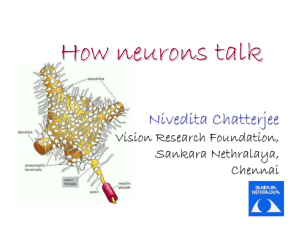
201fl
advertisement

Identification of Pluripotent Stem Cells and Characterization of Glia in the Planarian Schmidtea mediterranea by Irving E. Wang B.A. Biology (2003), M.S. Molecular, Cell and Developmental Biology (2004) The Johns Hopkins University, Baltimore, MD SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2014 MASSACHUSETT ISTo 1ITUTE OF TECI-NWLCGy SEP02 201fl C 2014 Irving E. Wang. All rights reserved. LBRARiES This author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author.... Signature redacted Irving E. Wang Department of Biology August 29, 2014 Certified by.Signature redacted Signature redacted Peter W. Reddien Associate Professor of Biology Thesis Supervisor Accepted by..... Michael T. Hemann Associate Professor of Biology Chair, Committee for Graduate Students 1 Identification of Pluripotent Stem Cells and Characterization of Glia in the Planarian Schmidtea mediterranea by Irving E. Wang Submitted to the Department of Biology on September 2, 2014 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology ABSTRACT Given their regenerative capacity, the planarian Schmidtea mediterraneahas emerged as a model system for the study of stem cell biology, tissue specification, and axis formation. Many aspects of the regenerative machinery have yet to be characterized. Although it is known that neoblasts, the population of all proliferative cells in the adult planarian, are the source of new tissue during regeneration, it is unknown whether neoblasts consist of multiple subpopulations of lineagerestricted multipotent stem cells or if there exists a pluripotent stem cell type. We developed two methods for performing clonal analysis to determine the potential of neoblasts: sublethal irradiation and single-cell transplantation. Colonies consisting of both self-renewed neoblasts expressing stem cell markers and differentiated cells expressing specialized tissue markers from single cells that we have termed clonogenic neoblasts. These cells are capable of generating all differentiated cell types in the adult animal and restoring regeneration in hosts where endogenous neoblasts have been ablated. These findings provide insight into the overall process of regeneration and the regulation of pluripotent adult stem cells during regeneration. Regeneration and homeostasis, the gradual turnover and replacement of cells in the adult planarian, both require the formation of new cells and signaling pathways to control their specification and function. The Hedgehog signaling pathway has been implicated in anteriorposterior polarity specification, but no role in planarian nervous system regeneration has been described despite that hedgehog is expressed in neurons in the brain. Although Hedgehog signaling is critical for central nervous system development in vertebrates and Drosophila, our data indicate that it is not involved in patterning the planarian brain. Instead, Hedgehog signaling regulates gene expression in a cell type identified as planarian glia from its localization to the axon-rich neuropil, expression of planarian orthologs of astrocyte genes, and branching morphology in close association with neurons. Evidence of both the existence of planarian glia and their regulation by Hedgehog signaling offers the opportunity to dissect glial cell biology in a highly regenerative model organism and understand the evolution of the cell type. Thesis Advisor: Peter W. Reddien Title: Associate Professor of Biology 3 4 Table of Contents .. 7 Chapter 1: Introduction ......................................................................................... 8 --------------............. . ... Foreword......................................................................................--9 I. G lial Cells of V ertebrates and Invertebrates................................................................ 20 II. Adult Gliogenesis in CNS Repair and Regeneration .............................................. 34 III. Planaria as a M odel for Glial Cell Biology............................................................. 43 IV . Content Overview ................................................................................................ 46 ...................... Figures................................................................................................. 56 -.......... References.............................................................................................................. Chapter 2: Clonogenic neoblasts are pluripotent stem cells that underlie planarian regeneration................................................................................... . ---------------....... A bstract..................................................................................................... .. ..... Introduction................................................................................................ Results and D iscussion ................................................................................................. Figures........................................................................................................................... M aterials and M ethods.....................................................................................................137 A cknow ledgem ents..........................................................................................................145 References........................................................................................................................146 73 74 75 76 86 Chapter 3: Hedgehog signaling regulates gene expression .--.... ... 151 in planarian glia .................................................................................................. 152 Abstract .......................................................................................................................... 154 .. .. Introduction.................................................................................................... . --................ 