.
At each interval the first reading only
was taken as the equilibrium value for that period.
I-, -,.,
7 --1-~·
--i.
I
__
II
ý--ý-7 ------_--
-_
,~/~
;
--
ý
I
ii.
I.
II
- .....
ý , ý- iI ý
,ý
-1
i
- .
ýT .
i ,---
I--
i :- _ iý :i
- -, -
.
t
. I.
.
.
-
- .[
'- .."
_
,
-
.
I
'
- __I _- _ _
,
i
-
_ :
-- 1A
- - - I.-
-.-
-
ý --ý
ýý--
- ·;
-
I
_
1_
_l __ _ _ _
, ,-_ I
,illli-;;i
ý.ý-- I- l--
;IJq- ý- .--.1--.- _t
-ý._r
---I-II-.-'.
ý_'!-----,
_:---_________-1_
!_i___'--+'-+4_'._ýý-----7
I
,
-F-HE'l--l
1 ' ý I1-I:
ý..
i_-__
-----14-,--: f_ý______4_-7 -l!
ý"I
ý.
ý,ýt- ý
-ý-!
-7
4 -ý -ý- . _L I
_-ý
ý
ý -
I I
I
,
,
,
-; - _-; ýýý--_- ------ -, I.
t__l__LQ7-3_,O-__
_-1__r_-_
;-7--ý--,------"--:r
t---- -- -ý_'_ '
I __-_l'_ý______.__0:ý'
_-__...
_ý__---,
__;ý_'
ýý.1_'_.
-ý-.
.
--:!,
----,
--ý-ý,__;__ý __'.
I_____
ý
,
+
-_l_._ý__l_-____
-:ý
__
'l-T
-=_-_Wl_:_:-_'=-.--A-:---+ -----.ý--_--,
.IN
t:_
.1
;II------,--T
--:------ý-ý-ý__
--1
ý,----'___!-;-'-_
ý,
h
-I
11
1
j__.
_
_-'-;__-ý___'_.___.____
_
--_
_l__.A
ý_ý
-_'_i___.
I_1
-_ - ____L
ý--.--___ý__'
__ý__--_.
_:_-ý-ý_-'---ý
i
I
I,
I- I_ý__ý---i-,,
_--------_--._--_:_U4-=::-ý--.
___- ,ý
-[-ý--4
_;_
, ý-1. -__-ý___+----t____-_'---ý-7"-r--_
ý_
ý
,
-_ ý
.
-_ I - --
-I -
:-:::
1
i--ý
i
1
:::
1
-__ý-ý' _ _ _: ,
-I_ - _ .. -
-ý - -
ý
ý- __A -1
t.
-,-- ,--- - - - -_ý , ý -
-r
-
-
i
] ;
I
-;:
ý
ý
, __
-
.
-i -i-:--·
Y
!_::.i -A -i--i
__ - -
- I I- ,.. -. .
-i
-4
_ ..
ý_ - .-
-
4.-
-74
__ _ _ _ _ _
- i -I
:ý::
.1i::
I
I:
'. -:::::- I .-
7
i i
-!
~
-__ýI
_I
-
.
;;
_ý
= ý -::j
-
-
__:I__ _l_7 - . ý-7.:
;
_ -ý I - -, _4 __ ý_.-_._
_ _T
7-
,
i
_
, _'-r
-- _ ý
_
I-ý!_-:
j
.
- - -ý--. , -, _
, ,,
.
-i:::-(
I II
;
, _ý__ i
, II
:-:
.
- ---.- - i i -L-,-..
-I
- - - , _L _1 i -_r_ _
- -i
:.
:
-
ý
ý.
,
ý 1
:
.
_ _
i
rý
, -- , .
- 1
-- ý_ý
'_4--t ---; -'..
-, - -ýt i; 1 , 1
ý
ý 1ý
,
:
- f i ·-
' ' _
.:
- ý- __ , 7
: ,
_ - :1 ,
-'
:7i
_A'ý
,
.
'-
-- i
.
--
I
i
._l - -- ý -4 - -'
:ý ý '_-1- . -
,_
L ___ý
::i
t
i I:-
. .I
ý
I
,_
ý-
,
ie
ý--
.
--i-
I:
I- I.I__1- ý,ý---.. - - L -
-
.4--
ý-'-_ 11-- --
- , i_ l
I
ý
._...
-
'_1_1__l'_.__
-__
" i~~~~-;:'i~ _'__ý _ - _ ý :.-:-, -
I
f 1-1 1- -
~
i-- , .- , - -1___-I i _
: -i
_ _' _
_ : 7 _
j-i
--
- _-
I t ý,
.
' .
_
t ý-
. _"
"o
-
-iC I
t_
I-
ý--1- - -r - r _- --- _____ i -- r __
ýý-T - -4 -
I
-, _- I
, 1
I.
-
-
X'.
, ý,
·
1 -! -, _ .ý 1
- --.
--. _-_ __L 7 :
_ . - ý._1 ý.- -ý · .1
,,,
-,1 _:_ý
; -------4 -- _--: __ ý_
__-'
--, _ ý . - I __
'
. -_
I _ _.
...
- 7 ,- ._ý
I
ý'
_._'_'
ý__''I I ;
_=
-- -I
ý.
,ý1 I_'__--ý4
,----- -,t-ýI~~ý..
_-- :-- -'-_ý
- .-, -- _,
-.__
- :ýI......
ý __
,_ý_
F-..--.
'
.ý- --- I-ý -----.....
_ý
,f__ I.-. -ýIý-1---.1-1-1t
?%'ý_ __ ý,
I
't- - ----ý-- J, - 2 ý'ý
I....-ý_,------- ý--- ý-:-'-ý
;--._ý
Iý-- - ý- ý; .--.-,-!
-- ;ýF:
- ý-l-_
I , ,1 - Iý1-1
!-,__I--ý_i___. _ I ý- -_---f-j- - - --- ýý---ýL__
tý _ ,
,. I .- T __ - -.-- -I - I __I - I ii '_
- __ý__
, 1 ,ý:
I r ý,
__
-_
i-_-' _;_
___ ,- --ý_ '
.- ,-,-,- I7, ýI! - , , -,. - - ji -- I I-.t_ _ ,
O .----------r--- -,-1
I r :I _- 1-1-; I r I_- ,ýýý7_:ý._
iI - t - - .ý . ý- - I ý. .1ýý
-I - -_ I
ý.- 7.;_-ý_ -- ý_ I, I-iý ___Z
;-------------iý- - --ý--- ----, ý-- -- -- ___:lN'
.. . - I .
71!
ý - -_
.- -.ýý-ý .-.
- - ' _ý l_ _ :-L _ ý. ,
ýý
ý ý I
! ý _ ý ý- ----- - ___ I . .
___
4;___
_ _1I __ ý I . -1. - -
,
-
ý
_;_ ___I
-1-:
.-7
I
-
_ ! _ .-
__T
- -i
ýi
Iý
I
.: ý 1- .-i- I _- - __
_- _ _ _ _
t
,
I
L Iý . __ý._.
- ,
'
-ý
- - - ,
I
- -- ' _ _ :_ ' _ l ' 4 ; _,
,
--I- -
-,
ý ''
iý---.- i -----r--···
:
:-L--r---- .
-.ý1-1-__
I'-,;-- _ý
--t--'-7ý-'', __
--i+__
_-f-_-.-__'ý__'_
lL__
--' -._-1
-___
-ý
I iAQ
iý.__-_=j--I - i-1
ýt,I--jN-"
_ýý_'_
i__
L-_
-!-,---,
i tl
- Ht '-:--iý
!
7 -[-,-::- ' I - -, fI
-1 _ ---'- '-"
- ' _1
"._:--- ' -,_:- -I,-I -'', _'__
_r I0___'
ý -:
ý 7ý ____--_L _ I_
I
, -4- 1 -7
-,
-_ ,- ._ý___
, ý-.___ýi7:ýpjý_
_____ýý '_
-ý
._l- __1_7 7 = T"--.---.ý_11_
__
t--ij
- I ý-ý'-A'
___
ýI I- -ý_i
- __
'_
---'--I-['-.
ý_-:-,
-r
__
I
--ý6_4 _1-1-1
--_'-`
,
_ --__-_ý
' ý I---_:.;___-__ý_-__
_- .--- __i
_- IlT----N.
-_ I-1-I-ý
i -:___
I I- -_-i--ý_'_-ý --. ,---,-.
- Iý
--- "-:
! _i_'_r'- ; r -.--. , _t____4._
1
I I ý 1ý 1 , i , i -_ - -. _'__t_ - _. -,-, -- IF ,,r -, - i__'
-. - , ý
_
1. ý'__'
,
_
_
ý
t
ý
_
_
_;i
"
I I . __! -1 I -ý _
ý
__ -- - - _
E., -_I
I ý
--j- - L__
-11 -1 ...
i- L- , -- ý : I I___I
I ;-- J. i -,-,-,.-
'- -*= - -_ý- -L-V: - .-, --;_ -.1 I, 'ý__
i _
ý_ý i __ -_;--------F]
-t-- '_7-It- ý _. I . . __
ý_' - -ý I-ý-! ý ýi -- - ý:
i ,- ý - - , -! ýI:
-I
..
ý__'__
-,
,
-ý-__.__ -,I----:
.ý__ '_!____i'_ __W,
_._+'.L__'_'__ý_ý_i
ý , ý.. -:
- ý'_7!,__ ý Iý I, - - ___
I, I _______i
I I -I._ t:.t I
4
- 11
ý%
I~~ _
_.l____-.
---I
.
__
__
.-." -I-i týI __q::Zj'
- : -1.1 - __
I __--;:-- _ __
-- ý
F ..: _ý
- ý.
o I
;
_ __ __ý-
-__---.--.
- -- i -ýI1,ý :
I
ý t
I
i
- -ý _ 1
ý-. __-ý- ý 7 - - - -j ý T --'
_
-I- :-- 1 ý- - - -- ý
-_L
4
. "4
- I '-'-"-I
-- i ; '-:·- 4
' 44 _
V.
;'
j ,"
ýi__i_';"__.:,---_J_-ýý
I _1_t'1_ý
I..
Iý____ý.:__
I
,
_____-L__-___4_j__'
---'-t-ý
=
--__
-'----1__
-.-'-_----ý-'
_
__l
-,--I,----ý
-'__.__-___i_:_
-__._
-ý_:____
I;_-_'_F-_'ý-'
-_+--1,'_'_I!_ý-___L_.[I
---, 1-j-il'
__
ý
-;'iv-A
ic ..- . ·- ýI I - . ..
.
..-... I . I .1
-i _ I_ý
_
____
-t
_
__'.Lý
-_
___4
t
_T_-.-_
-------_
_L...i
I
I
i
I"Ij-/- - -- -'1
-;-:-_-_-_
, __
ý -l--·iT-!-;ý ; -, , .,
I
LzV'l.v S %i
I- -':, ýi- I f-- I- i-ýýýý
ý
;
,1 i!
I
.- TI -.
..
:
"I
-I
.i
---.__!-ý- 1- ;ý--ee
, ,-X-i_,--
t
4
_l. - -
-
ý'ý ___"_
_L
l -79.7 -*-~i
-~~
11
ýi_'
. I---I-_---,-- I II
_;
-1 -- ý
ý-----.--.
- -_
I 1 i-t--ý---;-'-i__-ý-'-'
_
--_:ý
_'-'-_-l- __ll_--j-IFEE
-1--_ý-ý_-4-__ý_ -.I',-- ý i1_=-_.
J
ý _, , . I ý - ,-1 _
i _ ýý
-1
- --IA
-,--_
t - -- ý .
~_. ý---k , ---1'/
-14ý:_
Iý , "O ,
- .
4:.
I- __:
--
.
-1
7
_1
ý,MLP-Z-L4RL·_1A1_d7
p-
- I
-
ý---!--
I
I I_
-
-
_ __ I- . ý-I - ý - ý_
-,
_
ý.....
...
.____ _
I-
-
I
I
' I__
I
-I I
. - I-1--- . - _ :
I --
ý
_ , _ __- - I I.- ----.
_i-I __
-
1
- - i
i I1 -,-
- - ' _ _- -'ý' ' I :=ri
:-
,ý
-.-
t 7 7t7 =
ý____144
_'_--'--ý
,j- ý, __
,--..
I- ---_ -______'_
I. ý_1--7
____
_ýý__-___ý
-_
.tý I,
I , Iý''__
t-,.-.-L--TI;_.ý_1-1
_.!""Wl
_1
_
ý ý_ -7
1
-
t
ý--,--___
- --j---7-ý=-_ý_'_:
1ý7I-----.
-_ -,i _'_
-,-'_----__
ý------,_____.!'___
I ___
11
-. ---:_. ý,--1
__'
_-l_____l-I-___ý'
--1ý4
.'T-F.=1-ý-7
_'_
ý__
'_i_,
'-I,--ý
---.I ____:
11 -- _1_.I-ýi____ ýi ýi i ý II - :
I717-1
-. -.7
_'___
,
-:--1ýj
I
-1_I__
ý- __i._l_-_'__l_
-.:
-.--=
_
I
li_.__1
__
- ýI-'_
ý'ý-z
ý-1I-__ý
I ý.,-Iz- 1ý! ý_---ý-:-,- 7N,
-ý
ý
-I
__
.
-.
-.
_'_
,
-1
--'
-_
-, I ---__ -_
-1-___--;.I .-----.. I- 1,'_ý--.ý-1-,-- -__
-, .-ý---. -:. ý----H_'_- -.-iI-ý
---:::A
-; ,
---,
.
ý
ý :_.--,.-ý- ,ý -I
,ý__
--__
,. I,__
,_.-_
4L_
-- .__
i-,_ý--4--_-,
I
,
-I
-I
-I
_
_
:
..- ......n - _ý- .- ! . 1ý. ,._-
I
-
-i---
i---
-,---------;
,-
---
_-1__
_
-
I_tý ý
ýI 7
o r, -
1-ý-
ý .
__
: I -.
r~i--:I
ý--- ýý-
i
-ý-i
ý_'t-* -
I-
.
- --.I-,- _. I
-
I -1
. I.
ý~0
_1--3
·---- ---·- ·--ý--·-----.-- -I---_ _--_
z
-
'
I
-.-
- .
-
-
.
-
.,
-17IV.
RESULTS
The results are pre.sented in
graphs in Figures 1 to 7.
the form of
In each case the abscissa
represents the molal concentration of the dispersing
agent or electrolyte present in the solution or
dispersion.
Where a mixture of sodium sulphate and
dispersing agent was used the molal concentration of
the agent alone is given with the corresponding concentration of the electrolyte below it in small
numbers.
The ordinate represents the property or
characteristic which was determined.
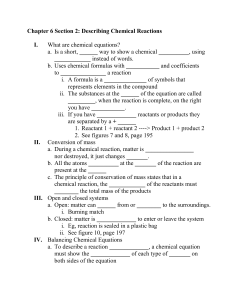
Figure 1 shows the effect of concentration
of the dispersing agent on the dispersing power.
It
will be noticed that in very dilute solutions of the
agent plus sodium sulphate the dispersing power increases very rapidly with concentration to 0.01 molal
where it begins to increase less rapidly until a
maximum is
reached at 0.045 molal after which it
falls rapidly.
In the salt free dispersing agent a
similar result is observed with the maximum coming
0.08 molal.
In low concentrations the dispersing
power of the salt free agent is
less than that of the
agent plus sodium sulphate, but as the concentration
increases, it increases until a maximum is reached
which is
greater than the maximum, of the agent plus
sodiuml sulphate.
It
will also be noticed that up
p/I o-r
L)a/Al
iC~A0A
MA
PI-/TAIYL /'W77A N/
ji-fePJ/;
0,
Vp
it~~yI-etA~e
Cc~:bo::rdyox-..4*j-o
#"4rare
d/D~
w/t4
.4
~~ohr·te
ImiQ'e
T5E 501 C17/TI
6*/'/t~
-
fi
~ee
f~'o'i'-
N:SOJ__
a:.~._
J0
J
6
5-
4
0. /01
'oQ5
N OLAL
C ONCE)VTRAT7OA
-
_
··_
·
·_
ýý,
------.----.
..
.I
.. . .
I .. .-:--,:-:--.
.1
-;.. . .i-4
, - i ----...- - ..,.-..
!-,, ý-------ý..
.:-.-.. ...: ..:- .-::-;. ..., .:-... ..
1. ..
-...-,- ..-.-..i-.-.-....:
a -!-.----4
. .
-. ...._.-.!--..-!....:.
- .. .. - ; .-. .;...... ..7. .!.a".-''-. . -- .
......
-,
;.-.....--..----'
_---.:_ :~IT.1
. .. : . .I
.
-:.,
- -t . i. I .......
. .
F
..I:. .7..-,...
... I...... .-:
:- -- ..
i-il
. .-.-4-!.-!-:-.:-.-:
.......
: :~-:'. P.-.. ,.-. ...i.
IiI..- ý-....
.
4
- ,ý .
t-I- .
.
. . !i·l ýim. :.-. ;-i %-.
'..
...
..
,
;..I-.- .
., .- . i T
: i :-- .
:-, , . . :. .: .. .
. .
. : I.!...1:-ii .-?-! - 1 f ý :,- .I T
I,,.-I ..-.. Ii
-:-.-.ý.-.
i;-l- :ý-,.1 i ., ..:-., :..!
.. ....
- t --t i:im--',".m--ý
I
.
.
..
.
1-7
,
i
.. I.-M.-:.- - .
. .
. -ý:-ý.---- ý:..':. -;;ý:.-;. ý-ý.!..ý---j-.ý.---.-.ý-,-.i,-i---:
. I m.
ý-:
!
r.-..; ,- - ---. I, 1- --:-i-. 1Awo%
. : .. ý
I ý-. .1 i - -... -- ....P..
- . -..-... ..--....... .- I
jLLi
I.... .I .......
.-- - -.-- , . .. - - J.
.
.
: I
.... ... ..i.ý .., .t :..: .: ý...-.I
A ...
.--.. - ý. -...
III ,..ý..ý
I
. I..
:. .
..A.---;..-....
F. . ..-.-..i
- .! -- .i.--.-.---.--'-. --,...i--.-I!''
- -.--iý- --.. . '--!..,
ý -:.
..I. ------.
,
..
L ....-.-.ii-;--- - - i..ý_.;...
1
-...'.
i
-:.:
? ..iii--- -. - ---.... I .1
- - -. ; -- -:-; -: .
.
-1
- :-:
, , I-,.;.-j.--.
ý.-
...--......
:....
..
.........
:..I
. . ....
. ....tI....
. - I I . . . o-b
iý--.::--,,,--.-.---.i.--L-. .-.
-- . ;...-; ,-:: : -1 -:_-ý.L
j".-: :..:
--.-'-....-I
ý-.-I....
...-.li--!
ýý
.. -...--.-ý
.......
- -.-. -,.j.!.j
":
777=......;
..
:.
.
.
%
.
,
ý..-ý
..
....
...
.
.
....
!..i.-I
_
:-..-ý.i..;._...
_
r --_:
i-,;--:-4
, _:! _ý.__.-.-.
, .r-.-:_,
I .:,!
iI4- ..:ý_
Tý
. ....
...- ..
. -; .'. . ...;; .
-..- l
- L.4
a-%-!
-I;-t-i
-- .:;..
:....
'!!
'..
.
ý, .. 4...
.....: .., -- -M...
:. .:. ..-T.! -- :-ý-:
. ......
..
- - -+-.-.:...
--ý..,. -.;ý.
V.-" --.-i--ý......
.....
i-;-.
-- - i: ,:.Lý......
.--..
.ý...
-..-.ý-47- "-ý'.'. ..-....
i :
- i-,
I.
i.t ; ...
;----...-:-:-T-r-ý
.
I
. ýZ:ýj--:-7
ýý-ýý
..-!
.....
...
:.-i
....
ý.D7.: I;7ýý. -:...
i.-..--:-.-!..-..
1.1--....i
:1--ý---i4...
4--. i-i; I ý . . -,-, I-.-..-.+
ý;.1.
r-- -1. .!..i-L-.;--.-- i - . i - -.i,.i : -.- -- 1 '- ;-I--
ý 7-7ý
-
:
.-
..L -.ý-i- .ýJ.ý .
!.
..
I1
i ...
... . . . I 1: Ii
.
...
; -. . .- -; -- ...-
.
: :
:
!
- - . - .. .: ! . - I
.7 - ..-. . .... - i.-
..
. t
; --. -
- -I r .; .- '!
.
,
_ ~__·~·· _
. .1
; ,a:
: --
.
.
. I.
--- l.
.
. .ý. . .
. . . i ý-'
-
.
..
i
. - 1..
.I..:- -..
.
:ý1 ý,
.
:
_
...
! ..
- I
...
:
.. - .
. I .!'T.1
- -·---; -~
:.-.ý i. : I ý 1.
:
. .
:
I
;
i . ....
.. - -: ý. - - ..i - -,-. - . i- . .ý
I .:
,
. r :-
, 1
..
ý.i . -:- . - ...
.
. - - -! .-
.
-. -
: .
1 : :L .-; - _:_!.
:
.
-ý . .
...
1..!
r.ý ýz'".1,1; .
..
..-+ý
;-..-.-..
i-.,..-,---I...
-I---.i..ý.!-.
i-"'
....
..
-..
_:_;
-ý----.
r-;-;
-,.---il..
i.-,
-.-:
I.-..!-i.I
-,:--.,-, - t-,.----4-.
:---:-T-.
: i---; i ---7 : ,- -- - i.-. --.ý:i...-- i--- i..i .. -I,......
:.-ý
.i-,
...1,
.-_LL
.4.-----:I-,
-ý--ý-ý-i-..:-.+--:-:;
...
4...
.----.-q----;-1,
4ý
.L
ý-,...,.-t-i
ý...ý! .--..-Lý-..
. .-. -.:. T 1 -1 .......
:: ýý
. .j---4+.
. : If
. . .-L.I
..
...
-.
.
I
.
.
ý --- --:.,..11- .-..4:
--,!
- - -1
'-f
-tý."T
i .-4
ý-.-;-.ý-.--.
....:..ri .T,--T.ýý:::-jq---ý..[:;-:
~
1--11,
. . Pý
.- ý;
I-J-1
ýL--,.----ýi-...".."..".."..".---ý!--.'. ;rj..
-i----
-.-:-"4--.,L- -,ýýý'.
-,,'--,ý!,, II.11
ýJý!
- , --..-- - .- ýJ
.11
'. I. ... I. - ..-r-1.
-,aniw
..-I
-;........
-t-1.
I-I.-,.---.ýý7
...ý.... .-.ý;i -I
,- - -;--4m; ;....
-... .. --so
,.
--;-,-,.-4---.,.
_ ý-Tj-,-.....-.
: .---:.-i.-i
-i...
ý-!-.,.-!ý-ý....
1-.-. 7-;-..-,.
.1ttt
-.. - - ...
-- ;._.....
.:... I .ý I .
.. --: .,I-.;r..ý--, --ý- 1.ý
.M-.
.". ..-!-..
-..- :.
.! ,:ý .1
. I---.---. I:--ý-Iýf-- :r .-. --:-ý+
, . rl. : .L
ý-.
-;-ý-ý
-:-ý4-"-i
;---r.
.
;
.- ME7,-.
..- -' .= . :--.- ý.-..-....:--:-4-i-- -4-"- i ---j_:_.,..
!
-4-4_ý
..
.
.
-!
I
..I - -riI .-.IA-,.-,.--..-.
-4".
,
. . ,--A--LýI..- ---t-ý-jý...I. -. I.. ,---.
.mI ý ;ý.! : .---..
ý4ýI i. :
'7ý
-.ý
;-.-I.-:-.ýý
T
-,.-.. ...-;--. .. Iý ---:-L-L
7- ,- f-- :-D'-ý-'-,=F'--.
L-
· _·.__
..
.. .
!
. .·-:-
I:
.
. ; ý , .ý .- . , I. ; j. ;-. ,1. . . . .
' :. . 1 1 . -
--;
: !ý
..;:.,
4.--,-4,,.I
'.-, .,
: :-ia: .-i-;.+ !.,-1
ý-T
. :-. I,
;-:,-,,
. .:Iý-,.--.
: f.: -...
: .. I-.-.-...,
. --....i
I .. r-i::!.-!-i.-..----.
L--..
,.-L-;...i.
--.ý
..0-:-j-,
1ý,.
;:-ýýý!.--tti--.
7ý'
,-;.
-----..
-i
ý-..-......
,.,.:
i-'
-,
iý-!
.,
_:
-.,:....-Z.L
---..
..
..
!
..
.7w.'
;.-,-., I- - .rt........
--..-._ .!.ý...
....
.. .
. .
. .....
.;......- . ý1...r..:- . - ...
ý:. ;
. .
'.., - .! -...].-
. .
.
:..-....
:.......:.F.1.
i ...
--ý7.:,..--;-.
:--r.::
'...--m7fý--T7-j:l
-1
I':;:..;-!:.-:-;.i...-.
. ý-..;:...
i-,-I--.....ý-.ý... : . ý-.2I-:-'-....
;
...
...
--..
t-A.
.-.
,
-..
tl.1-,-. -..-..
-...
-7
-I...
ý
1.
--,ý:
a
-......
:
.
.....
m
-.....
4
;..
[
;
.
.
.-I
ý
,...'r.---ý.,;
-ý
ý.-;
....
.....
-7
_4
...-.
-r
.7ft....
:...-.-.
..-.....-.---,.
.--"-.
-.:
.
-.-.l
;I .-..-.I
...
..
ý
_;
.
-ý
.
.
.
..
-.j
...
;..:
...
1----:-r
,:
..
.
.
:
i
-:.4:-,--',:7
[-EE,
.
:
-.
!,:ý
-,
-!
-,
..
t
-.-'. .II . , , . .. ; ý! ý-- -r-
..... -- ,
..
-
, -- -
-
- -
.
% . . -. - -. - .. - ; :
- - -i
.
- .- ý - ý ý. ! _
. &
- .. -
-
...--
-
1i
-, :-I;
,
:-~
. ..--.
i...
- ..:.
:
.
. . .
:
I '. .:
.
:
. .
!
..
- -
- - - ý ý-
,-..i.I
. 4-+"-;.4
i--.-i-...-..-t-,..-=
--.
. .%
.-. . .ý-ý!-:-Hrl-!- ...I
ý;
--!
.1
.r'.: :ý..--..
.'--rýfý:--:
.m
..I
!,
.--.:;-4.-ý-4,..
. ;-ý-'-.
! . ..-C71t:. : ..7. -.j........
- I 1-I.,-,--r,
. ......
. ,LI.L.-dL" .-,-II-,!,.-.
I.;EPA%
I .--., . i..ý..
-+--.ý-i-------.!±.-.1;`- .--:-i
., ,-.....
ý
m: . .m .
. . i : : : ; ; ý %flW-....
II
- : T.!I..4-..--.-,
- -- - .I
------TT
J-77-r-T
I
.
7.....?
.
.
.
%
.
.
I
:
.-I.;-...-,.--,-"
-P.--ý--.,
I. -:. .....
ý1.1.-.I
.
. -..;
-.-!..
_7
.-i.-.:
r
.-,IT
.
= . :,;---.-.-.
,-ý
-I-. ---r I : ; I ,....f
: - . ,...:-.;--.----..I I
.
i-1
;---.-!-...
........
- .-:-...
-,.-ý-.ýý
-.---A
ýA-.-.i
1.i .......
-+-1--i
. L--.,
:-- .-.
..!..-.
'..-..---.
.-...
.- !-L-l-,.- .
..,-j,.,-.-!---.-.-;-..-.;ý..--ý-.--.--ý-.ý.-,-.-i
:
ý. - - :_4-j,:.: - - -- , - .:..L....!-;-.,--.r..
..
---..-.---I .
. :!;
I
... .
...I
;.;;--'----....--ý -ý-;;---- -l...". ` i ,
, ý:. .ý4-I - ...-,-..I t -:-T
ý.,-ý-....:_m
--..--.-.--..---,-..ý-.%.::ý.-ý
,
...:.:
--- .- . . . .. .. --..-- ., ....
1 , i....,
r ;-:-.-!.-.: :.
.1 .....--.. ,-'- ;. i! ý-. ... m.,..!.-.-..ý-"-",. ..7. . ---.;.-;.-,
1.. I-.
i .- :.....-..... ý--.-4-1, L
4 :-ý-4:,'.
--;---.7.-.1
-,--i-,-.;,--.
,
ý...
! . .-I.-.,...
:,.,...I-..-.
. .....
L-4-.--4-.".,---m--ý---.ý.
.
; . I
. .- 1.-...-i..-!-F..---:._;
. r- ý-..
.
-- .- :...,.- -..ý -- ... !;-.I -,-.. I.-.--;-.-.
. jý ;
i . It AK ... :
..
-.--- :..i.".;,.-;._.
f
;
.
.--.
,
ý.
,
,
. ..
. I......
. I- lý.. - :-.
.- -V
. ;
ýi--.- . -..
-.- 1-4-- .4 I.i. r .. .--- 1. !--.. .1--:. :...
-;--.1
,F-i-ý. ..;
- ý.-t--.'L
..
-7-:-'-'!
7
-!
ý---!.-!
-.:..
:-ýq-.......
--,1
--!--;:
.;.---I
.% . .1 1 :--Im. ..
I -:. - m..-.I--.
.
- - -------L.1 - ý...
. ;,. ý ..... .7
.
ý-ý...
. i ý-. . ý-ý:
ý-,ý"!,.-,-.-,=. ..!-I- .
ýt'-ý-4, ;:-7: . . -7.- -. - ; : ;-%
;- .ý- -77 .
-:
I ..
. . . -.. .. -.. ....I... - F. . -..-..-..
I ,-----.--,,,-ý.. .. -.- 6-"--,--.-ý
,. . . . ,-,-.
.. ;
. . Iw
: -.6 :I i- 7- - - -` , ý- 4-.
..
. ++ý-;
-- .. -.4-T-..-r;-.T=': -rf;_I
.:I .-4 i--"----.. -:..:
- .
-_;.-;
--..
.N..ýI-q 7il.ý-.;
.rp.,--t-tý,...
ý,.-A
ý ý. - .:, -;
'1-71.
:
.
.
.
.
:
.
.
:
7
.
-. 4ýý-;
.-.+-I-i,-,---ý-iT+--ý:,-,-.ý.i..
ý-+.-.
F;,-:...
'--i-.
i
-...
-..
!..
.
I
... ..I . : ....
...
. . .-L.ý_:
E.-ý.ý.-ýi....;..!6-E.
17--.4
1-.: :j -1------..
-.i---ý- - :
'. 4.
".
-,- ý ;- - 6-r - - -ý- . I
.1...-- ;..-.
..
W ! .-.1.I..-- -.-. -.-..... I . ----- -.- -- ý.-ý-;
- ......
..
.....
i----i-.
.-..---ý-.
".-..-..--v-..-.-ý-4-.-.ý..
ý ý
4-ý
--....-.--., +-11-11%4
i.-......
i! -.
-ý-.-,
.7-4.-ý,-,.,-.:
ýý-.-.ý.
- 1 ý:;----J--
. : 1:ý
i-.+-ýý .i.-.-,-:-7-+-ý-ý-:';--;
.ýZý.
.1 ..t-.-iL-ý
2- ý-....4ý. . ......
..ý. -. ..-,;.-ý'..
F.--..
.-.7
ý-,.-ýý%ku!-,--;.
--.-.
!- ,-.7. ..-;.-.-*ý
-.-.
i
...
....
!.:.:
..
......
...---LA
.-....
ý..
---;
ý--.-;
-I-L
I ---.
.
.--..:-77-7 ! -ý-:-ýý
I .
.- - !
- .--. -;....:;l ---.
.
ý
.;
-! - - --..----, ý
'.
!ý ill3jN-.
-.ý _ - ' -.-- ,. ..T---.-..-..-.
.
. ;--. ý- - - ;.-.--:-j.
-7;-:; ., .I.;.-.l-..-l-- -- .ý . - I, . .- .
. -. -1-1.--..i
.
--%
.
.
.
l....- ,...- - - ,, - .:- ... "--.!-.-,--,----.
- : .ýI. .:,..,-..-...-ý,--fý-. ý-.-.-:
:_.
.
M..
-.;-i--.
-.:
;- -- .-. . .- . : .. . .....
. I---ý-..-.
. - b.-. ..
.
-1.
. -1
; -.-.- :-I
I
I-, L
,
.
,
-iýý
-,
_
:
-,
-.
Pý
-..3
.-ý
:
.
.
,i- ----. ..
I
--.O ...
ý-...-. . .. 1. . - -.- --i . - ! .-- ... . .
: T
1-i ý - -;--.....
.. .. 1. ... .-: ..:
...--r-., -- .-......ý .-ý:.-.; -'..,I .-,-.L..:.I M.
. ý. :-;. I.. .