157 Results..................................................................................................... Discussion........................................................................................................................175 ....182 ... . Figures........................................... M aterials and M ethods.....................................................................................................216 .... 220 References.................................................................................................... 227 C hapter 4: D iscussion ................................................................................................................ Cells........................................................................................228 Stem dult A I. Pluripotent II. Functions of Planarian Glia........................................................................................232 III. Roles of G lia Cells in Regeneration...........................................................................236 ...................................... 240 IV . G lial Cell Evolution...........................................................o 243 V . Conclusion .................................................................................................................. References........................................................................................................................244 5 6 Chapter 1 Introduction 7 Foreword Until recently, the most complex nervous system known, the mammalian brain, was thought to produce no new cells during adulthood. Recently, however, numerous studies have demonstrated the formation of new neurons in certain regions of the brain and the potential for neural stem cells to regenerate damaged portions following major injury (Lois and AlvarezBuylla, 1993; Nakatomi et al., 2002). Glia are an indispensible cell type that comprise the majority of cells in the mammalian brain. Their roles in the development, function, and refinement of neural networks are only beginning to be touched upon. Understanding how these cells, characterized by their highly specialized and elaborate morphologies, are regenerated and aid in the regeneration of neurons will benefit the treatment of brains compromised by traumatic injury or disease. Planarians, free-living flatworms, are noted for their remarkable regenerative potential and provide a tractable system in which to study glia regeneration (Morgan, 1898). Whereas mammalian nervous system regeneration is limited to replenishment of depleted cell populations and reformation of certain structures such as myelin, planarians can regenerate any missing piece of the nervous system and adults can form new brains entirely de novo. By comparing postembryonic gliogenesis between mammalian systems where regeneration is limited and invertebrate systems where regeneration is pervasive and accessible, much can be learned about the conserved mechanisms shared between both and the reasons behind limited regenerative potential in vertebrate glia. 8 I. Glial Cells of Vertebrates and Invertebrates Specification and Function of Vertebrate CNS Macroglia Introduction to glia subtypes in the CNS Vertebrate glia in the central nervous system can be classified into two general classes based on their lineage. Microglia are mesodermal in origin and act as resident immune cells in the brain (Hess et al., 2004). Macroglia are ectodermal, deriving from the same progenitor cells as neurons during development, and can be subdivided into at least three types based on morphology and function: ependymal cells, astrocytes, and oligodendrocytes. Although microglia are critical for CNS response to various types of damage (Hanisch and Kettenmann, 2007), I will focus on the specification of macroglia from radial glia and the biology of postnatal astrocytes and oligodendrocytes. Developmentalorigins of neurons and glia CNS macroglia and neurons derive from neural stem cells known as radial glia. These cells were originally identified in the developing brain as glial cells because of their bipolar morphology and were thought to provide a track for migrating neurons and axons. They are now recognized as multipotent progenitors and as the primary source of ectodermal cells in the central nervous system (Malatesta et al., 2000; Noctor et al., 2001). These cells form from neuroepithelial stem cells in the ventricular zone, receiving extrinsic signals such as Notchl and FGF2 to suppress neural and glial fates and allow expansion of the population (Gaiano et al., 2000; Smith et al., 9 2006; Yoon et al., 2004). During brain formation, radial glia divide asymmetrically to form neurons that migrate along the radial glial processes and form the cortical layers (Noctor et al., 2001; 2004). Oligodendrocytespecificationandfunction In later embryonic stages, the radial glia switch from neural fates to glial fates, producing oligodendrocyte precursors that migrate to the outer surface of the brain and spinal cord. Oligodendrocytes were originally thought to only be formed in the ventral domains of developing CNS structures specified by the morphogen Sonic Hedgehog (SHH) and the transcription factors Nkx2.2, Oligl, and Olig2 (Wegner, 2008). Deletion of oligi and olig2 genes results in loss of an entire domain of the neural tube and formation of astrocytes in place of oligodendrocytes (Zhou and Anderson, 2002). Recent studies, however, have demonstrated that oligodendrocytes can also derive from more dorsal domains of the neural tube. Intriguingly, the progeny from the dorsal domains disappear at late stages of embryogenesis, suggesting only a temporary role during development (Kessaris et al., 2006). Oligodendrocyte progenitor cells (OPCs) derive from neural progenitors by repression of neural fates through Notch signaling (Grandbarbe et al., 2003). Many more OPCs are formed than are necessary, and will either form mature oligodendrocytes in the presence of survival factors such as PDGF, or undergo programmed cell death (Barres et al., 1992). Mature oligodendrocytes are primarily involved in providing insulation for axons, allowing dramatic increases in action potential propagation speed through saltatory conduction (Kaplan et al., 1997). High metabolic rates are necessary for creating myelin sheaths, leaving the cells 10 susceptible to oxidative damage (Juurlink et al., 1998). As a result, oligodendrocytes