I !..
I- . !-.:....-:--.. .!-..--...
.....
. .:-..-...--.- -.ý.,
...-..
.4
--.T-1..
-ý
...
.....
-.ý ..: .. L .. .. ..- 7ý . ý .. ,
- :.....:-- - - - - ý .. .. . . . :... ... , .
I .........
7-"- . .-T....
....
iI-:-...
..-.--.
.... ., 0-.... . - -.-- .7.- . . :. - r
: ...
. 7 ..,: v ...
:m...
'.7---- - ..- - --. .-1. , '"I ý.
. i . . .. .:-..- .-- . -ýI
. .-- f - I--- ------.- -!--'- '"'-".
.--- i-. :.. -; -- I-..---...
:..... - .:.. .... ..-:
.ý.. --.-.I-ý- ,.-- . I- .-.-.- .ý-7---.; .--- - . .. .. .
ý------ ... ..;....- --...
.
......
-I
.-..
";
-----ý
ý .- I;.4 , - -- .
.
. .
. .
-.ýT-..
; .ý.ý,-..-.ý,.-ý-4-L----ý
i--=.,,-..44,-...
. -. .:.
-. -i---;-,..
;- + ;..........
...
: -ý
...
....
.. ":7--=
7. '.. I---....
,-.r-;-..i-.-:.
; , . -,7=t----,-,
.
=,7-='-7,-7-rI!':ýý7-, :I=.=',-.
ii"'-i: --1
. ---m
i.....-:.t.
I--:.-.....---.-,..!.-....L..
-.1- _m.
--4
--.
1. .......
.7
.,---.
-.-i-.i-ý..--,-4-i--.-ý--.-..-,..I.
.
..
c--;-;-.
.11,
.
.
..
. ,-ýýI"LT-m.
-.......
..
;
.
.-1
l-..-.-..-wJ
F-:,--.If 4--!11
-,......
:;------.
a.q-1-:
..
-.
I-,.1..,-.-t.i....
.-ý-.--..i.J.-.7
L.-:
. ,.......
;-j
. -.---.:-.--..,
ý.:l-'
;.--...!.7-.,
.---.
-.-.,--.....
. .I--.ý-.
.. :....,...., ý-ý;-I---i., , .ý.-.:7-T.I L-:-...q.i.-ý
. . ......
I!, I- -;-;-.!.
i i:...
:.1..-.4-.ý-. ., ;-: -r--,.-t
11
ý-:-.-ýý-!--;.-.;-.;1...-.,..;.
i V.- - -;--7--i
r-7-'-'1---- ...-... 1 ;..; .;. ý:
:-.- , ý--! ý , ,!--ti -7ý --..
.
. .
-i
---j
-...-....
ý
I_-.-.-.....!
.;-,....4-ý-; ý -.-.. 4-- II I
A . .-.
.-T-.- -,. 1ý .
- -!--..-.
----.1
.1-i..,
--lI.4!.......
-.-..
...-,--....-,
'.----l-...---.. ý.......
---------.ý
..
=+-....
-;.
ý.I.,.
_..-....i--1-1
;...
.-..
ý1--t4-.-I
--.....
,.-.:-,
--;-..-!
:.-;
_
-.-,
-f
-..+
+
:..
.
-ý-.--....
,
!.------.i
...ý....
....
..-...
-.-.....--..
. ..:l.-.--l-4.-.
,-.-!!
i....-., ý--.-.. - -..-ý....
-;1--- --....
-,
.L7..;-!.;
-,-.-ll-;....-...-,ý
! -ý..,-I-.-.
i.-...-..! i ý.. .....71-..
--....L- .I...
--!ý-;
--.
ý.-ýi.-.-4
.ýI-_.k.Pý- .:
. .- - ----I ..-.--.-.-ý---, : . - --. :- . .I. .1.:. -:-.-.. :_ý___
. . 1'. .3_ ;
-;
.. . . .
.
. . . -- .-;
. .- : . . . . . . - ....-....-. . .. .. - : - -.- ..--.
..
r ....."•- '
- . .m..
. .
. - . . -.- ý-; -L - .. . . .
;• i
,-- ..t- :- T :-r . - - -- --- :
-ii_ _:.i
-/ --i : "
. .-_.:_
-..-•. .•.•:.•-j ,. i·-••..-- •. -'---•----i- .---b-:--'--:.. . t "-=-jt-,q-r-•"'-·- ·
• "._".•
....
:"
T•
- _!-.I....-' -i L- -- ".!
... .
".. .. .. .. ',.
•_ . .... ...' ...
.. . !-. .... !i.;
;-...-.
". - '
•
-•
-- -- -,i
-- ....
-. ii- . +..
. . .
. . ....
'".
--.
,.··. .--.-t
,.
-I-•- : • - .. ...
- . . . . .. -i'. ;.... " - - -1
.. . ;• :._i
i ... . . i. "2 7.
-- --- "
-".: ...- .i L -/..
- I -.
- I-- I. . .-.
"--" ' . .......-: T_..
..
-.. -.ý---;TT
.L
• i .I, -.. ....
-,
.
- -I.-'..
. . ý - ..--.... . •: -L
" .. .. .- "- i ... ..
•
i ..
,
. ."-' ;
•i'L
i . .- -;- I. .
..--. '
-. .E. .. "--. . !.. . . _ -: . .. . . ...
.. --......
-" •.- . ..- ..-. . . ... ý...;. _ ... .. .....
. .. ý. -.-..
... .. .., . .-- i,
I...".l. --.!.W%.. .A ' . • .. ..:.
- ..
..
.. . ;
•.
•
:
..
. . ; ! ... . . .. .
.. ; ..
. . "
-...
ý "
...- .- .. . .. : .- .
i - : ,..
- _._t.
.
-- .-;
-L
-..- --- .
-ý
-; L .- -.
..
.
. I. .. ....
- -- . ! . . , . .. : .. . .
. . - . . . , -. .
..: . - . . '
. .
. . ..r I . .. • ' . . . .. . . .. . . -: .....
. .
.... L.
:......
. r.._.-. ..
..
....
-.- . ...., .-. . , .--... .----..- . . ........
!. -.--
_.:ý:
.
, ..I.
...
....-_
o. .. .. . . i - ; . . ý .....
. ..
1 '.· --- - --i;--·...
...
ý .. ., .........-..o . ... . -, 1
_...... •-_ ....
:::-•...
.. :...._
• . .:"
. . .. ... i-;:l..
; " "•
..
"_
..- L_ '• : .--.--.
.. !.. ..
"..,....
II.2 ..
.. -;A..
- _.Y
.-."L ...*'I.' J....-" _. .
. . .... ' ...
-.
.L.'
-.- .[.- _.. :.--.
. ;" ...
-•!"" i '
. . .
.
: . ...
0 -i"O1'O
". ]
"
. - .; • '
'
! ... . '
. . . • . ..
.
. zF: :.ý ."ý
.
.
.
..
i
-.
.
i...
.
.
"
•
•
. - : -.. ., .-- . m.: .. - - L j . - --:- -- 7
- ý . ..
.. I
'
"
!
". - "m
" "
"-. - : - . , .; .- .
.
;. " . .. " . . . . : . :.
. .:
. :.
. . ' . • : -.
... : - ... . . . .
. .........
... f....
-"
I.. .......,
.....
..
..__,,. ... ,:
-
-
_
-18-
.to 0.04 molal both of the curves resemble adsorption
i
Uotherms.
Figure 2 shows the variation of pH with
concentration of solutions of the dispersing agent
and of carbon dispersions made with them.
The curves
obtained from the agent plus sodium sulphate show a
maximum at 0.045 molal, but in general the pH does
not change much.
The pH of the dispersions is very
nearly identical with that of the solutions from
which they were made.
The values obtained in the
salt free agent are considerably lower than in the
agent plus sodium sulphate, and in general do not
show any great variation from the dispersions made
therewith.
The effect of the salt free dispersing agent,
of a mixture of dispersing agent plus sodium sulphate
and of pure sodium sulphate on the zeta potential of
the carbon particles is
shown in Figure 3.
The zeta
potential in pure sodium sulphate rises to a maximum
of 55 millivolts at 0.003 molal then falls off,
rapidly at first,
then more slowly, and approaches a
limiting potential of approximately 30 millivolts.
In the dispersing agent plus sodium sulphate the
zeta potential rises to a maximum of 63 millivolts at
concentration of the agent of 0.019 molal, then falls
off slowly and approaches a limiting potential of 53
~
.
. .. . .: :... . .-..- ..;
:
a. -. .
.
.;. .
.
.
'
i.
..
:-i-.
7 i. i
..;..
..-.-ý. 1.!..i. .
1--.- Emv
..
,
-L
..--^
--.
;ý mI-ijiTr;
,
,
-
*.
7..
--
-! -.
Lv
I ':
i. 1.
--.
,--..,-; 1- 1...
..:ý'-.-i..:ý! -j"I;:..:
,.L. ;;.. .
.I.-.-,---.4.. :..'.
ý.7,
i-.!..--..--.
-t 1i::
-;-1--,- .
;- ;
- --1
I :-.ý-.4.. -. .-.---~-!".;!
'..ý.-:-'..
. -:.
-1
iýi.1:i. :: !,I'...:
:s:.!-ý · : . .:_ -.-,
..----.
...-4;-.
2- -A!;r. /'i'C.-:. le. :!.-.1-i-;.
::: -1--.--;-;;-i---'.-;-m
;-!
_iýý!
::-.-t-I -
'
...
%j :. .
. ..
I
- -.-.
.-.. .
-7-
,
-
- : - - ;
.
.
:
i..:
-.- ,- j..
. .
'
..
.
IIN;
. .
- i
. I
, ItU~ --
..
. . -... -
,
.
. mI .
, . ...
I
i .
!
.
Aý
I-
..
-
. I
I
- ..-
.-4
,I-TI
...
.- . ,-,-!-.-.. . I,
-j-' !-' ,_; .
,
.
: ...
.
.--.- . --..'-. . .'-:J-z. ....--;..
..-: . . I. '.--,.!-......
··i:
~:
-..: .-.-- . ::;::::l:;~::I~t.-ý:-!:-f-- ....'.;-..... . .i.
..-.
j,I"II-7-. . 1-i ý.?-.--..
...
-.1-i
--,.-..-..-.,;_..:._i...
: ......
1-:,-:
I I-L-i-i
i,--.------..--t
i..F
:.ý:",
i-i
. -1
I-..: I..Z
-*iili_~
.~;...i
-
i
.
-···i.-----jS~.-.:
'!j
-l.--1.
:ý ; : ý.. .;
I 1I -
..
! !-!-
. ; -i-.:
-;;
.
.l.Wi
..
.
'- L4..!
..j-l:-j
-- .t- -;--
t"
.
--
f-
-
.
. .. ;
-·i- -r · -
,
-
.
.j::._
.
-...
;4-T - . .. :i IH -II-,-1.
...
"
1. ..i- I,..., i~Ii -1
. --:...
-: ,-!,L- : ..*-..1 ,. ! I ...ýý. . i 4:
-I..
-:II.-7....
......
. - .
..
. i-ý ..
-. * .
--.- .--
, ý: I
1- I 1
i . -, ;1:-:I: :ý-Tt- i -Iý- --
I
- .. -.1---...I --.!.i--.
1-4, . 1.:IT t ý
.-
-,-ý
.
.
t.. t .;
...
...
, ; ..
.
0
ý j--- . .f ý:
i'
. - .- !
..: tLý
-
..
: L 1-
;cii-i.
...
~
~
,
; -; . -
.
''
.
I
.I
.
!
.-
-1-.
i--:. -
.
...
I-
I_..
- ..
.
.:
:.
;
.
:I
-
- .
:..:.....
-!-.
i, .
.
..
.!-;-;. ..1 .
,I.- .
.-..-
.-,.-;.-
;....--- .1
---I-i...--'
;.-;.1.-.-:
i.---.
'I :.''
.
....
.-..]-
.
.:.
.....
. ..... - ..... .. .. : .....
..... .
ý;.:...: !-!' ..ý...:i .;-...
'..-=-.... .. ....
--.-:-..--"ý;
.:-.iji.--.
.-.-;ý--: --- .--.
'. q . .. - I
; . . ..-. ...--. . . "--;.. ' . : .: :. :. . . ...' . .
: --. -: - .-.:.-.
. .....
. ..ý...
: :
I A ... - iý
.. .ý
7ý p-: ý
... !.-..-!D ..-' ...-....: "-L
...
--!;i.i..
I
..:.;.,-.
.
. ... .. . ... .:.-I
'
.I ..-..-..
. .. T::
.. i-..--i ...---..;....iýý
.:--.
.
-.-...
,.!-.....
-.m..
.;ý
. !.....
.
a
..
.
:
. ..ý...
-ý
,
,!
. i...
..! ....i-.ý ;..i_ x._.;..........
.
:
..
a
'
I
I,.1. .......:.-;.- ...I- I- d..
. . .....
.- -...
..:. .;...
- i- ...
....L
.
. ....-..
.
.1
.,-..----...-z
.:
.
ý
..
;:
;
.
..
.:
.
I
.
..
.
.m.
..-..
.
q
.
;..:.:
...
;
;
...
L.......
:.....A.j
i
.:..L.ý...-....
.
.
...
'....-..--i.
.
j--.'
-;
...
ý'
I
.-...
.
-.
.
--"'
.
.: ..- i.- . -: ;. ..- ...
:
.
.
..
I
.... ..
..'
-.......
-..
.
.
.
.
-:.-.
'....
.- - J. _.... . ---..---. i ... . . j.1 --- - -..-------.
% A. . .--.---.-..
:
I .
.-. -.
ýI !
-...
...:I..; . 4f :-.; ; -.4ý----ýý
I - . , . . . ý ..; ... : .::.. . -----..---- -ri -.-----;-ýý-.ý....
- 1, I--,-..!...
-----
...
... .. . 1- I.
...
:i ...
..'
, ; ..: .'
....
. ý...
. ....
.
: ..
---:.i -"'] .--i...L-:...-...
I .
r.....:.- ! : -. I. ..1
.:
: .M.
. I:..'
!;-.-.:
. :_
..ý
ý...-ý,:::.-. .Iý-.-;. J1. . ...: . -.:. - ..:...i.----..-. .-;-.....-:-;
1-.
-.- :. :..
..-.
II !..:ý; . . :. : ':-4:
......- I -- -- .- . . ý;
.!.-..
A
..-1
..;.-.
. .....
..-: :. :-l..- v.-.-- . .. ........:. - ..-- 1..
; . - .Iýý----: - :- - - I: ..ý; ..:..1
I ' - ' ::..1
.,t.: ...
-- . . . . . I :l.-l4,ý -';--...
-I . .:...iI--.
. ... ..11.-:-'-........
,..:--.1!. ::, L.
: J.. .:- !.ý...- . . . . ;. P. :. 0.-.. I.. . .. .
i ...:.. I .,.. I'
. . I ýIi. . . . .. ý.....-t-I . -!: i ..... -1
.-- ,... .....
..-..
..
.
.1.
..
..
A
.;
il
i
ýrý
..
.:
ý
. -..-1.
.
.
.
'...!
ý
. I. m
. -, . ;. ý....
..;-....
!---. .-.
-ý
! --I..-: .-.....: ; ::
.-,-=
. .-.--..
. .........
. -ýý..... -!- .-.-. . ....
I.-.;.....
!-!.;-1
. . ....; :.-...Jj..:
. ;!......-.
.: . ...Iý:...
--......
-I-:
ý.......
4.- -: r.'P.. !.----,. -:.. :.- -...- . ..; - ...-I =0i; Ii i r : 0: .:..:
.
.-1: -i..:..!
-ýi....
' : 1Iý.;
I-...i .; m.
... &
. . - d-;.--I.--1-1
:-"
; . : . .. ......--. - i-.... ýýi I . I....
.--.;....-,
........
4....
.:-:
;.:
.-...
-..Iý:
. ;I.r.--;
1.-.11.
-ý..:
..ý-...
I..,.;-..--..
1--ý:._4.J
...lI.m
-:.:
1
;
...
t...
..
.;...
..:
.
z
..
.;.-1-' .
.:
:.
.
!
..
;
i
:
.;
I
!
....
L.J.-;.
i..I
.:
.
1.1,
.;.
-I
-;.,
!.-.-.
:
J
.
.-..
.
.
.
.
..
.
0...
-;
-..
I
..
........
:!-..
ý
..
17
I
...
i
.
i
..
.
.
i
...
-...
....
:
..
..:
;
.
1.
m.
I
.:.
i
.:::
:ý
-...
!
I
-.
;
..,
...--..i
L
I
.-i-..:.-I
'
'
1.
.
ý
.
.
:
!
.
I
:
m
:..
q.:
..-;
.'
.:
.
..
.
;
.
:
'
.,
-..
;...
..
...
.4
..: : ;0%.
... I ..- ..- ...,....-.-.- ..........
..
1
- . .-ý
. -1-4-. ..--..-ý ý- ,---7--.-.-..-. -,-- O !--ýý
. .-. - -ý --- .
.I.. . .-i-: . . .-. :...
a
.4
.
Id
-
......
.
I
.
.
't..
ý
.
:
.
.
-
.
-
-
.
-
.--
-
9
-19-
millivolts.
Both curves-are typical for inert
surfaces in simple electrolytes.
There is little
variation in potential in the case of the salt free
agent, the curve being a straight line sloping
slightly upward.
At all concentrations the potential
in the salt free agent is higher than in the agent
plus sodium sulphate.
The potential in pure sodium
sulphate solutions is considerably lower than in the
solutions of the dispersing agent plus sodium
sulphate.
The surface density of charge on carbon
particles in the same dispersions has been plotted in
Figure 4. The plotted points were calculated from
equation (2); the smooth curves from equation (3).
In low concentrations the surface charge density is
greater in solutions of the dispersing agent plus
sodium sulohate than in the solutions of the salt
free dispersing agent.
At 0.02 molal the curves
cross and the reverse is true.
The surface charge
density is considerably less in pure sodium sulphate
than in the solutions of the salt free dispersing
agent and in the dispersing agent plus sodium
sulphate.
It will be noticed that in general all
three curves take the form of the adsorption isotherms calculated from equation (3).
. .. . -. I -! . .. - . I . . .. .I
1--ý-
... -. .
. .m
-ý -: ! I- . -. . ..... - . .. --;. .--
I.
.
.
.
'. .
1. ...
..-- -. . . : -- : :.
:I .I . . . ... -- :7 .
!-. , ý.-- 1 : I.
;
.:----ý- ----.-'.. -!:--
.. ...-. . . .. ......-
...
...
... . ý.
ý. .
: ý, ; .
. I .
;
.-- : . ?
-7 . ' I
I I-- ---
.
III.
'" ..., -.-,.7ý....
...
;..-ýi1.-...-.. ..
...
.
.
.
;..-..-I---..
ý_:
_-_--- j
.
,
.
-:
1--
! 1.
. I·f .
'-.-
..
:.
:. , .
. F.
. . - i..
..
.-
. _._ r - .-: --%.: ...
I . I
I:
: .
I
.
:.. !.:
: -- _ ..ý;.. . .1. . - . .. .- . -- . . .
.
i ;
.
.
-:..
-..:
..
. .. . .!. ý. .I . .
.
.
__
.----. - 7-.! , 7..--r... -1i-- .1..i...... - , Ii .
.
.;
.!
.
,
.
·' - (:
·
: .
.
.- ;i...-..,:-.: 1 . ......! -;ýý-l...
.:...
ý- 77 !...-.-- 4.;
... I.,_- .- ý-., ! ! ., -. - . - : , -. . Io : I ..
,--.- =
..
-;
: .
I:
J ; .ý:.1
...
.. - ZL .1!!
- -1 ;
., :- , i4 !
-
j- 11 ..;
M&.
.i
:!
.
O·lff
I ':,D-l
.;
. .. .!
.
.777-,-- -ý-ý--ý
- ..i I I !i-;---!.
..
r."
,
.
. mi
.. JL, ;;;;;;;;;
-.
ý... ..!-.jLm
·~1T.~..
;I
..
- ý-,
Puý -- 'N-!A.---7
'ii-ý ...;;:I
:.....
..-iA
. :-.1 i...-.
........;--ý.j.-.-..-...
- .j...
..-.
..
."..
,..
lt-;-!-i .ýý.
1 ...
-....... : .+-...
;..: :J
-".-!...:-:iL
......
. :I..
...
1...
-. .;j:-iý-.ý
iI.
.ý;.m
.i., j.!ý.:...
...-i.:,,-.....
i~i: l~7 -' 1t---:._..~t--i,4-
:
:ý
. 1
I.
. .
!- - ·i
--'- .- -ý --. :ý ;. -L ;ý,F. ý I- . ... - -,-i ý f .. .i_4 .....i; _:- ,
----L.I-....
:. I;L.a.;.i
- ' ' 7,'
-ý
.. m
. .1'
..
.
-i ,.
- ?- -
. ...
7...
...
- ..
-
.. - I- .-:!ý-- - .-,-ý ý-
- --
;-
.
-- ,.: 1
-
-
I - .I I
-i ;
I. ,. :-: i
;ý:
, :-. ;
.. ..
I-
.
.
.
'. . -
1.11
I
!:-7 : : ! ;-; - I - ,
ý.
a
, r- --:-! --. ;
.
o ý.,
-- ,4 i---:"i
-
-I
-V7r-' -I -'-;of
: -;-:F,--s
...ý-.;-:. -.-...
.- ,-..
...
-.-..... ..1.-...1H - , i i..v.;._
:. .. - -. .
ý---- ....: i . - ý!.:: .'I-..-!!
""
i .
- .
.
ý-:-
- ý-
- -
- .-.-ý-'-z
·-
i
-e
.
i
" ......
.---
1-
-ý.
-. . ....
,
I
:7
.
:
.:
, :'L
.i-I v
...
1 , : :-,
! - r --
.....- .
....
: :
-- !- - ,-
. - L --,- -... 11.
.--
: ; ý :-- . .
;.+ -
j
,-. -
: --
i
i
,
-
i
:-
ý.. : I 1.
:
.
.
--,
: . : I -Lý
.. - I -!.;-;...
ii
.
- ..
-iT~
- :.-.- i.--: %
1.-. _i-..- ..-..
i.
r
-, -I
; i
--ý--1 -I
J.rl- i " f : : - I..
j4
.
I-7ý;ol- ý:ý
-,;
:.
.- -
~i
.iri~H !--.. .. . .
,.
1 -
:
I- .,
!-I-' . V!
r-i
~
.
0
- .
-
. .
-- -@- -
. I,
I, i
..
-
- .
. .
1
! I .:~:
-:: -' - '- '·
'
-..
4
-
,I
; .
-
;.-
--
.I
,
·
· ·--1- -I-I
'
.
;
4
ý - - --t --..
. eq
.
-
;
i!ý
--
.
4.. -
. .- -I- i-, - i
.
i-ii--ý-
. .- ý-i.. ..-1:-, - -;-ý
-1m .; .-;--.-;. ;.. . -.
,.. .:. .-,.-,
--
-..
_I
f_'406-
.... , . 1. .!..-.
.
- -..
. . iI
I-j
I .: I.- r~·~-.
ýý, i-.iI:~~:;::::
---.- r :irri
. -.- i·
.
.. : , . . .
-I -.
.
;-·-.:,
,- .. ...--1, - - - r - ----- r-,-, I
_L
....-.
:.,
_......
ll--i--r Ii......i ---ii--i·--;
. m.'.... . .........
.
. "-:. : ..
.--:
- ý-.[ .:.-.':. .,-1
r .. -,.
7
1-r
.......·:i-:I:-_`:::
: . .-...--_;t,-:
-- ýv:--.--ý
- - . ---.
.
--q.-1.~
.
.
.:
. . - j LI . . . l-. I
.---..
.....
-.-.-.-...
.
.........
..
...
-.
.
-,#;:
.i.
..
.
:
..
....
-!-;---.
,i
.....
.JJI .
-.1 -- . - , t- ,,
! :- i..;
--.rý
;..---'.~-;-l-·j
, ,,.
.....
. :-- -,- -I---. ..I. .,L-:.. .I
,ý:,---ý
.-----... 1.......:.-.
. :..-. -I --:-1- -!i ; :-:.
.ý.
L----. -;-4.1-:: - irý--:--i
. . j -Ll._
7..
--.I..
-L
-L!.
:!g
.
L:.
I'oi
&
""
.
-I---,;j
...
-.. .-.
'1
.; , I. i ,,
;4
--r - --. .- -. .I A
.--...
., .. ... L
... ý..,-,...
..-...
. --,.... ....
..:,
.1
1.! .-I:i I- , 1...ý
iýII.. . .
. 1... !ý . .;ý
.
i -.. ..
..-,...--,.-.
. .:1
. .i....
.1-1
:---!. F
:.l.
,.....
, .'.-,......
.1-il-m...
. ., ....
ii- ,....I,...;,i-......
,....- . .I........
:-.-.-ý...... .!-. - ....... ý1 . . ... .. ...
-- ....
1-. . -..:-.1 .. ., : .: ,.I,
.
; . - .. fI..
. .%
: . i -ý
!i!-!.-.
------!..
: L- ... . .. .ý.i ..
...- :. ..:
.-i .. . .. .. :-ý ýI"-- .- - I, .. ..-, .. -'t -:.
I -........
:- ........
. .. ..., .... .ma-.- ý.r
.
;........-....
A- -
.
.
- - . ---
ý
. . I. . :.. .. ,ýa ! . :
:. . .! : . .
.. ..
: 1 : . :. : : - -,
:, . -. . , . . '
I. .
. m
-, ..:].ij
- . . ..
'•
. . . .. ! . .-,-!,. .. -. .;
.. .
ýý
mm mm..
4 .....
. .. I ... . .
i
.. .j
:;
.
.. Iý -...I
. .t. -.I- ... iIt...........
...!...
.I--TI-- :--!-.. ! i.1-4ý. !
-::. .I .;. .....i...
ý: ;1-1i.m.
.
!
,
.
.
. . I- -
;
i : : .. . . ... !. ... I - - ! : . II ... ..:
. : ..:-...---...--.. a:.-......- i-i -...-.....- -..---.--i1.I . .i..-..------.:
.
..-..;,.:i. jL. .. : .. .ý:!I :. ý .. . .. .-..-. !; .: .:. -. ... ......... - .I.ý...... ... :..; . ........ .!II: :I . :.iI : ..---;.-:!ý
..- .....: :....I:.ý
.. . .
%
.. . - I .......
...I -1 - . - -I!I ,.- - . .. ý-1
ý. . 1. . .. - . : i . .... m- - . a.
. L;w-..- ----I; . .- . .. .; . - . , --.
. m
I
i
I.-
. i
. .
..
. ...
:
.
.
-.--
.
..
---
. .
. ..
I
---,i
.
:
.
:. i
.
.;
. I . ... . .
.I
.. ..---.-..: . ..
.
......;---....-.,.-- ,.
.......
.- .ýI.
:
.
..
,
....--...
..
:
.
.
!!
. I,-. ..
_ý. !,_...1,_4,__,
ý ;.IV I
-.----.--_ ____.
..
f AiLtz
ý- i ..
Figure 5 shows the 'variation of surface tension
of solutions of the salt free agent with concentration
and time.
The readings taken to a constant value show
uniform values of 73 A 0.2 dynes/cm. at conceitrations
ranging from 0.005 to 0.06 molal.
After the latter
concentration, the surface tension begins to fall until
at 0.10 molal it reaches 72 dynes/cm.
The values
taken at definite lengths of time after pouring show
that after standing for two hours there is a marked
reduction in surface tension which varies considerably with concentration.
At 0.01 - 0.02 molal and
at 0.07 - 0.08 molal the surface tension drops from
73.5 to 58.5 and from 70.0 to 55.0 respectively.
These are the most extreme variations which occur.
As time goes on, the variation decreases and the
curves become more cyclic in nature so that 31 hours
after pouring into the vessel, the surface tension
varies from 68.4 to 71.5 to 68.8 dynes/cm. at concentrations of 0.05, 0.06, and 0.07 molal respectively.
This variation is very nearly constant for the
concentrations ranging from 0.03 to 0.09.
However in
extremely dilute solutions, the surface tension rises
to 75.0 dynes/cm.( 0.6 greater than the value found
for water) at a concentration of 0.001 molal, then
falls rapidly to 69.4 at 0.03 molal.
In general, it
_I·_·_
r'------`--'-----'
_- I 1-1
' 1- :ý - . . :------"--
ý II
rý'__
.7,-
I ý I I~1.1ý
I I
- I
74
i .
ý
i- ----,- -- ,I_ - .--- I- I__ . -- ,--- _____'_
ýt '- --ý !
I
ý
1ý
7:ý
:, . .. -ý ý II - .
ý- ; ,
I ,
- I
,I
____
L__.-----__-__.___'l._ .- __
.ý
- .
- II I ý I
ý
.'_ _._._
___= __
ý
i
T I
I
ý_
-I . _f- I . . :
ý
L
I- I .ý 1 ý __ _ý__ ý.
ýI i
__
--- - -______- - ______
ý .ý I ýý r I
I ý . -1, ___ ý I I -i - - I I -ý ;
ý- .
ý-ý, . --I ý- - -ý : ý I ý
: ! , - IL ' I Iý ý ý - ý ý __
ý .Iý__ý_______
,
.
. .- I
- - _I- I
I-I I
ý
, --I
. 7 ý'.
! I.: . - -1 ý _. - , i - -I
ý
Iý . I I . - I .i. I I
--- : , ------ - ----I
ý---- , : . ý4
I__
I - . : .7 ý I
I ýý
- I
ý
Iý
-1
.
i It
.
:
ý
;
iý -.
.
I I i ý. ý
.
Iý :
-_.__'_'__7------------ý.1 . ý I - !_ , - , - I , , ý - __
I
ý
ý
1
,
.
-ý
IFI - ' I ý ý: ' _,- I I- ý_ý: L- --- -I -ý . . I
_-, _ _.
.-- _____ I -, _'__
_.I - ý I - ý
I- I
I
1
_··_·__I~·_
___~_;_(_·_
· I___·_1·-1--··1·11 ··-·~ --·- --i
I
ýý -
- _
. I .1
;
.
-- - _.. .-,- ..- ;-!-: i - ý '_ 4; ,I_
ý '; - - __
ý .ý ý---- _.F1:
1-1
- I 1, ,
I ýI
_-: ý i: -::_ _'_Fý_' :ý , I - .'_ý . I - L . I.- I .i I I .- ; - .
ý
ý
I
ý.
I
I- I .- _- -,
I __
i _:
I
I. ý , ý - -; - 1
I .-I - _. ý!
.
I I
-.
I
.
ll ý
ý
".
.
i
ý_
-. , - ýi - *
I
1
I
I
____
----__l_____ - __ý------ -_----__-I - --.I I --- _- . - -- -, - - - -- ---i __ - ------I
I -_,__
I __ ___ - -------: ____ . -.,--i
ýI
I
I .
I
I:
I I I
IL I I IIiý
I iý . .
I
- ' I II I - . :i
I
I
__' I I , I I . I. ! ý . !I1 - . I I
II : I
:- I ý , 1
i
!
,
,
:
ýý I ý ý ,
ýI ý j
I I .
I
i ,
I ; ,
i 4 1 1
I
i I
I ýI I I
I
17
I ý
I
.I
-I __-___
.I I I
II' ---. L--- _ý
--i.---------ýýý---.---ý----,ý.ý..-..-----.ý---ýý
-.--i- I__
II__
.
iI . I I - --1
I ;1 .
i
I
I ý
'
- II i -- , ,
I
J
i
ý
:
ý
i
i
1,
ý'
ý
.
I
t , i
' ýý I ,
!
I
ý
,
1-,-"
ý
_J_-'__'
-L.
ýý
:
1
;'
,
1ý
..
,
.
ý'
ý.
.T-i
-ý-_-- .-- i-I--- - ' T- ___4ý__* ;___
I ý
ý
:I
11
.'
.
ý
I
ý
I
i
,
.
ýý
I
I
......
)
-----I
__
ý
;_
I
.
I
ý
.
I
.
'
ý - , I-,-!-. I- - ' ý
Ia
4.
- 7 ý_... _ f.L
I
1 '6 "__ý__*
Iý
I I
,-- - - -_
. I - 1 __ I ;
I .
i.
, 1;, - ý 7I
.I - I
i IIý ý ;ý I
I--I----;
I
. ,---,-,--FI .: I- I - - -1; _- _;_ý;- -- -ý- A
I
--1
---.1I --,- ý . - ___- +__ ý_'__ .-,-i_;__,
___ý__
__r_____.____ý__
ý
Iý ___;
. I: .
ý I.. I ý i, - t ý
. II . . I ; - - ý
. ý-11 I ý..- - i , ý_'- - .