are hypothesized to be replaced regularly (Young et al., 2013). Astrocyte specificationandfunction During the late stages of embryonic development, the radial glia detach from the ventricular surface and either migrate into the brain parenchyma to form astrocytes or persist in the subventricular zone as multipotent neural stem cells (Merkle et al., 2004). Astrocyte differentiation is a multi-stage process that begins upon activation of Notch signaling in neural stem cells by adjacent, newly-formed neurons expressing the Notch ligands JAGGED1 (JAGI) and DELTA LIKE 1 (DLL1) (Namihira et al., 2009). Activated Notch signaling triggers chromatin remodeling and repression of genes involved in repressing astrocyte differentiation, including neurogenin 1 (ngnl) and neurogenin 2 (ngn2), as well as expression of astrocyte genes (Hirabayashi et al., 2009; Sun et al., 2001). The progenitor cell, now primed to adopt an astrocyte fate, can respond to the neuronallyreleased cytokine Cardiotrophin 1 (CT-1). This factor activates the JAK/STAT pathway, leading to transcription of astroglial genes derepressed through the actions of Notch signaling (Fan et al., 2005; He et al., 2005). A protein involved in the unfolded protein response, Oasis, has also been implicated in astrocyte differentiation. Interestingly, Oasis acts through ER stress-mediated upregulation of gcml, a gene encoding the vertebrate ortholog of the fly glia master regulator Glial Cells Missing. Production of Gcm1 results in demethylation of promoters for astrocytic genes (Saito et al., 2012). 11 Differentiated astrocytes are generally subdivided into two classes based on morphology and regionalization: protoplasmic astrocytes of the gray matter and fibrous astrocytes of the white matter (Miller and Raff, 1984). However, recent studies point to an even greater diversity of types (Bachoo et al., 2004; Cregg et al., 2014; Freeman, 2010). How these different fates are specified is of considerable interest; it is unknown whether these different fates are determined in the neural tube domain from which the cells emerge or from local signals where the terminally differentiated cells reside. As the most abundant cell type in the mammalian brain, astrocytes perform a variety of functions, including clearance of neurotransmitters from the extracellular environment, formation the blood-brain barrier, and modulation of synaptic activity (Sofroniew and Vinters, 2010). These cells are noted for their long processes that either form end feet on vasculature and neuron cell bodies to provide a blood-brain barrier, or branch into many peripheral astrocyte processes (PAPs) that form tripartite synapses (a synapse consisting of a pre-synaptic neuron, a post-synaptic neuron, and an astrocyte encasing the synaptic cleft) (Derouiche and Frotscher, 2001). Near synapses, neurotransmitter transporter proteins, such as glutamate transporters GLAST/EAAT1 and GLT-1/EAAT2, accumulate to uptake excitotoxic glutamate from the synaptic cleft into the cytoplasm (Anderson and Swanson, 2000). Intracellular glutamate is converted into glutamine by the enzyme Glutamine Synthetase, a necessary process for preventing neuronal damage (Zou et al., 2010). Another prominent astrocyte marker is the cytoplasmic intermediate filament Glial Fibrillary Acidic Protein (GFAP), which is particularly useful in labeling astrocytes in a reactive state, as will be discussed below (Brenner et al., 1994; 12 Condorelli et al., 1990). Many of these astrocyte markers are not astrocyte-specific; GFAP is expressed in radial glia, and glutamate transporters are expressed in both radial glia and oligodendrocytes (DeSilva et al., 2009; Hartfuss et al., 2001; Shibata et al., 1997). Ependymal Cells Radial glia also give rise to ependymal cells, ciliated glia that comprise the barrier between neurons and the cerebrospinal fluid of the ventricle (Bruni, 1998; Spassky et al., 2005). More recently, however, it was suggested that ependymal cells retain stem cell-like properties (selfrenewal and differentiation) and are capable of producing neurons and astrocytes upon ischemic injury (from oxygen deprivation) to the brain (Carldn et al., 2009; Johansson et al., 1999). These findings are controversial as other labs claimed that these studies incorrectly identified astrocytes as ependymal cells, and have been unable to identify proliferative ependymal cells with ultrastructural resolution (Spassky et al., 2005). Little is currently known about ependymal cells compared to astrocytes and oligodendrocytes, but it remains a very interesting glial cell type with regards to participation in nervous system regeneration. Overview of invertebrateglia Glia have been identified in many invertebrate species and well-characterized in the two major invertebrate model organisms, Drosophila melanogaster and Caenorhabditis elegans (Hartenstein, 2011; Oikonomou and Shaham, 2011). In fact, glia have been found in almost every Bilaterian lineage through electron microscopy studies or via identification of glia-specific proteins (Figure 1). Some of the major exceptions include hemichordates, bryozoans, and 13 rotifers. In most cases, the evidence relies on the cellular morphology and spatial relationship with neurons. Many invertebrate glia have what are described as primitive features, such as nonensheathing morphologies, which some speculate may be more similar to ancestral glial cell types than vertebrate glia (Hartline, 2011). C. elegans has several distinct glial cell types: socket and sheath cells that support amphid sensory neurons, CEPsh cells that send processes to interact with synapses in the nerve ring, and GLR cells that form connections between head muscles and motor neurons (Oikonomou and Shaham, 2011). In addition to providing support to neurons, glia in C. elegans are also active in the development of the nervous system by secreting axon guidance cues (Wadsworth et al., 1996). Drosophilaglia specificationandfunction Drosophilaglia are the most well studied of the invertebrate glia and arguably the most relevant in the context of understanding vertebrate glia. Four major types of fly glia have been described, each consisting of a number of subtypes. The surface glia are flattened cells that undergo