:-- - t, ý, i' - ; I II
, I __
ýý
___
,
-,
- ýi1
, !:
. . I ý iI ý . ý ,I
- fý I , Iý ;
;- .-I-- _'_ .- , . - -j- I . f -- , -- - - [ !, ý -F
I- ý ý -1-1 .. - - - - _'_
,
. ý ý: i ý
.I I
ý
-ý -- Iý-'
__- -. Ii- :.-- . Iý , ý - . .- ý r I! II ,- , ,ý,II
i -, ,11, -ý ,, - - . ý.jý-iI, ---. ý- -'- --.I ý.....
jý - .I i I :;
ý I.
iI .; I _'_ý
_ ._ý
-i 1I-- ý -ý I ý ý __
I
1, _7_ _ , 4, rI
ý
I
;
I
I
I
t
I
,
.
ý
T
.
I
i .
.
I
. ;
ý
.
ý
.
i
.I I '
ýý'_ , i
ý
ý ý- I_I - ý-c
I
L_;
j I-1
.
ý
__
ý
I
I
ý
I
,
I
,
.
i-!
. I
;
I - I- _ . .
. - .ý
, ý.
I
.
ý
ý
7
--!
I
.
I
Iý ___ý_________.
I ,I ,: ý' ., ý Ij ý -tL
I
- -- - - - -1 ý- - I, ý-ý , ,. 1ý ý ý. i. .ý T TI ---_'-1- - 1 -_ F-i -ý.
ý _ýýI .,I '_ _; ý-_ý I ý-1 -_ I_II.rr;;-I -- - , I- --- - '- ý' ý' ý F
ýt ! I ýI Iýý
ý
ý ý - ý-:
i
.
:- -- ýý I ' t , r I
o
I -,.[
i
_ I -_ -4-7__
III ý - ý I
';
F ;
r
.
--ý- ---- -------- - ---------
I
I
1-1 I
;
:_
- ýý--
ý
. .; .!
ý:_-.. -
, , _-_ .----,-,
iI 1, -1 I, ý-_-ý ,_-_--ý 4. - - ý
; ý, _:- ---i ý1ýI--.--i -_ý
: IýýýI-_
- -7ýý-1-77
. .7-ý_'
ý-,- ýý-,rIi ý- -:
ýI - -II--_-ý
I . , .ý
II _ý_
,Iý,ýI, ýII ý .ýý: ýI, !7:i
. .. ... . ýIIII-,. . ý ;_ , - Ir ý-ý7- ý':-;
-7 *,ý-ýIý I - -ý_
ý , , -_,I. ;---- ;ý- IIýý;; :qR O-:_
!
'
,:
I I. III
p_',Eý'A?_'fz'1t.I
ýolýII7
'
__7_______r_; -ý, , __4 IV
, . ý,P-1-114,
, 1ý,LIý- ý/_Yal'/V_:_
Iý- -7_ _liýý_/ýx
I
II ý --- --:- I-I-. -1i___ý-, - ------ I_
-, ýv
' , - ,__
-- 11,---. .-- L-11
ý- I,-.-.ý,ý' .Ii
-- _ q
i- _-1ýý,-::-.-ý--I i , ý-i
! I ..I-I ý____Tr: IT- -,-; -ý___
-_:
I
I
- ý.. i
I
_ý'
i
'cl _'-ý:'_'
- 1____
, i
ýT - f-t __ý-j=
7AD-ýI -/_V'X
-,4-r- --ý _r
-- __'__O_.',ý
--- ''
. ! _: , I T:7[----- -,--. _j, , - I t-ý
: . - ý 4ý_
- 1______t _ __ý_;
. 41- - -
._-._' _R_-_(;74If -7
ý-.W...---.-,/-.ý-ý
-_-_-_.ý----s-.1-&-ý
,-.ý-R-,I-,III; D_ý__
ý-I,
ýI
____C_,Y_ý
. -j'-__'_I 1,j- ý-1. _ý;'
.--- ý -r-ýl-:,-1-4-".
_ I__tET--_
ý- j,I_-L
_
_
----j-;-:_,-,;--i ý- ý
Iýý_________Lý
I
, I - II
I
ý
ý___
ý
_ Iý
I
_.
..
I
ýA' -0 _vi
_*&ý&
ý_
LA__''
/ýI ý qw,eýe/;, Alit
ý-.--ý-----i---.-----------,-------1-
_ý - i - ý ,
' i
i
I .
I -I I
ý ý ! ý- - I , ý , ý : - - I -- ,
I -;
I I _.
.
i :-_-ý
1--ý;
ý I-_'_:_ ýf ý r ý _ý - 7--_ ý ' I .
II
i 1. I
_ý
ý - f i - -; - I, ,. ý ,- j - '- F ; ý,
ý ý-1ý ý ; 7 --l;- _f ý. !_..' :. ý i ' - . ' I _ .ý _ý I
ý I -1_ý___
I _ýýý
i__. ... ý .i.: .
. j-I_--ý , _ý,ýL__,ý`
'-7-.-"
'__ i - -. --ý ; _. !I
- 11I- 1 I;-ý
--ý ý I
ý ý .; 'I ý. __ 7 . - - I _ýI I -_ - _ :---- k-1
-T ;_'_,-.,--,
I
ýý-11
i - . I_. - - I i1.
- 1
I___
..ýý
I-I.ý
I ýýýý11- _- - -;- - - - : - I -i; ' __ __ ý ý
7 I 1.
ý
:
:
ý
I-ý
ý
-"
,
ý
,
- .
ý ý I I_ ,
- ý . . I -11
ý i I- - .
1 2_- - -- 1-1-ýII
- - - '_ . ,ý ý, . .-r-..-- -J :-- i ý-!
.
- - -t- -i
" ;14 I 1-, - I_1 -.
,ý .; ýý'ý
- 1,
__ I__l- I i I
f-I -,ý ._ 7ýý. _ý_ ..I - . .,- i _* II ý I Ic - I! ý - I! ,_ '
. . .. I ý_
ý I 'r.,
_ýT
i
-:e, I "
' ' 'i
Iý ,
ý
- L_ -ý
i
'
_'t_'sow
,
r_-,I
A
,
c
1.000
I
:
X.O_
IL
ý
A
L
L
__I '
ýý ý
'I ;;ý *
. _ý_ý_ý_'
Ij - -. i ; 4 - I ý I, ý .- :-I ; ý
,
r ---I'
1- 1
: ýi f - I1- ,4,ý _ - __ ý ýi '_____iý .----, __ - " - ------I_._
I ;
-1- Ll ý
Iý I I II I . . I I, I-I .I ý I : ' ---*- I
_4_ý___.
_ -_
__Tý 4 :ýtý 11 . 7 r ý- . III I ý
- .-- - '-ý ,-[ ý j - __ __, , t --,. -ý4k ý _-._I
I if
I- : I ý-I __ __
-i - _'ý
- -' -__
,
ý_ý
_-,
___
ý
_
_J-ý
:ý
I
-,
T-7
77
,r
''
ý'_
ý
L:
_ý
I
i
1
_ý__
'
:"-_ý--,
,- : ý__
' I -c : I_- -. ,. ýI --- 1--- [ .
, --- , ,- _-1
,:
F-I
__i __
I - 1-1 I 7. - _ý_---ý
- _r* _.'
- i
I
_,_
, i -- T-ý *-[_ý11 ý_- ,1-i-,
I _ __ ' - ..., ý-- 1-I ,
1 ,ýý
3
1_Lrio f
--- ý
ý
_.
-1
ý
ý
I_I
ý___
-,I
__4
L44
-1
I
t---:
iý_
'___
-_
f--;
.__
ý
Ao
t
--,
.
ý , ýA , ý
4 I 1 I 4II ý__ý
I-,ý- _'
ý , - ;.4---a- - -1: -7-'7_'_'
ý-'-_-_-_-ý7:-- ---i 4_ i
__ ._ýý
'- -!- : _'_" ý_-.-- ,,l
. ý
_:ttj1±7."--J= _[ý-!. - t--ý' ý- - ' ---- I ,--ý____1 __ý
-_ ý E _!'ll .-ý.:_____ý__
__ -_
! I
!-,
_ i -i -I-.ý i 7 ý
- -- -i- --I 11
17 - '. ýi ý II ý , ý II
t- l_:_ L ý -, 1 j, --, - - ý, _..i 1-1ý __i
-7 :- -I I ý-7 ý__ 7 "ý- : - 7- - ;- -ý-f - ý ,
, - !_
I I- I I .
.
-1_-___;I _ý
-, - I I: - - _.
1:
.I I f __
. ý Iý I I - i ý__L__!
.I; _ _ I -1I
- _4- _i;_
'-'-ý-"
p___ __ I I - ___
.
ý
-_-__17
ý
_'_
4
ý
.
I
. I; ,I II
-:.--,L-_!ý 4__
_ý_ I :- . I. .. Ii Iý
---___-- - ,- Iý __Iý_
I : I : -'; :: -ý-'-_`
I --: II- -- T_
__
4- I , - '-ý- __
-;'1- -- -11.ý----- ý.- I' - 1 ', . 1I ;-1- -- ___- __
-_!'. - ýýý.- __'4.__!
__ý_
____
_____
ý_;
ý-1--:_
I _-1-1_L,
_ -' ___'_ý
-,- __ý_:
--.--- 'L _f-i.:
Aýý_
-,1-11
_ __;
1 '_ý
-,-.
-,--.
ý !- - - ý-7I '_
_- 1..,--.ý -ý ý___
-_'ý
, - ,ý.......
------: I_
.-, - -1
,ýi - -,I i- _L
ý I, - ý__
____-._l_ ___-- ýI--Eý` _'
I ýI 11
i - r-'._'_'
__-__ý
: :__
i ý
I
i
,
4_ý
ý
i_
_
,
.
.
i
f
,.
I_'.
'
I
I
.1
.
ý - : , II
- _i
- ---. - - -'ýý _ ý _'___--__l__._V ,
i _.: i
' -- [ .
_ý-! - -- ý : 7 _ - , --- I
, Lý.
ý ý I- ý '
ii7- ---.
i-,-,- ;+'
..t- --- l ý--ý --- ;-ý__ k _II ýýý ý-f -ý _': 1
ý ;!
_ýýý ý
' ;
7
ý
'
-7-;
:
-ý.
.
v
ý-'
"
ý
-.
lt
- [ ýi T____
-, -I-,--- I I , ,ý.
-4- -, ý,_
_ýI ý-I -- -,ý- ,_ý
I - _ý
. ';- - ýý_
- -. t_ý4_:--_ý_l__.+I
ý ý_T
ý ; ý ýi ýIý-" :'- ý:ý Ir-_
ý. -Iý-1_'
I- .; __I -iý__
'' ý-- ý
-__ ý -I. - I- .-_: !I-.-, - , __ý
__i__...+
; - :-: Ii,-.:
. . , ._,,-_.-_-,
i _ - __
, . 1, ý _;
;I~.L
I :_- --I -___
t7_.-_-_.
- -.,-+--___
- ___
I -'L- -I T--,, ýI I- ý-:-..--j
..-;T-.I , -11
ý, ----;- ,ý;---I- ý ý:-------.
- ::I'n:7ýý_
-- :: _-ý_
_7 - IT11
;- ,-:-..-"Iý _:,L_____l_7
-___ ýi___;ý:
ý- -. ýIL-__.,
I
I
1 =- " ___ý_
- --_ ý__ý
ý __r
-- ,i-,- ý
. ý.
1 1 i.I_ý
--r
- - ý__-;ý,; ý_. L. il-, 1, - _I-L
_;_
Lm____.4_l_
__j_ .__'_ -ý_
-- -'- t--"
ý--- ------I I ý:; ý- -. _.- I__- ýý- -L
j
-_
'
I ý__
- ý+L, -, 1ý--i
ý-_L
I I- -4I- - , 1, i_ý-,--.,- ý-t-_T
7 , , , , -ý--_ý-.
- ,
, ; . ý- , .-i
,--- _ , -,-V-l-E ý-l
"
- -__ ý , , . , - -_
I 17
,
:
ý_
Iý f
, I I., ... 1, , ý-, - - -I - - I - ý' - :--- --- i- ,= 7-E E.- __I Iý - i -_ý
__L--;,-i A ý ýIý
ý__ , f- _=ý'
I
i - ";
II
-ý'
I- - -I I , ý - ! : I . ý-_
_1- .. ý-L, - .. I , - !--;_
- ý ------- -__- - 1----.ý - .- I ýI.tt- - ý_ff '-,I
: : _ýý:
-_
,
i -- ý ; -- ,- , ý1-1 , -, _;'
4 -'Aý ýL'I- - I _ý_. I
1
-7 _ý ý
! - 7- ý_ '_ý_
I . ý
--_ L. _ ý I'-- i ___ý ___.--, ý ýý,f
Eq:_p
'_ý_
ý
ý
ý
I
I
- ý
T- , ý ý .
fI ,..
r
I
.
!
I
I
I
,
I
ý
:
1
77
i
7
i 7 1- ý.
ý
:
-.
ý-_ ,- t- __4I - -i - I
1_1 -, I -1
- I
ý I ý III
11
I I! ; - I
!!
I
..
.
ý
I
I
-ý, !_-4--'=;_
- ý-- --- - ! '_____'__'_._ý__
_;_', ___ý_ý__..'
; I-7 _ý--_ I~!-ý F:
-rýrl
__
I Iý ý_r
- i -1.1- _-- - Tl-- ___
- TL
_:
L-1
,__'_
i ---- I
......
._ .11I-,
- - -- I--. -L___
-ý- _-, I._ý
- ý .1ý_L
_ . __
14
.. I.-I
I_,_;
J-,, -__
I ,_L_--ý_ , i- - - I4 L_ý
I _. -11: : Ii11
,_i.
i I- -_ý_
i--, - ý_r'ý
, _ý__
___'
____
1,I- ý....
'.----ý i, ;__;
I-----I-------____'
I __ý 1-ý-_!
, _r
-,-, _:_7-;t+ý-ý!-ý--i
_.' -__
7___ý__
ý -. - -_
I
I -] T '-.-- I--. __ ,_ -_- .! r- I:-__-_..
7-I '-:L - -1
ý
. :T
_:-, -,-7,-" ý , T
ý i
.LI
I ý ý. I . I i,I
, . I ý_ -L
ýý
I I- ,ý-I I 'ý .
- --ý - - 11i
-1 _ F -1 .. .L 11
; .I: 11 1 11, I __
, : ý_- ý, ,,-- , I_ - -1- ý-' I-,
1- II
I
ý:_.
I
:
.
ý
i
.
ý
'
----ý
_,-ý
A
',
;-'
,
ý
ý
I.
.
:
I
I
ý
i
ý
;
_
.
'ý'
L
__
ý
_
.
:
:
'
11_7_1
,
L
*
I
I
ý-i-j-_1L,
Iý_
.
ý
i
-)
- , I -4-1 1 ý -1 - .I___:
ý........
:ý_' _ - _ý;
:--- 7 1t- - 11
,
-:, ý 11 ý . ý 1-ý_ - I
:
I
I
I
,
ý
I
..
__
-ý
(
71
7
-ý
I
i.I,
,
f
'-4-L
.
j
ý-!
,ý
I-'
__
.
--ýý_!ý'
-I
-:--,-:
ý
I
__
1"
-_
I
ý
-_
[
---:
4-4
,
;
11
i
.
ý
,
--_ýT 7
I -, ', -;'
- : ý_
----- ,--. I - - __
I :;
-, ' - ;I
__ý
ý__- :ýý
I ýF
I- - I I_! I - __ I - -1.
.-7 __ý. _ýý
I ý-1 i II- __
I I -f
1 - - - - 7- - ---------I i
t ----- - "' I- - I- I
; ý ý-I ý ! ; , -ý
- _7 L
I
!
ýL_ , "-, _ý: .Iýý: -7 ;
, :
I- I I I- I, - -t--r ý __
, 7 '_
:
-1
I F - ; Iý_- T_
, 1..
- 1I-. .ý 4--ý_--ý_
. I ý I - __!
ý-_ ý . --.'. .ýI.r1- i
. I 1-, ý; - -- -i I __: -i , I I : ,
- - -- .I. iý -. ý-I ý I'_:-_!:7.
..... I II - -_I-ý_tý'
I'_I-1
,
ý_
"
7
-.
i
,
ý
_
ý
ý
.
_
_
____
r
,
'
'
f
:
---I
i ___ýý
_ , - __- - ý _ ý.. -;_ - iI - -- ---ý ýý .-. r-_, , ý - . -_ ,I_ .ýT-- -iý_
- I- --ý I- - __
.- - ýt ý, ý , . - -, - -, r- ý__.