endocycles to increase their size and connect to one another through pleated septate junctions (Baumgartner et al., 1996; Unhavaithaya and Orr-Weaver, 2012). These cells form a structure analogous to the blood-brain barrier (Stork et al., 2008). Cortex glia extend lamellar processes that wrap cortical neuron cell bodies and provide nutrient support and gas exchange (Freeman and Doherty, 2006). The neuropil glia are the most diverse type of Drosophila glia, assuming roles such as axon isolation, trophic support, debris clearance, and nerve bundle fasciculation (Doherty et al., 14 2009; Freeman and Doherty, 2006). Members of this class express glutamate transporters and Glutamine Synthetase (Rival et al., 2004). The last class of glia, the peripheral glia, are associated with the peripheral nervous system and perform similar functions to the previously mentioned types (Banerjee et al., 2006; Freeman and Doherty, 2006). During embryonic development, Drosophila glia can be divided into two classes based on their germ layer origin: the midline glia that derive from the mesectoderm and the lateral glia that derive from the neurectoderm (Bossing and Technau, 1994; Coutinho-Budd and Freeman, 2013). The transcription factor Glial Cells Missing is the master regulator of lateral glia specification. gcm mutations result in a switch from glial fate to neural fate and overexpression results in the opposite phenotype (Hosoya et al., 1995). Studies show that expression of Gcm results in chromatin modification in neural stem cells that prime them for a glial fate (Flici et al., 2011). Thus, the involvement of Gcm in chromatin remodeling is similar to the role of mammalian Gcm discussed above, but in fly Gcm plays a much more central role to the specification of glia. In lateral glia, gcm expression activates two genes, reversedpolarity(repo) and pointed (pnt), that encode transcription factors that promote glial cell fate, as well as a third gene, tramtrack (ttk), that encodes a transcription factor that represses neural fate (Giesen et al., 1997). Another target of Gcm, locomotion defects (loco), encodes a regulator of G protein signaling and is necessary for glia to form proper connections (Granderath et al., 1999). The lateral glia cells differentiate into cortex, surface, or neuropil glia depending on which neuroglioblast or glioblast they originated from (Hartenstein, 2011). The midline glia follow a different developmental pathway than lateral glia, using signaling pathways such as Notch and transcription factors such as Pointed to control 15 differentiation and function (Menne and KlAmbt, 1994; Scholz et al., 1997). During development, these cells control axonal midline crossing through the expression of cues such as Slit and Netrin, a function analogous to that of the vertebrate floor plate (Mitchell et al., 1996; Rothberg et al., 1990). At later stages, the midline glia population is split into the anterior midline glia, specified by Notch signaling, and the posterior midline glia, specified by Hedgehog signaling. Whereas the function of the posterior midline glia is unknown, the anterior midline glia persist into adulthood and wrap commissural axons (Watson et al., 2011). Evolutionary Origins of Glia Evidencefor a common origin A major long-standing question about the evolution of glia is whether vertebrate glia and invertebrate glia evolved from a common ancestral glial cell type. The implications of this are whether the study of invertebrate glia can offer insight into the function of vertebrate glia. Although there are noted exceptions, complex features are more likely to have arisen once in evolution and lost in extant organisms of the same lineage that lack them than to have arisen multiple times in different branches. Comparison between vertebrate and fly glia reveals a number of similarities in both morphology and function that support the hypothesis for a common origin. The surface glia form the blood-brain barrier and cortex glia provide trophic support to neurons, which are both functions of vertebrate astrocytes (Hartenstein, 2011). Additionally, Drosophila surface glia and vertebrate Schwann cells (peripheral nervous system glia) adopt a flattened morphology and 16 form pleated septate junctions (Baumgartner et al., 1996; Boyle et al., 2001). Drosophila neuropil glia wrap around axons, a morphological characteristic of oligodendrocytes, but function to maintain the extracellular environment, a functional characteristic of astrocytes (Hartenstein, 2011). The function of common genes expressed in the glia of invertebrates and vertebrates can be used to infer the function of ancestral glia, but few genes are currently known to be shared. Three mammalian glia proteins have been used to attempt to identify glia in other species: Glutamine Synthetase (GS), S100 Calcium Binding Protein P (S1000), and GFAP (Hartline, 2011). The presence of Glutamine Synthetase in multiple branches of the bilateria lends credit to the theory that glia evolved originally to have environmental regulation functions (Anderson and Swanson, 2000). S100p is an EF-hand calcium-binding protein that can bind to and inhibit polymerization of GFAP, but has not been identified by immunoreactivity in Drosophila or C. elegans (Donato, 1999; Hartline, 2011). The use of GFAP as a glial marker in invertebrates may be unreliable because it is a member of a cytoplasmic intermediate filament family that expanded in the vertebrate lineage (Erber et al., 1998). Cytoplasmic intermediate filaments expressed in other cell types may crossreact with the GFAP antibody, leading to false positive results. Additionally, certain species where glia have been conclusively identified, such as Drosophila,lack cytoplasmic intermediate filaments altogether (Goldstein and Gunawardena, 2000). Therefore, although it is tempting to identify glia in invertebrates based on immunoreactivity with anti-GFAP antibodies, it cannot conclusively demonstrate the presence of a glial cell type. 17 Evidence againsta common origin A common requirement could be the impetus for cell types with analogous functions to arise multiple times throughout evolution. Astrocytes and astrocyte-like cells are likely to have evolved to fulfill extracellular environment maintenance that neurons are unable to perform. If glutamate, an excitatory neurotransmitter, is not taken up from the synaptic cleft after release from the pre-synaptic terminal, it will continue to evoke post-synaptic responses, cause surges in intracellular calcium ion levels, and result in damage to the post-synaptic neuron (Manev et al., 1989). Neurons are intrinsically poor at reuptake of glutamate because of shifting membrane potentials that affect glutamate transporter activity and high levels of intracellular glutamate that drive diffusion of the molecule into vesicles or out of the cell (Anderson and Swanson, 2000). This innate inability to transport glutamate presents a need for another cell to carry out this specialized function. The functional and morphological characteristics shared between invertebrate and vertebrate glia may have arisen through convergent evolution. A line of evidence that supports this is the lack of developmental similarities, specifically the known transcription factors that specify these cell types during development. Orthologs of DrosophilaGcm have been identified in mammals, but are found at low levels in the nervous system and mainly participate in placenta development (Altshuller et al., 1996; Anson-Cartwright et al., 2000). One group reports that upregulation of gcml expression by the unfolded protein response results in demethylation of the GFAP promoter in astrocytes, but the loss of this mechanism in mutants only delays the differentiation of astrocytes (Saito et al., 2012). Gcm in flies is also involved in plasmocyte differentiation during hematopoesis, which would suggest that Gcm is not glia specific (Jacques 18 et al., 2009). These results raise the possibility that Gem is a general factor for chromatin remodeling and was co-opted into regulating glia gene expression independently in vertebrates and flies. Mammalian glial specification factors are also present in Drosophila, yet are not involved in glia formation. The Drosophila Olig2 ortholog OR controls motor neuron axon projection in flies rather than glia identity (Oyallon et al., 2012). The lack of conserved specification factors between fly and vertebrate glia suggest different origins of the two cell types. In order to gain a better understanding of glia evolution, not only will glia need to be identified in more species, but they will also need to be better characterized. Comparisons of development, gene expression profile, and function can provide better indications of homologous cell types than morphology alone. 19 I. Adult Gliogenesis in CNS Repair and Regeneration Reactive Astrogliosis in Vertebrates Function of reactive astrogliosis Terminally differentiated astrocytes outside the neurogenic zones of the brain are capable of responding to brain injuries during a process known as reactive astrogliosis (Sofroniew, 2009). In the brain parenchyma, resident astrocytes are normally non-proliferative and can be identified by their expression of GLAST/EAATI and Glutamine Synthetase. Upon traumatic injury to the brain or spinal cord, a series of events unfold that lead to the formation of an astrocyte scar (Burda and Sofroniew, 2014) (Figure 2A). In addition to upregulated expression of GFAP, astrocytes near the lesion experience a short burst of proliferation within two days following injury, as evidenced by cells labeled by BrdU and the transient expression of cell cycle markers (Barreto et al., 2011; Buffo et al., 2008; Zamanian et al., 2012). These reactive astrocytes also undergo hypertrophy, an increase in cell body and process size (Buffo et al., 2008; Li and Raisman, 1995). At the lesion, reactive astrocytes deposit proteins such as Collagen Type IV, Laminin, and Fibronectin, forming a barrier of extracellular matrix near newly formed vasculature (McKeon et al., 1991; Silver and Miller, 2004). Dedifferentiationof reactive astrocytes During astrogliosis, reactive astrocytes have been shown to undergo dedifferentiation to a state similar to neural stem cells found during development (Robel et al., 2011; Vaccarino et al., 20 2007). Reactive astrocytes collected following traumatic spinal cord or brain injury form neurospheres in culture that can both self-renew and differentiate into neurons and oligodendrocytes (Buffo et al., 2008; Lang et al., 2004; Sirko et al., 2009). The dedifferentiated cells activate pathways, such as Akt/PI3K and ErbB2, previously silent in astrocytes and express radial glia markers such as Nestin, a molecular profile highly reminiscent of neural stem cells (Feng et al., 2014; Laywell et al., 2000; Yang et al., 2011). The basic helix-loop-helix (bHLH) transcription factor Olig2, which controls glial cell differentiation during development, is expressed during the reactive astrocyte proliferative phase and conditional knockout of the gene in astrocytes abrogates their proliferation (Chen et al., 2008). Although reactive astrocytes are capable of differentiating into oligodendrocytes and neurons in vitro, their potential is restricted to astrocytes in vivo (Buffo et al., 2008). The mechanisms that limit reactive astrocyte to unipotency have not yet been found, and why these controls are in place is unclear. Regardless, the multipotency of reactive astrocytes makes them an attractive target for in vivo stem cell-mediated regeneration of the nervous system. Activation of reactive astrogliosis Healthy astrocytes are activated by signals released from damaged neurons and from immune cells that invade the lesion. The type of damage, such as from hypoxia or inflammation, results in differential responses as indicated by different reactive astrocyte expression profiles, suggesting that either astrocytes are capable of sensing certain types of damage or that there exists multiple subtypes that each launch specific programs (Sirko et al., 2009; 2013; Sofroniew, 2009; Zamanian et al., 2012). Additionally, the proliferative response of astrocytes is dependent 21 on their distance from the lesion site, indicating both a mechanism for confinement of astrocyte scar formation and for regulation of astrocytes by local signals (Barreto et al., 2011). A number of factors have been identified as activators of reactive astrocytes. Epidermal