, r__- . ,L-1 ýIt _- ý ý_"-A
- --i: I i___
- ý; -" ,. '_ - i__
I - __,I'-Iý - ý - , ___ _
__-!-__ iýý-ý ý-ý!_ -1. _, ,I ', ý , __
I] _1__ý
___.
. ý; - --- __
- - ____1..iý .ý_; , - - 1 , I i _ý . ý , - .-I
-ýTý-!ý-- I ý- -; ýýýL
I
7-f- - - : _L-__.___l_
- 7 - . ----.-I-, ý. _
-'___ , -, ýý,
i - _ -ý. . . I
I i 1: ýI - I 7 7: 1ý-,- - --. ,
, - -,-- 7I L-;.. __
_T._ý_ T- _L -, , i-,
_ý :1ý -7 ý
, - L- -- - . --i___ý
-i . _ý-_
I~' __ I-,-___
- , , ____ýýLý:
. . - - __ý 1 i ý :-- ______I1ý
- ý ,i ---_-7-, III _
___
___ I , - -f
I I
ýI
. I ý
:I
.
II
_ _ý____L__
__ý
I____
-,
i; - ýf.. .ý _-I , .,I ý ;;- ;_Lý_ý
. _1
, --- --1- .. Iý: -- _,
I I ___ :-!_
_ ;-7"-_-!T-.
__ ý . ý ý ý . I 1. ý : : I ý - ý . ý
I
,
ý
,.
,
;
-!
-1
.
ý
ý
;--,
ý
i
I
ý
-11
__
:
.
1
7 ý " ,- ý
:
I
;
ý
".
-_
I
,
.
ý
-1
I
.
ý
:
'
ý
'
j
ý
,i__
---ý
. : : ý :f !
. _'
_t
- _.. -,-1
,
.
- I
- I ý I ý ý __.ý .T,- . ý.
-. -- Iýj -ý_;- --- Iiý1 ý, ý -__I- iI _7
,
ýý_p - - . 1 _411__
ý., _. ý . I p_
ý1 I-_ i ý.
_.
-- .
- - ,1r ý_:, -ý I!t ý - I- .: Ij _-_
--t-. .
- 11-_ _
. ý, _ý
--- I--- 1
--- I . ý ý-l , , - ý - -- - i. - ý " ý _; ý ý - Tý :ýý 1 ,-: ýl-;,.-;
o
_:
_ý_
ý
1
:: - :__
- _ r--. ' 1ý`__
I
7
. I I- - ; 1-- I- I- 7 I, ---.- 1.___L
___
- __i___l__ý._ _ __ _ .ý__ ,! -- -,-.- 1 - : ,
A
ý
- - __- '_
t ...- 1 _-.,. '.
. .. t . , ....I
ý
.
- - - ý : ý - :-1 -I - ý_- I -ý. . -__ý ,I - I ý -I - :_ý
ýI I I - -- - ý - -- L- -ý
. ý .-1 . _ _ý - I
."
-__
7-1
_ý
ý
;
,
_
ý
ý-i
ý
1
I~
- - ý i-ý I_ , ý :_.:ý'ý _'_
_r- - - __-ý ý_II -1. .- ý_ I , , _I..
- i' - - -- ý-'
- - ký
- --_ý - : I. ',-- ýI
I---- ý - - - 11.-, -: , ý. ýI ___--I_ ___;, ý_- ý-_- ; ".
ý_- - - 7 ýý: : : _- t ý.
I -- __
- ý
ý
I __-1-1
- '_ I, . ! _-m ý ýI --- - ; ý - , I I - . ::
I Iý
- I __
- l_'_.
- ý ý. ý . I Iý - - I 17
7 -.
____-_-i-_;__
_'__ .__ I
I-, -ý -.
I- -7.- : ýI
- I
- ý, -- tI- ý_ !_:ý. , 1i : ý _.ý_ i . I ; ' ; .. - 1
",- i
, - I
- I I
_._- ,
., ------ r_
_____
____._
I
-----------,[
I
I
I
___
-'
'
.
__
. F
. .:
ý - I ý1 ýI--- -:
. , ýI
, _ - . ý 1.,-"-. . ýýý :_
-7 - ----I
- - _Lý: ý- "
- ý __; * I 1_--ý-- ,' - ý :11
ý
Iý
.I
.
- I; . - - , ,: , -'
'- . t ýý - --:
,
-,
II
I
ý
ý
_
--' _T I -__
.
,_
t
I
ý
:
-ý
,
I
ý
:
______L
--.
-,--ý-:I
ý,
I
i- _ I __ __ - . i- , ý ýi -I_ - I i
ý
,
!
!
-,
-,--,f-----!-----=
.
__
__
- ý ., ___ 4 I
__'_
__
L
I
------ý1-1. -."- -- I I------ __
,
I
:
I
.
ý ý 11 - ýI ý ý -ý -. ifi -; ý. __
, I- 1
. I 1 -_rt rý-.1-1
I ____
,- - f_____
-i
- i ý ,ý- .--- !. - - *_.1_
:- . I ý i :
-. ý-_-,: I I - ----- : -- :-,
--.. i ": _T_-1.- ýI - -, Iý - j _ItI -- -___
-- - - - :i 7i- -1 . :- -i 1-1
L
_. _ ý-1 . i - ý - A-1
- - I ý. I
.
II
1---- -i
- : t, ---I. ,1 ; 4-I I
-I
II .
I
. ,f
'ý ;
. -_ I
_s4___ L_I _
_ _ Ii ,
ý ý ý__iý : I; ý7 __:
I
I .-i . - T
I I ý ý- .
- - -ý .. ___l
- __I
ý_!- -_ý,-. ,.I1-1
- - 1 - -I-'_... It ! ý__
-- r ý ,-- I! -. I, ý, L_ -I i: -_17
; - ----- 1, - -- - -- " .-- __
j 4-- : t '
iý . _: I 11 : ! - - __--- ý 7 j - - ; , __
-1; - __ __ I-r i . ;
:- - r - 7
1. ..
ý . ý.___ i,
._ý__ -i _1 - ;- i __- ý ý'_'Ti _ý ý- - _.ý -I - -1--------ý :. ý - ý
_
i ] ýý_____,_
, _. , -, - ý__i 1.I ý ý ,
-- - ý - I-- I i ;-ý " - 'ý-- .1, ý1
, ! , _ý
ý
I
-- ý -- ý
i
__
!
_L_
I
I.
ýý I
-_-_'-__'
- 1 -- 7 : -7 __1
ý
.
;ý
- . _'__
I__
_ý-1 1- ý I __ !
.
;
ý __I-1 I I
Iý
I-
yffli-oll
, , --- -- ý- ý
- 1' I
---71I-- - -I - - -___-I - ý_ý- 17
I*-,-l.--. - __--- --_ýa
- -ý-- -'ý_
-- L--4_'
_ý_--!_"!.'ý
ý -r
- -. ____
I _f __
:. 1,
ý--.-ý-_ _"ýý t - ,ý. '. ý-ý- ,
.-1
ý
i
I
_ý At
'? , 41k
I 'o
1
'
I
I
! - ý___ .;rý_
I __
I ....
ý4ý;ýý_"___`l
ý_____'
ýII - __-ý
'ýI __ý--,-,-----ý-1--I.-'ý-ýiýI[-ý---.
I:,......
-I
-:ý/'
-P.Aý_oicv.- I I I: .
I
ý_- _.- -__:ý
ý
7ý- _ 1- __
__
I : - - ýý: ----ýT-!ýý- -
I
I. . I .
I
I,- , ___._'
_'ý_______
-
.
.-
. - ý ý Iý f'_ý ,
f -
-- 7-1
IT ýIi-ý__';__
ýý47,_ý---
L,.-. _!j r Iýf ý-ý_ý__- 4--t-1I-I --7---:_: __ý
I --' - ------I , ý1T
I: ; : - I,ý-ý-,ý: ý, 1;-7ý_I_I I __i .----.
L-:,:-- -.__
,- ý-1ý-,- ý-: I ý-q:-'I-ý_-L
-- ,___ I : _-ýý
ý,-ý
:_i?
.-1ý__j=
-If--I-___..,ý'-: r
__-17ý
7 -I
o
F_
L____`ý'
i .-...
-.ý-ý._iI---__4-_ýý
._-ý-__-;iý
-ý-I
-__l
,--,
I~
-,-i__
I ,_11
I----I___
11
-I-ý-i-.-.-,.
-.1--l
I......
..
--11,
----.
_ -1
--,--ý
I-!
.;
ý
.ý_
__
ý
:1
__
4'.
:.-1
-- I
r--.
:
_It=
-ý-,.-:
_'______-.___I
-___._
ý
ý
--i
m
i I--!-,-,--_!
-ýý-i --_',-- --_
-ý,-----I ýlqý'___'
__:
___-i-J_-__-- ý-,!-.--,-...ýý
------._------ý_--T--7
"I
;
7-7'
, _.-.ý'-ý
ý_
'-'_ý
ý___'_fr-_ý';ý.
!_ý__
-1:.
I --ý, ý
ýI
--;-t -1
__
__+ T
-tt-ti-l-i- 1-- .-TI__' ý
ý! 1-ý
-
Lý.__ý
__ - -71'.7-1
F,
ý-,
-i
i__
--ý
-;
-7,l,'- ýýI-_-_
-,:-,--ý:1--lý
I
ýd
Fý^
-II,IýI-ý,
Le
ý
:--7-_
I
ý
-I
-_
ý_
_ý
F---.ý
ý.
.
..
I
'
'
'
ý
'
ýý
ýI'1-I---,
----i-,I- .-_.
; "I
-ý IýI--ý I- ý-_---
11
--- r---f
!,I,--- -.i-F
, ____ý
_____
-, i
F_ýý'----,
Wi _-'_'_.'-ý
,-ý_-_'_
-ý_ý_
I- -1__
_.....
-_ ý_
;-'_'
-;-----_-__:
-7::__
-Q
_ý
ýp
_:
____Aýi-__l_I
ý
_!-4 -______
;----I ý
----itý_ý
ý____---,-,--.I
--L!_
--ý--- -_.;____ý
'__'_7___
__
ý_'%ý_'____'
_____--:l'l
__
--i-.-.,I-i-ý,ý-ýý-.-,-,--_,-;ý----ý___-_
11,.. i__ýý-ý._-_______
_L_;_,+4_'_.__'_f__.__
_-_ýL'vý-,ý_'___-_
11-ltll._..l-_!_.
___,l_!__'_'ý-_Lt,ý
------,i ý---r
ý '-ý7ý!
-_-!
ýýl
- Iýi__
-...
:
ý
T
.__;_'
ýIý__I I
-I.
__ýý__ý4iq
'_
ii
-ý
-I
_--lI
I--I. "ý-Iý IýI___
_'_.
__
-.1-1
__l__._l--i
-_
__
-I-.
.I..____
__
II -- - ___
."
,
.
___________
'
ý_
-r--A:-_ý'
I'...
..... - _-___._._._ -..--ýI, I.'' ___
- ', -':i--___
il
L4
ý
-".---,I.
__-ý___._'
___;ý._'..__.._
__-.---I-ý
__._____
.____'
r----- .;ý"_-____
_ý
1-1---__-_i____NsL
I---'.-___ý
;r-----'___
,-ý-,-ý--.ý,-,-,----!----..,.,ý-----Kzý
---_-ý_
ý
;,
-------_
ý'ý-____
ýýL_; ýý1 _jjtý,
-ý
'__-___--_-______'___
--I _-4_-.-III- ___
f_ ý.ý7777_
ý ý ý1 ý, __
. ý, _
.
II I
Iý ý I -.
: -- II ý I I1:- - . ;ý.ý - - ýi ý- - -I -II , -I
ý- 'ý \ t
_____ý_
L:
I! ý
i
- 1- ý - I ý I - - i ý- -I : I . ýI ýý -1 4
__
.......
-,_ý
ý_
- .-Iti .-!-. -.. -.I- II.L , ---; . . . !I
ý -:-!
1,;- ..,'- ,ý:' Ii--..i
J-_:
ý
-i
I
-.:-.. '.
.!
_
ý
.
I
__
I ý.ý- _0
11-1-...
1:
Iý_ ý'
,
!
!
ýll_ý'_.____,
ýý__ý_
I?-o-------%J-ý_
.
ý.._- - . -1 I.... I
.
.- 4
.- _ _
; -7.--- - ----, --------ýýý- ýýI I I: . ý -11
ýýý_!, "
-ý ý_1 ;:L__!-,I -. ,. IIý .iý ý-I I .:. I- * -Ii' -- 11 - ýý Ir - Iý __
I-_ ,-'_
tý-ýI I-.-,-.----:'_ýt ý -- .- - IIý ýI I II ý Iý. __III I IIII;-.--. -ý-__-ý
.
!ý-.--.!-. I
--.
I
.
.I
rý_
ý
I
I
ý
I
_ý I
I
i
I, ý,
___-- -.----.T--':_
__________._ ýI- --"----.-ý--ý-----L-ýcýL----.-,-----k_
________7'_'_
-_7
--------I I I ýýI
;ý_1
--__
L-_
-__
;
,
I
.
_
I
I
f
-ý
.
ý
;
_
,
' ý__
. .'
;ýI . -- I. I_.
. ý . . ;:
: I
-i
- iI
I .
;;
. II
_. .
Iý
-- _ý
iýi . :I ý
;_ý __ II I- . ý I ýI
_ I. 11I I_-_
-.
ý
- .-- -_-___
___
- II
ý I
---. -.
. - - .I __ýý ý ý --1
1-1-1-ý-----.---------.ý-l-I..-ý-.--.
I
___
----------ý-------_
---.--.
,-7----'--'
-ý------ý_ý
----.
-,
I
_
:
_ý
-422_'_.__ý7
11
I
..
I~
I
--- I~- ';_ 'ý __ ý- - I_._'_-1Iý_ ý 1ý I . I! I .____
1
_. i
I ý - ý-1 I I- I ,I4
.--- - i-I
- I
Iý,--I- ý1'- Týiý' -- -_ ----.. I
I I
I - ý Iýl: Iý 1_I
.ý
I
_ ý' ý . ý__.
-- I - - . 1 I7
- -
-1__
7 :_'I _. I
I- I
. .- ý. ,: 7 . . .. I - .- . !
:
I I - - . . ýI I I
,
:---- - - - ý ý , - :- .- ý
ý_ý. _ý
-; ý.
1. . . - . . __- ý I f ý . ,ýýI .. .. ý !.,I ý _ i . . ,
Ii i
Ii!
- __--,-- - - - I _____
. 1-ý - -. --__-__-_____
ý ý
I
;
I
ý
I
-.--- I----- _______- - - -_ _____ ý_'7,- ------- -4
--. ------------ .......
-i--t ! , -.-.ý
-ý
-ý ý .-!.-ý------I ---ý ý-'-7___
. -- _____--la- .-1ý'__
..:II
ý . . I- I : __- . i ___._- ___ _'_ :7- ____
.. ..___
I-.-- -II
_
.
..
-ý
ý
o
__
.
ý
.-- -I-- --, -. ý- -- -- . ý - - - - ý,
I
.-1 - - - - - . 1-1 I;_ ., _ ý . I : ?_ t I ý . ý - I ý -1 - _ _1--ý
I
T
.
I
.
.ý
I
ý
ý
I
__
- -1 ý 11 -.
.
ý
I
_.
I- 1-_
ý
i
I
I
;
i
.
i
I
I
ý
'.ý
ý__I_
L___- - , _ _ - ý ý I
ý-ý,
_. - '- __- - ý :_7 _ý . ý
: __ _ ý I
, ý ý - ý . -i. ...;-, . .- - - " ,. ý . 'i
ý ý - I I . .- . ý __- ;
.- - _
I- - . - I I~ 11ýý- -.-- iý
__
' I-I._ý Iý - - -I
- .1. - ý_-11
__ .--.ýý ý i_ - - '_ - ---, . ,- __ ý -- ;--- : : .- I~ ~ . - L, , - I _ýý
I __; __ - I
ý ýý . _ý. ---,I ý - __
ý. -I - , --- ,- -, -- Iý
I
Iý'_________.ý_
.:
,
itýI - - __. . i -ý.
__
ý 14
i
___'_
__--ýý,
I
I
-, - ___
I
- ý . _ý_1.
I-.
ý
-,
.
ý
i`
7: ý . . ,
I ýI I ,-,
-ý__
'_._ý_l
-'--,
---ý;
_'.
1
.