Growth Factor (EGF), basic Fibroblast Growth Factor (bFGF) (Laywell et al., 2000), Endothelin1 (ET-1) (Gadea et al., 2008), Tumor Necrosis Factor (TNF) (Selmaj et al., 1990), and Sonic Hedgehog (SHH) (Sirko et al. 1990) have been demonstrated to induce neurosphere formation in cultured astrocytes from unwounded brains. SHH is found at greater concentration in cerebral spinal fluid (CSF) than in the brain parenchyma. Injuries that cause seepage of CSF into the lesion result in reactive astrocyte proliferation whereas confined focal damage such as from amyloid plaque formation does not (Sirko et al., 2013). Another factor, Fibroblast Growth Factor 4 (FGF4), has been shown to be released by reactive astrocytes themselves to stimulate proliferation of nearby astrocytes and may be a mechanism for expanding astrocyte scars (Feng et al., 2014). The variety of extrinsic signals that are necessary and sufficient for dedifferentiation of reactive astrocytes may be further evidence of a heterogeneous astrocyte population. Effect of reactive astrogliosison CNS repair Though limited in their regenerative potential, reactive astrocytes themselves block regeneration by preventing axons from entering the lesion (Silver and Miller, 2004). Normally the brain parenchyma does not secrete signals that inhibit axon outgrowth (Davies et al., 1997). Damaged neurons will attempt to regenerate lost axons by sprouting new neurites, but proximity to astrocyte scars results in collapse and formation of a dystrophic growth cone (Houle and Jin, 22 2001; Li and Raisman, 1995). Studies found that the overall neurite outgrowth length of was decreased for injured neurons either layered onto astrocyte scar explants or proximal to lesion sites in vivo (McKeon et al., 1991; Rudge and Silver, 1990). These studies also found that the level of neurite outgrowth inhibition was correlated with the recruitment of astrocytes to the lesion, and the presence of chondroitin sulfate proteoglycans (CSPGs) and Cytotactin/Tenascin on astrocyte processes (McKeon et al., 1991). Degradation of CSPG by Chondroitinase ABC (ChABC) allowed extending neurites to enter the lesion, suggesting that CSPG is the main inhibitory factor to axon outgrowth (Bradbury et al., 2002; Moon et al., 2001). Similarly, neurons lacking PTP, the CSPG receptor, lose sensitivity to CSPG gradients and are able to extend axons into lesions (Fry et al., 2010; Shen et al., 2009). PTPo is functionally redundant with the myelin-associated inhibitor receptors NgR1 and NgR3 that mediate neurite avoidance of myelin, raising the possibility that this mechanism is employed in neurons when avoiding oligodendrocytes as well as reactive astrocytes (Dickendesher et al., 2012). The inhibitory nature of reactive astrogliosis towards regeneration of the nervous system may at first appear detrimental for neural tissue, but astrocyte scar formation is essential for preventing the primary immune response from inflicting further damage. Ablation of all dividing astrocytes during injury response results in increased invasion of the brain parenchyma by leukocytes, leading to inflammation and damage to previously unaffected regions of the brain (Bush et al., 1999; Faulkner et al., 2004; Voskuhl et al., 2009). Astrocyte scar formation thus has beneficial effects at early stages following traumatic brain injury, but at later stages appears more disadvantageous by preventing axon regeneration. Some hypothesize that axon growth arrest during early phases of CNS repair is essential for preserving damaged neurons (Rolls et al., 23 2009). Interestingly, an astrocyte's capacity to form neurospheres and to permit axon growth all diminish with increasing age of the animal (Laywell et al., 2000; McKeon et al., 1991; Sirko et al., 2013). Oligodendrocyte Progenitors and Remyelination Identification of adult oligodendrocyteprogenitors Demyelination can occur from age or disease and results in impairment of motor and cognitive ability (McTigue and Tripathi, 2008; Nishiyama, 2007). Restoring myelination requires new cell production (Figure 2B). Previous studies have shown that remyelination can occur in focal lesions where oligodendrocytes have been specifically ablated by antibodies against sphingolipids or by infection of glia with coronavirus. However, mature oligodendrocytes were unproliferative based on lack of BrdU incorporation and were insufficient to remyelinate when all other dividing cells were ablated by ionizing radiation (Keirstead and Blakemore, 1997; Redwine and Armstrong, 1998). Cells expressing the proteoglycan NG2 were found to proliferate near lesion sites and differentiate into mature oligodendrocytes (Greenwood and Butt, 2003; Levine and Card, 1987; Redwine and Armstrong, 1998; Watanabe et al., 2002). These cells derive from the OPCs found in the embryo. Most OPCs specified during development in mice will differentiate into mature oligodendrocytes within the first ten postnatal days, but some will remain in an undifferentiated, perpetually cycling state (Reynolds and Hardy, 1997; Young et al., 2013). These cells can also migrate into and repopulate neighboring regions of the brain where proliferative cells have been 24 ablated by ionizing radiation, suggesting a capacity to regenerate their own pool (Chari and Blakemore, 2002). NG2+ progenitors in adult animals are distinct from OPCs found during development based on their slower cell cycle and proliferation rate, and are referred to as either NG2 glia or polydendrocytes (Greenwood and Butt, 2003; Nishiyama, 2007)). Under uninjured conditions, NG2 glia are thought to continually proliferate to replace dying mature oligodendrocytes (Young et al., 2013). The rate of proliferation and time required to fully differentiate both increase with the age of the animal (Sim et al., 2002; Young et al., 2013). Myelin itself has been shown to exert inhibitory effects on the differentiation of NG2 glia into myelinating oligodendrocytes, raising the possibility that the age-related decrease in proliferation rates may be a result of increased amounts of myelin debris throughout the CNS (Kotter et al., 2006). Differentiationof NG2 glia Upon demyelination, astrocytes secrete the mitogens PDGF and FGF2 to activate remyelination programs in nearby NG2 glia (Frost et al., 2003; Talbott et al., 2005). The differentiation of NG2 glia into mature myelinating oligodendrocytes is dependent on early expression of transcription factors, including Nkx2.2 and Oligl, which leads to constitutive activation of myelin genes such as Myelin Gene Regulatory Factor (MRF) (Koenning et al., 2012; Qi et al., 2001; Xin et al., 2005). Olig2 is required for differentiation of NG2 glia progeny, as deletion of the gene results in the formation of astrocytes rather than oligodendrocytes (Zhu et al., 2012). To identify more regulators of differentiation, a study examining gene expression levels in differentiating oligodendrocyte progenitors found the neural stem cell marker Sox2 to be 25 upregulated during initial response to demyelination and downregulated during differentiation along with transcriptional repressors such as HesI, Hes5, Id2, and Id4. This study, interestingly, identified, along with Oligl, two histone deacetylases (HDACs) that are upregulated during the differentiation phase (Shen et al., 2008). Repression of pro-neural genes and activation of oligodendrocyte genes has since been shown with Chromatin Immunoprecipitation Sequencing (ChIP-Seq) to be controlled epigenetically (Shen et al., 2008; Swiss et al., 2011). The SWI/SNF complex component Brgl is recruited by Olig2 to modify chromatin at the promoters of oligodendrocyte genes (Yu et al., 2013). Chromatin modification factors are in turn controlled by extrinsic factors such as SHH and BMP4, which have been found to drive oligodendrocyte and astrocyte fates, respectively, in vitro (Wu et al., 2012). Axonal regulationof oligodendrocytes Notably, NG2 glia have the capacity to receive instructions directly from the neurons they myelinate. In the hippocampus, stimulation of CA3 pyramidal neurons results in depolarization of NG2 glia in the CAI region, providing evidence that oligodendrocyte precursors can directly communicate with neurons (Bergles et al., 2000). In vitro, activation of glutamatergic AMPA receptors in cultured NG2 glia results in inhibition of their proliferation and differentiation (Gallo et al., 1996). Additionally, neuronal stimulation of NG2 glia through glutamate receptors causes translation of myelin component proteins and modification of the membrane in cultured NG2 glia, signs of active myelination (Wake et al., 2011). One source of glutamate that evokes excitation in NG2 glia is glutamate-filled vesicle fusion along unmyelinated axons, which supports the hypothesis of activity-dependent 26 myelination (Ziskin et al., 2007). An in vivo experiment using optogenetics to stimulate cortical neurons resulted in proliferation and differentiation of NG2 glia into mature oligodendrocytes as well as increased myelin sheath thickness (Gibson et al., 2014). The glutamatergic NMDA receptor, on the other hand, is active in NG2 glia but its deletion does not result in any morphological, functional or survival changes. Instead, NMDA receptors are thought to regulate the composition of AMPA receptor populations at synapses (De Biase et al., 2011; K~rad6ttir et al., 2005). NG2 glia response to neuronal activity has implications for the reactive repair of demyelination as well as possible mechanisms for modulating neural function during learning. Stem cell propertiesof NG2 glia The multipotency of NG2 glia is disputed. NG2 glia isolated in culture have been demonstrated to differentiate into both oligodendrocytes and astrocytes, but not neurons, even when cultured in neural stem cell media (Zhu et al., 2008). A number of reports using Cre recombinase techniques for lineage tracing have reported NG2 glia differentiating into astrocytes, neurons, or both (Guo et al., 2009; Rivers et al., 2008; Zhu et al., 2008). Another report claimed that the previous studies were flawed in their use of non-specific promoters to drive expression of Cre recombinase, and that in vivo NG2 glia only generate mature oligodendrocytes (Kang et al., 2010). Yet another report states that differentiation of NG2 cells into astrocytes occurs during embryonic stages but not in adults (Zhu et al., 2011). NG2 glia are at least capable of self-renewal, suggesting a stem cell capacity. Studies using cuprizone treatment for demyelination of the corpus callosum showed depletion of the NG2 glia pool after repeat cycles of oligodendrocyte loss and restoration, which was interpreted 27 as an inability of the cells to restore their own population (Mason et al., 2004). Other studies using focal demyelination with lysolecithin injections found that myelination ability is not attenuated after repeated insults (Penderis et al., 2003). This second result is supported by the finding that NG2 glia can repopulate regions where those cells have been lost (Chari and Blakemore, 2002). More recently, using clonal analysis, NG2 glia were shown to undergo both asymmetric division, forming an NG2 glial cell and a myelinating oligodendrocyte, and symmetric division, forming either two NG2 glial cells or two oligodendrocytes (Zhu et al., 2011). It may be that the original studies generated a non-permissive environment, such as one filled with inhibitory myelin debris, for NG2 glia proliferation and differentiation. Gliogenesis in Neurogenic Zones Adult neural stem cells Within the mature central nervous system lie two neurogenic zones, the Subependymal Zone (SEZ) and the Subgranular Zone (SGZ), populated by adult neural stem cells (NSCs). These two sources of neurons and glia are essential for maintaining the sensitive olfactory bulb and for memory formation in the hippocampus. The neurogenic zones are comprised of three cell types. The B 1 cells are slowly dividing neural stem cells that give rise to the transit amplifying C cells. The fast dividing C cells in turn form the migrating neuroblasts known as A cells (Doetsch et al., 1997). The SEZ neural progenitors then form a continuous chain known as the Rostral Migratory Stream (RMS) that supplies the olfactory bulb with fresh neurons (Lois and Alvarez-Buylla, 1993; Lois et al., 1996). Although this is the primary role of the SEZ neurogenic zone, recent 28 studies have shown that the NSCs are capable of contributing to gliogenesis, particularly in response to