;ý
ý
ý--:1
I
, !I
__ ; I ý" Ii ý i.
!- - --- - ;. I __
- - ý- 1-1_,- -_.-- ý--Iý-'-- , -."- ,I_._ý
_ -. __II-.''-,.
I I [--- i, -----. - ý I I
ý ý ý.,
--- "
r-7
-ý_! ,--I--1,II __
- 'I ý. _I 1: -. , ]
I -------.ý.- -.-_ ý - . - .
I
: ,., - j
1------______
ý ,- - -- ý__: ' - . i- - - . I- __- I .ý
: t
-- 7- ý I t ý ___ý7 - - -I ,I - --ý411 1 - ----_
___ -- _-_
-_____ý w-, . . -I--_--__
__._--ý--_
1
-- -
:,-_-, 1, ý-- - -1
--
4--t------'-----
- -_ -,. ý'.- ;, 7
ýý-- Iýý_ .- ,- I i _: _Iý __, Iý_I
-- l- I_- ý I . - I .
i- - - 1-1 .
_I - . .: -.I ý
' I-, . , ý,q: --ý_ -_- -- i
,------!- r T7-ý'-'
i
- -"ý
- --_
I~I-I.-ýý.;i ----...- 1-1-!
--- __
--i
ri---ý
I - ý_
I-_
-iýý-,.- - -.1-- ..II-:ý--_
I-, -___'__
- - 1_ý_7_1
.7
ýr
I-,---: - ILý-ý-:--ý- -m.
I -ý__
-I
7:
ý
-ý
-_
-)
- ý ý _- I - . . ý ,
,
,ý
ý
i
ý1-1.
_I
,
-I
-,
.
.___
__
_
._
_
I-___t____
-- ___' J.-.ý
I5__
ý
-,
.---.-.,
.. ý _.I I :- -------4ý,ý- - ý ' ý
I
i
,
,I I _.__ii_..'._ ;___
- ,.-i , I
!----.-,
ý__.- I ----.IýI- _- _'_1--i-1
_; ____ ý-____ _ -, -, --- ___- ý
I
___
___I ý
--11_- .- .ý ,---- I
'7-
-ý .11.-.- 7 ý ý - I!- - - -- f'_ýI ý-ý ý
;- __. ý_: '__
-.- 1ý ,- - - - r I "I ý __
i
I- - : -I -- . - - _ __ :_.- -. - __ ýt - : ; . -__
ý, ..ýýi -_ ý
:--":-, --ý-*1-,-,--- -
I ý ..I.: ' '_'__
-_- _ _
ý - - ,ý
ý
. __ ý" : ý ý..
Iý - - , - - ýI
: - I' I.--- ýý I ý_. _T'
- - -rl - -_
_- ý-ýI- IIý-_- . I
- - --'i- ý -11-11
_ -ill - I - -,-- I -il . II I . - . ;ý
I. ýý'
- -,--- 4 - -- .ý-:_.ý_
- _ .- 4 1-- ; . : I - --ý- - T ; - ý ; ... , , _' - __I___
ýý - -ý -- - -l -- _ _ ,- r , ý . - ý ---- -____ .
1.
. ---; 1 . -::ýji-I-,
7- __ I I .- - II-- - -- -4h
1- : ý-,1- -'- -,_ -Ii __
__ - - ý
. ; - - -- -.------ - - -- - -ý-_ .. - .,-. . - .
f
- -- ý-- 7 - -.- - . t ý-L - ,
I ý ý I . I -1 - . I ý : ý
; .. - , ý ýd : , ,-,I - - ý, -.
_ -- .-__---I,..--ý- . .
___ý__
-_
_'____
i---__-_______
;
---_
ý_
_1'___
-,-----, --.
- -i- -ý- -___ ý'_ ___ 1 - _ý _ ý ý . - - . ý . 1. - . I
: I - - -_ ý__
- -ý--ý-- -i - I - - , , , - . ; ý. : - - - I
L- : ý.- ---,- , __, ý ! ý ý -- I- -1 . - -11- - r _ý _1
I
I
ý
.
,
.ý- ý
ý i
.
i
k'
;
_ý. .--- ,-,i
- -_ --- - -. I .
!_. -. - I ý _ I ý .1 ý __-_ .I I I 7 [ I , -_q- ;_ 1-;_---- .; _ - -:, -j ý ýJ)VO
I
.
- : .ý. I-.- ý - .
ý.7
1 -.. I ý- - - :- ,
-ý . . .-i-.- ý - jI - , , _ .- L -_,-Ii_- -- - _--- , - ý 1 I7'I- . , I
I
ý
- ý- -.- __. ý_ :
- ý i; ,-ý_
__ r
__ - - . . .
I ý -; -1
ý- .
-.,1-1 . -7 .- ýý_
-ý-- ý___;
, '.ý
. .- .ý __ ýi . ý - I!_ I, , . 1,
' . . - . I__. I -II 11
;_
; ý ; ,__ ý______
,,,.1. . __ ý
1-1. I L_ýý
ý - I - I __
__ - ý---_ .- -_ -- _. -i
I----.
ý I- ý - I----- ý ý- ý-I--.I- ý ý -- i .1 ý
-- I
--i I I . I
_ -_ - . - . ý
I ,- I
-1 - I Iý - ,
I I. - - ý
I
Ii
.
7
I
- - ýI
I
I
.
._;__
I, ! - _ , . - - - ___- ý. - ___ . ý
ý;
ý __ ý
;
,:-. I -I ý - .- I ýý
. I ý - - _1
I
.
I
- I~- - - ý - - :-"--,-.
:
- t-.1- ------.-t-.
. 1.
- r I~ . ': , I.
ý
I~ -.-_l_-_.____
ý-.- ý--- --- 'I-- ._ý_
I
I1.
.
ý
,
,
1
- I- . -_ýI
I~
-49-0
-7
,
-.
I
I i
-_ ý
ý.I ý . . 1.
I ý- I- - -_7I". ---- I
i 'I ý-.-, __: - -, ý
I. I ; . :.iý :'
..1 - . I
-ý
I
I
ý
ý
;
m
11
ý
I
ý
ý
-,
ý
II
ý
ý
.
I
:
ý
ý
i
1- ý .
- - ý - . _ -- ,ý ,
7ý
I- I
II ý
I
"
ý I
1. I I -I- . -I
:1I :
I
I
000,
1
AM
ýI
I
.I
-3
s
'
.
- I - ýý__
I I
- - -1 I I -- 1- _ ý---i__
ý
-C
- _ý
i
Ir
-J
I
_,In_
_^1_7 L_
__
:
ý-- - _ _ Ii-, - ; :
I -- - '-I -1-- " ý1-!-'
I --_:-___--.; -- .--,-,-;
.-,
--
_-ý--
- __.
-- .I i -
I
I-
I
-6-a7, , ý'---_-- ý-t_ý -__ ý.
.1- I . 1-1-- ý'-T10,------ -------_
- --ý- _.
I I . __
-"
t*. , ý- ýI_____L_'
,-".-i__ýAo_
-. . 'a ,iýý_
;- , ý --i
_--______:________2....0I--:---. -, ___
ý ; r
;A-'- -_
-II >-I
I.,
F____ýY
D'e-M-S-Z
.ý-_-__-___'_.__'___!
- S_"
f_- I-I---------,11,..- -,I
- .-.ý._ý4CA-0
ý -F----.
I-:,---,-.
I 1_-__-_-_
ILýoz'
--_ý,_'-'-'-_I____
ý__ ý-'-___-_
I ___1-1 ____
.100---c
,I- ,-/_/
./_O_'.._'____i ý
-1_,_:
_.__ý.IýIý-:ý,1ýiiý.
Iý--,-_ý-_4;ýAE
-_-.-.---.1-1--____
I
.
.
I
;
I
ý
.
ý
I.____.'_.__
ý
ý
.-:
ý
.
I
jI
ý
__
.I-ý- 11
, - ýý, I.- -,-.1!-.
I -I _.._._'_-_____'__
--.-I. -.---- .ý_---_:_ - --.----i-ý-.I - ____
I-..--.-..-__
-__-_.__._______''___:
___-__
__-_-- - -I--1
ý___
_____.__l-__ __
_-__
ý-'
.
I
I
.
ý
I
I
.
I
I
,
ý
I
1
,
ý
I
I
ý
I
I
ý
I
I
ý
.
,-_1
. I -.----ý .-I 1-1 . I "-----I
- I . ,."ý-.I--,--"ý-,_.--,.-,-.-.-ý,---,-.---I.. .,--1 .- -I-,---.,-ý.-ý-ý-j__
ý'_
1____.____-_ý ý,.ý-----"-ýý--.
,--------_1_-J'I___._ __.______'._
_I
Fc,'. 7
ViScojvr/
AT
2 50
I
0
D 1b ypAtAy/ l
A- A/
7
etA aCe 4z//ojpate
+ /31%7.
.O,
9.00
o~0A'IOLA L
CONCENVTRAT/ONV
O./O
,O,
-21-
will be noticed that after the initial reduction,
the surface tension tends to approach the readings
taken to a constant value.
In Figure 6,
a comparison of the dispersing
power with surface density of charge is made by
plotting the relative dispersing power against the
surface density of charge.
free dispersing agent,
In the case of the salt
the dispersing power is
directly proportional to the surface density of
charge up to 0.08 molal after which concentration
this relation does not hold.
Similarly the dispersing
power of the dispersing agent plus 13% sodium sulphate
is directly proportional to the surface charge density
up to 0.015 molal after which concentration this relation does not hold.
Figure 7 shows the variation of the
viscosity of aqueous solutions of the dispersing agent
with concentration of the agent in
them.
It
will be
observed that the curve is a straight line and that
the viscosity of a 0.1 molal solution is approximately
only 15% greater than that of water.
-22 -
V.
DISCUSSION OF RESULTS
In general the data obtained from the salt
free dispersing agent do not follow as smooth curves
as those from the agent plus sodium sulphate.
This
may be due to traces of barium sulphate which could
not be removed by the centrifuging process used in
the preparation of it.
Reason to believe this is
given by the fact that the carbon suspensions made
with the salt free agent were a medium gray shade
whereas those prepared with the agent plus sodium
sulDhate were black.
This would indicate that the
barium sulphate was adsorbed on the carbon particles
and as a result it
properties.
might affect their surface
There is also a possibility that some
thiocyanate was not completely removed in the extraction with alcohol, but since the test for it
so sensitive, the possibility is small.
is
The
presence of enough alcohol to affect the results is
also improbable since the dispersing agent was
thoroughly dried at 105 0 C.
For future work it
reconmmended that a supercentrifuge
(e.g.
is
one of the
Shar-ples type) be used to remove the barium sulphate
instead of the tube type which was used in this case.
The zeta potential curves
typical for inert surfaces in
(Figure 3)
are
simple electrolytes
which do not reverse the sign of charge.
The zeta
potential in
the salt free dispersing agent is high-*
er than the potential in
the agent plus sodium
sulphate and much higher than the potential in pure
sodium sulphate.
Since there was no dispersing action
in the pure sodium sulphate and since the maximum
dispersing power in the salt free dispersing agent was
greater than the maximum dispersing power in
plus sodium sulphate,
it
the agent
would seem that there might
be a relation between dispersing power and zeta
potential.
If
there is
such a relation it
probably is
extremely complex because there is no simple relation
between the curves of dispersing power and of zeta
potential.
Since the zeta potential curves were typical
for potentials at inert surfaces in simple electrolytes,
it was assumed in the calculation of the surface
charge density from equation (2) that the dispersing
agent behaved as a simple electrolyte of the unidivalent type Na2R
2Na÷+ R,
where R is
radical dinaphthylmethanedisulphonate.
the organic
The points
obtained from these calculations, and which are
plotted in Fig. 4, very nearly follow the curves
calculated from equation (3).
Since equation (3)
describes the course of the surface density of
charge on inert surfaces in
simple electrolytes,
it
would seem that the dispersing a•gent behaves as a
'then rises, and falls again.
This peculiar dip in
the curve may be due to ion antagonism between the
organic radical of the dispersing agent and the
sulphate radical.
A consideration of the method of measuring
the dispersing power leads to implications of a more
general nature.
It will be realized that the quan-
tity termed relative dispersing power is also a
measure of the stability of the dispersion.
It
is
possible then, that the stability of a suspensoid is
related directly to the surface density of charge.
Figure 2 shows that the pH of solutions of
the dispersing agent varies somewhat with concentration of the agent in them.
hydrolysis.
This may be due to
The fact that the pH of the dispersions
made with these solutions does not differ much from
that of the solutions themselves indicates that
hydrolytic adsorption is negligible.
The surface tension of solutions of the
dispersing agent is considerably reduced a short
time after pouring, and is increased as time increases (see Figure 5).
It may be that the initial
reduction in surface tension aids in the wetting of
the to-be-dispersed material by being adsorbed
mechanically at the solid - sclution interface and
-26-
that as times goes on, a 'mechanical desorption takes
place which is followed by the ionadsorption discussed above.
In connection with the surface tension of
the solutions, it is rather interesting that the timeequilibrium values approached those taken to a
constant value.
Perhaps the slight stirring of the
liquid-air interface effected by the movement of the
ring of the tensiometer through it at short intervals
of time caused the equilibrium concentration of the
dispersing agent in the interface to be reached sooner than when the concentration of the dispersing
agent in or out of the interface was allowed to
proceed by itself.
The viscosity - concentration curve (Figure 7)
of the dispersing agent is
a straight line over the
concentration range which was employed.
This would
indicate that the dispersing agent is not a highly
dispersed, solvated colloid, like gelatin for example.
VI.
CONCLUSIONS
A consideration of the results indicates
that the following conclusions are probable.
1.
it
The dispersing agent behaves as though
were ionadsorbed on the dispersed particles which
increases the zeta potential and surface density of
charge considerably above the values obtained in an
ordinary electrolyte like sodium sulphate.
2.
The most important factor governing the
dispersing action is the surface charge density to
which the dispersing power is directly proportional
in dilute solutions of the agent.
-20-
VII.
1.
zECOMMliVIENDATIONS
That a study.be made of the stability
of dispersions in ordinary electrolytes and other
dispersing agents and that the relation of it
to
the surface density of charge be determined.
2.
That the actual adsorption of dispersing
agents on carbon particles be measured with the
object of checking the results of the electrokinetic
method.
This might be done by an ultra-filtration,
colorimetric method.
-29 -
VIII.
APPENQDIX
1. CELL DIMENSIONS:
A. Depth of the cell
Lower
No.of
surface Revs.
Left end
6.0
14.8
12.5
12.2
Center
2.2
2.4
3.8
3.1
Right end 32.4
33.9
32.2
31.6
Depth :
Upper
Surface
AverTotal no.
age
Scale divs.
31.5
34.9
34.5
34.7
325.5
320.1
322.0
322....5
322.5
26.2
26.5
27.3
26.7
324.0
324.1
323.5
323.6
323.8
6.2
7.3
5.2
5.2
324.2
323.4
323.0
323.6
323.5
3 9969.8
323.2
323.2X0.002 = 0.6460mm.
B. Width of cell = 12 mm.
C. Distance between platinum electrodes - 35mm.
D. 50 ocular micrometer divisions : 0.09mm.= 90
microns;
using 40x water immersion objective and
binocular eyepiece.
-
--
--
777--
p
__n
l~i
-------
,1
'-----------------3---
'
~--------?-ii---------i--c~-il-i--i-~---
4
-
____
4
4
___~_~
4
4.
:t;:i:
z:z:i
A
-
-. 4,,
.2-4-.
iu
~~--T1
I
.'i
A
r- 77V
7
7-7A7
i;}v
-t
I
21
1
I--
KT
:
4 17
IL
T-I
~~ ~
rT___1__1__T_-__
i-----t-AA- :-~T
T-
~ ~ AL 4
_
4,
-
.
.....
Ff
____
____
L
__
.
-C
-- A
4 - 2R-
Z-___
-
2
.--
".-:-.7
-I-_
o
--
--- ~-------~i i--~r~~__
-·--:: 1
: , -
---- 4
i:
:1~-~
___~_~;
i ; j.:::::;::_l. -~~:-:l:i:
.:_~1.;:
~-:,:~:::
:j:::
b i ......
~i~:-~:I--~~----
.
-
--
--
I
o0. /
0
-~---------~--~---------
-
~Žt~2&A L~CJ~A•c1fALZYAJ2LQAL
77
Fiy.9
+ /11
VOLI.S
rNA7/41
SRO 4ND
GA
C /RCC// T7 D AG
M - L/NVDE/MA NN
EL E C TR 0 ME 7etR
--
144
ct
IT
i~N
b
~
N
N
144
(~4
N
Ct4
~%J
-30-
2.
CALIBRATION OF CATAPHORESIS CELL:
(1).
(2).
Vzn i n Vptwith
nvolts Vznpt
=
(3).
with
V
(4).
Vpt in
scalept
pt
scale
divs.=
0
volts Vzn= 0
Vzn
(5).
Vptin Vpt = (5)
pt
volts Vzn
IT
(3) - (2)
9.90
9.95
9.96
10.00
1.65
1.75
1.85
1.85
1.85
3.80
3.90
3.90
3.10
4.25
-0.50
-0.40
-0.60
-0.70
-0.70
-0.95
-0.80
-0.75
10.00
17.00
17.00
17.08
16.95
17.00
-1.10
-0.55
2.15
2.20
2.45
2.55
2.55
4.75
4.70
4.65
4.20
4.80
1.90
1.95
2.16
2.24
2.24
3.86
3.83
3.80
3.48
3.89
0.192
0.196
0.217
0.224
0.224
0.227
0.225
0.223
0.205
0.229
10o 2.162
or
The voltage drop /cm.in
V
Zn
0.216 av.
0.22
the cell =
X 0.22 = V
X 0.063
3.5
V
V
= Potential dron acroes zine
-
t
-
-------
--
-·1R
----
--
"
I
I----
lPt-trnas
-r---Y1-
YUVYI
platinum electrodes.
-31-
3.
CALCULATIONS
=
A. Depth of stationaty layer
Equation of Komagata
(1)
7 = * b 1 ( 1+ 384
where y = depth of stationary layer from
the central axis.
k = ratio of cell width to cell thickmess = 2a
12
: 18.6
y
..
0.646
2 •
1 (1+1.246)=
3•
186
0.191 mm. from
central
axis.
Distance of the
Stationary layer
from the bottom
or top of the
cell
0.323 - 0.191 X depth of
0.646
cell
-
0.204 x
z2 66.0 scale
divisions
on the
micrometer
screw.
B.
Cataphoretic velocity,(
v )
pbserved velocity in microns/second
v
voltage drop cm. In the cell
= observed velocity
Vzn
X 0.063
C. Viscosity
N
-Rt
No
R ot
o
where N - coefficient of viscosity
R = density
t = time
At 250C,
No
o
.'.
N
=
=
0.00894
= 1.000
0.00894 x R xt
To
D.
Zeta potential
(in
D
e.s.u. )
1 cgs e.s.u. of potential = 300 volts
• "=
4r x 1000 x (300)2 xI v
81. 1x10000
S1394' v
millivolts when (v) is in
microns/second/volt/cm.
-z3
-z
rz
(~f)
a
a
g
0
a
'S
%
'0
'a
1%
'a
i
1
4
a
0
W
N"
I
0
''a
C,2
qJ
~
LUI.~
0o
C2
4)
IN
-
+
4-1
UI
(D
N
4-0
A
4J
ed
Cd
fr4
ab
0
'a
C..
C
4Z
H
0
~II~
(94
N
4:
N
"a
~
*r-4
Oh
04
tJ
0
.9..
O
cd
00
Lj
'a
0
0
N
43
'a
4.)
N
O
Cd
"-
0
IH
0
"a
qj
jC-s
aH
'0
-%
~.
HIi1-1
I~00
~
f%4
*
(j'4
a'
'a
'a
C~J
"a
'a
0
~0
II
0
0~
N
-'a
94
-34-
F. Adsorption curve for surface density of charge
q/ ÷ (3C
For example in pure sodium sulphate,
=
2960, and
0.0005, a-
at
c
at
c = 0.002,
0* = 4860; and therefore
the slope (q- (') of the curve equals
4860 -
0.002 -
12.66 x 10 5
=
2960
0.0005
Taking another point; at c S0.03, 0-=
.'.
11700
..
10 512.66
___0.03__
x
11700 = 12.66 x 10
and(
..
x
-
11700,
1 +
'-0.03
74.6
12.66 x 10 5
x --- c
1 + 74.6 c
DATA
1.
Viscosity of the dispersing agent plus 13%
Na SO at 250 ± 0.100
2 4
(1)
Molal
Cone.
0.0000
0.0004
0.0009
0.0014
0.0018
0.0037
0.0092
0.0138
0.0184
0.0276
0.0368
0.0552
0.0736
0.0922
(2)
Time (t)
secs.
95.5
95.8
96.3
96.6
96.9
96.2
96.2
97.7
97.8
99.4
100.2
102.5
104.5
106.5
(3)
(4)
-t/t4(2) 95. 5
R/R
1.000
1.002
1. 005
1.008
1.010
1.003
1.003
1.019
1.020
1.038
1.046
1.070
1.090
1.111
1.000
1.000
1.000
1.000
1.000
1.000
1.000
1.001
1.004
1.007
1.009
1.014
1.018
1.031
(5)
o
0.00N=
O.OO894x(3)x(4)
0.00894
0.00896
0.00899
0.00900
0.00903
0.00897
0.00897
0.00912
0.00915
0.00934
0.00944
0.00970
0.00992
0.01024
Viscosity of pure Na2SO4(from International
Critical Tables) at 250C.
O.100
0.250
0.500
0.750
0.00930
0.01038
0.01097
0.01229
2.
Dispersing power:
Salt free agent
Molal
cone.
0.0000
0.0005
0.001
0.005
0.010
0.020
0.030
0.040
0.050
0.060
0.070
0.080
0.090
0.100
Reading
% Absorption
1.7
10.0
5.0
9.5
10.5
12.0
12.0
15.0
15.6
20.0
21.7
24.5
15.0
18.5
2.1
12.5
6.3
11.9
Agent t 13% NaSO04
Molal
conc.
0.0000
0.0004
0.0009
0.0014
13.1
0.0018
15.0
15.00
18.8
19.5
25.0
27.2
30.6
18.8
0.0037
0.0092
0.0138
0.0184
0.0276
0.0368
0.0552
0.0736
0.0922
23.1
Reading
% absorption
1.7
1.7
3.2
3.8
4.9
8.3
13.8
14.0
15.0
16.3
19.5
19.0
13.0
3.5
2.1
2.1
4.0
4.8
6.1
10.4
17.3
17.5
1808
20.4
24.4
23.8
16.3
4.4
-3?-
0O
0
H
tO H COD
to n
Cl-
S0 0
O
0
0 0 0
H LO coC\2
00
a:
UC
C\ t.) 1
0)
0; G
000H
0:
0;
0)000
HHH
Cd
%0
OHH0)U-0)toOQ
0
W
0
0
m
0
n
4
m
C
m
n
co
400
00 02
H- G)
(a
00 C. (
CDO
*
0 0
0OOMOHOO
*****0
Lo
41 m a H
***.
m
-
o
0
E0~
N H
0
H
H
0
a o a 3,* 0) 0CF 0* 0" 0"
0)0()0)j 0)w)0*0)0w0)0)
CQQC
H- LOO
0 000
OOH 0•00
000
000
000
00000
o om
00000
-38-
ito
LO-tD
O0
L%
LOH
141O
0
0tr
LO L00 D
-
C-
to (D(D
toLO
LO
tO
LO
LO
tO
E >
SH)0
O • CNo N
r-IH
*
0
0')0
41C)
o
W1-4
o z
4-D
144
0 CQl
Hd
4)
H
4-+
\
N.
r-H
mCIO
*
0
ogo4z~ C
SC
4-:>H
Lo
*o
n
0
LO
•
4•
CD
*
•
000
0
0
0
Im"14414
0
0
*
O4
4411
0
*
1 n Il
U
o10
0
4-D M
U1) : E
QE! $
-
,1
00-00
W0
0
0H
0
0
0
to W)ta to Lo CtCtcC
0
0L
tOto
Cot Co
C
00
M
a00
-
toc(0 Co(0(
cd Lo
E-4 .,":
wl
a00 a
oo'
00
00 N9
00
OO
oo
00
oo
Y
a
•
N
14 144 0
CQ
CQ CQC QC
s
000
CQ
HHQ C 03
a
to
Q
a
0
-39-
SLO
lov
n n
-LOO)
LOLO•L O •j
If
@
8
*
•
0
nn
0
LI,,,
i.t";
no
Ne LO
0Y 0)
*
0
cOcO
(3OO
C)•-4 NC)
0 4--D~
d0
OP\O
0
n 00) CO tO 0
n3 aOD o Co)
COn
YD0 144n 4,
4.' or-4
II n
nn.
COt 0
tO V) E-
w
Nw nN
1L0 o0 (o
Q ww.
0
0>
OH
0 a)
.H
.•Lw-400
moo
LO
too0
to yH(tOE%-
(0 0.
o
E404't
,--
000
0
CDn)
000
0%?
w. C'.
0%?
02C.
to
o00
00
0
00
CC CQ
0*
a)* n•0 OD*
0)00
0
*
0
0ý
r--
(0 i
Lo (D L~oo
10 00
0a 0 6 0o
0')
O) 0,2
0) 00)000
CQ C,
10) C? ) 02 LO
Woeo0
co OD CO
C• 0.
aL~O H
00
00
*
00
0
•
*
0
-40-
'4.
Surface density of charge calculated from equation
(3)
A. In the salt free agent.
Molal cone. of
Disp. agent
Molal conc. of
Na2 SO 4
0.001
0.010
-
0.030
-
0.050
0.100
-
B.
e.s.u./cm.
1840
16500
40200
56400
80900
In the dispersing agent + sodium sulphate
0.0009
0.0092
0.0276
0.0552
0.7360
C.
Surface density
of charge
0.0014
0.139
0.0417
0.0833
0.1111
2300
17200
33600
43900
47700
In pure sodium sulphate
0.001
0.005
0.010
0.030
0.050
0.080
1178
4500
7250
11700
13400
14580
-41-
000
00
00
cDO
.0
nq)0
C104
n0r 00U-
(D
OCH
CQO
C)0
O3O
U)
n'
c-0
IH
OHH
000
O0
OO
Ha
r-t
-q•.!PS
B
a a r-i
D)C\ W' ~ 4
CD H-.CO
0:.Cý-!
HHr-
HH
O
CQ
COvn
C- OQ -0- l- -O0
• C' 0 V- U) 0 (00
~0U0O
N H ,H -r-IOnn CQ
H ,-I -
to
a)
)(
H
Hai)
b0
C
*
I40<
CD
a)
C•
O•OCOOUO•OOO
LO°
X, ,
O.
0O
tO
U) CO
L ) tLLO
0.01o 0
to
4-
02
0H
00
t
4-D
~1)
0
OO •
0
LO
0
0) . 4 •O C\2 0 0.c) M0 •0
L (0 LO (0 (0 (0 L.- (IDtoDto to E%-to.
00L
OH
00
OO
OO
o00
00
O O
OO
0
00
OO
00
00
(
01 0 00
0)
00
00
00
00
OD
cc
.4..
0- ,- o
o,: A
000
000
000
00
r-oo
00
OO
*o
oo
00
00
00
00
OO
&
oo
a
-42-
OO
000
HH
o -4 L-
rOOOH
c00
•0a
0
,- L,--I44
O>H
OOH HHC
00
C to
C0O
OOO
OO
c
OO 0
LO HCI
4'
~HH
0
m0
0 *
00*
0
0H0G')mOm
r4H H H
to
L
COt
CQ
0
LOuLo
N' GO NC\2CQ 2
S 0 .* 0
CI C0to
t
ot
.t
to cO 00
C\2
HHH-i
H
I
) C' C\2 CNCCQ Nc 2
L t)
*
°t
*
0 f0
00000
00000
LO
-ICD
>4i
Q
0
0O
CQ t'O0
HOf-0
0
U)LO
00tO0
QH1
HHnHC
tio
0
ýl0
i
0ýL
02 0O 0
(0 . 0
0n 5
O
-Cpc
**o
wC
I
,-I
,SLO
o,4U3-j
)L
OO~o••
0
4,
H C2Q wU')
0000
0000
OOOO
OOOO
0000
OOOO
0
C0*
0000
0000
0000
,--i
Cý-,-i 4
OO-
00
0-~0
00
OO
OO
oo
00
0
000
000
OOO
***O
HHr- 00
OO
00
OO
*0
a0
D00
O(D0
L'- C12Go2) to
(00
0
0 rd
OOO
OOO
OOO
000
tO (7 O,-D,-I
OOOOOO
OOHHOO
000000
000
0
00 0 00 000000
tO OO
00
0
00
*
-43-
CO
C-- Cn
00000
tr) o o Q l
,-.-.,-i
toO0
HCQ
L
OO
r.00
t
o
totO
n:0
OQ
v
H-
H
O *
C%2
0
D. o-O
0
to
LO
0 m
*0
U-)
0
00
0"0
moto.t-!-HH
HO
CQ t
to14
I,x0J
n
n LO
U-)
(aDCO to
OO
cJ
a.)
0o
0 O•
wC O
CQo o)
-0 to o•-
toHHH
(DCQ0
*
S
Hj~O'N
D
*
0
*
CQCeC
0
I
HH4
r- -4
O
r0c,-4
0o-44.;4o
1
iO
r-conHOotCQH
(1
ho
LO
0
O,2
0
0
oH
00ap
*
O*
0
0
0
CDOD
00
00
00
HC
00
oo
OO
*•
000
coo
000
. 00
00*
ooo
00
coo
00
oo
00
OO
0
OO
°
0 4oA
4_
0 0ord Cii
0
00
00
00O
00
oo
*
OO
0
0
*
00
00
00
00**
OO
-446. pH of solutions and dispersions.
A. Salt free dispersing agent
Molal conc.
pH of solution
pH of dispersion
0.0005
0.001
0.005
0.010
6.61
6.46
4.63
4.44
6.26
5.62
4.66
0 .020
4.82
4.98
0.030
0.040
4.51
4.92
5.01
4.96
0.050
5.32
0.060
6.03
6.11
6.12
6.34
5.10
5.21
0.*070
0.080
0.090
B.
4.75
5.70
5.70
5.84
Dispersing agent + sodium sulphate
0.000
0.0004
0.0009
0.0018
0.0037
0.0092
0.0138
0.0184
0.0276
0.0368
0.0552
0.0736
5.93
7.39
7.30
7.30
7.60
7.93
7.78
9.00
8.88
8.98
9.00
8.54
6.51
7.41
7.41
7.94
7.50
7.70
7.72
8.35
8.63
8.88
8.85
8.46
-45-7. Surface tension of salt free dispersing agent
Equilibrium surface
in dynes/cm.
Molal
conc.
Reading
taken to
constant
value
0.000
0.0005
0.001
74.4
74.7
0.005
73.0
73.1
72.8
73.0
73.2
73.0
0.010
0.020
0.030
0.040
0.050
0.060
0.070
0.080
0.090
0.100
74.4
73.0
73.1
72.8
72.8
72.0
2 hrs.
74.4
61.2
61.2
70.1
73.5
58.5
63.5
58.5
61.9
62.3
55.1
70.0
60.8
59.5
3 hrs.
14 hrs.
12 hrs.
72.2
75.6
69.5
74.9
75.4
75.4
73.
75.1
74.4
73.9
73.7
61.3
69.0
61.9
68.1
68.4
67.4
63.6
70.2
61.3
72.
72.4
69.4
71.3
68.3
71.6
68.9
72.2
69.5
69.5
63.7
67.6
57.2
72.0
64.3
66.7
67.5
69.3
74.8
-46-
LITERATURE REFEIHENCES
(1) Abramson, H.A.; Electrokinetic Phenomena; A.C.S,
Monograph,
p.
75 (1934).
(2) Ibid: p.110.
(3)
Ibid: p.188.
(4) Henry, D.C.: Proc. Roy. Soc. (London) 133A,106(1931).
(5) International Critical Tables vol.V, p.15.
(6) Mark, J.G., and Russell, W.F.: I.dia Rubber World,
Sept. 1,1936.
(7)
Reilly,J., and Rae, W.N.:
Physico-Chemical Methods, 2nd.
edition revised, p.476, Van Nostrand,
N.Y. (1932).
(8)
Robinson, C.,
and M1ills,
H.A.T.: Proc.
Roy.
Soc.
London 131A,576 (1931).
(9) Stewart, A, and Bunbury, H.M.: Trans. Faraday Soc.31,
pt.1,214 (1935).
(10)Thomas, A.N.: Colloid Chemistry, 1st. edition, p.99
McGraw Hill, N.Y.
(11) Treadwell, F.P.,
(1934).
and Hall, W.T.: Analytical
Chemistry,
vol.
II
p.404, Wiley, N.Y.
7th. edition,
(1930).