brain injuries (Marshall et al., 2005; Li et al., 2010). NSCs have much in common with radial glia from developmental stages as well as with astrocytes from postnatal stages. These cells express astrocyte genes such as GFAP, vimentin, and S100p, and are capable of forming neurospheres in culture. The neurospheres are capable of self-renewal and differentiation in astrocytes, oligodendrocytes, and neurons, both in vitro and in vivo (Doetsch et al., 1999; Laywell et al., 2000). Tracing studies indicate that NSCs are capable of differentiating in vivo into astrocytes and oligodendrocytes (Levison and Goldman, 1993; Levison et al., 1993; Menn et al., 2006). This result was elegantly shown in clonal analysis experiments where clusters of labeled cells stemming from one progenitor were comprised of astrocyte and oligodendrocyte cell types (Levison and Goldman, 1993). Specification of glia in neuralstem cells Olig2 is essential for specification of glial cell fates in NSCs. Cells where Olig2 is overexpressed are prevented from adopting neural fates and entering the rostral migratory stream, whereas cells where Olig2 is inhibited fail to differentiate into oligodendrocytes or astrocytes (Marshall et al., 2005). The fate of the progenitors appears linked to their destination, as NSCs contribute to oligodendrocyte populations in the white matter and both oligodendrocyte and astrocyte populations in the gray matter (Levison and Goldman, 1993). This multipotency in the cortical region appears reduced with increasing age (Levison et al., 1993). NSC differentiation is also dependent on its immediate environment. Culturing NSCs atop a feeder layer composed of neurons results in increased oligodendrocyte formation. When a 29 feeder layer of astrocytes is used, proliferation and neural differentiation are both increased (Song et al., 2002). This effect may be because of contact-mediated inhibition of glial fates, as reduction of Notch signaling activity by deleting both GFAP and Vimentin from astrocytes resulted in a larger ratio of neurons formed from progenitors (Wilhelmsson et al., 2012). Activation of glialcell production Injury to the brain increases proliferation, migration, and differentiation of NSCs in the SEZ (Nait-Oumesmar et al., 2007). Following ischemia, recently proliferated cells were observed translocating from the SEZ into the brain parenchyma while simultaneously upregulating mature glia markers and downregulating NSC markers (Zhang et al., 2001). Progeny tracing of the gliogenic Nestin+ cells in the SEZ shows that these cells, which normally favor astrocytic fates, can also become oligodendrocytes and neurons in response to injury (Li et al., 2010). Normally the B 1 cells produce neurons, but damage from demyelination is sufficient to direct more NSCs to proceed down the oligodendrocyte lineage (Menn et al., 2006). Perfusion of exogenous EGF into the ventricles elicits the same upregulation of cell proliferation and differentiation as damage, suggesting that this molecule may be involved in injury response (Craig et al., 1996; Gonzalez-Perez et al., 2009; Kuhn et al., 1997). Interestingly, NSC cells in the hippocampal SGZ do not respond to EGF application, providing evidence for the heterogeneity of the NSC population (Kuhn et al., 1997). FGF2 has also been identified to regulate NSCs in culture, and can function interchangeably with EGF but stimulates lower proliferation rates than EGF. It has been suggested that varying concentrations of EGF and FGF2 may be used to modulate NSC kinetics in vivo (Gritti et al., 1999). 30 Glia Regeneration in Invertebrates Adult gliogenesis in Drosophila There is increasing evidence of glial regeneration in response to nervous system injury in invertebrates. The two most well-characterized invertebrate systems, Drosophilaand C. elegans, belong to the ecdysozoa, a bilaterian superphylum whose members have lost most of their capacity to regenerate (Bely and Nyberg, 2010) (Figure 1). C. elegans lacks all somatic cell proliferation in adulthood, but Drosophila CNS cells have been suggested to divide following wounding (Fernindez-Hernandez et al., 2013). Programmed neuronal cell death or damage to the antenna lobe caused proliferation of repo+ glial cells near the wound site. This response was dependent on Eiger (Egr), a TNF homolog, and was restricted to the first week of adulthood (Kato et al., 2009). The interface glia, a class of neuropil glia, are normally capable of dividing during adulthood but remain in GI from Prospero-mediated inhibition of CycE. Upon injury, Egr promotes expression of Dorsal in the interface glia and, together with Notch signaling, results in reactivation of the cell cycle. Dorsal also promotes expression of Prospero, which accumulates and causes the cell to undergo differentiation. The newly formed glia close the wound site and repair the neuropil (Kato et al., 2011). A number of interesting parallels are drawn between the Drosophila and vertebrate responses to injury. Egr is a homolog of TNF, which is involved in reactive astrogliosis, and Prospero is a homolog of Proxl, a neural stem cell factor that promotes cell cycle exit and differentiation (Karalay et al., 2011; Kato et al., 2011; Selmaj et al., 1990). 31 Examples of glia regenerationin other invertebrates Damage to the nervous system results in the recruitment of blood cells to the lesion site in insects and molluscs (Bale et al., 2001; Howes et al., 1989). In cockroaches, loss of the surface glia that form the blood brain barrier causes an accumulation of haemocytes at the lesion site (Treherne et al., 1984). These haemocytes provide a temporary barricade while nearby glia proliferate to replace the lost surface cells (Smith et al., 1987; 1990). This process parallels the formation of the astrocyte scar, where leukocytes invade the lesion site early while astrocytes proliferate to form a barrier. In the leech Hirudo medicinalis, damage to the nervous system results in almost immediate expression of nitric oxide synthesis genes in ensheathing glia (Shafer et al., 1998). Because nitric oxide can act as a neurotransmitter, this response may be a means of signaling to nearby neurons. However, ablation of ensheathing glia cells does not affect the regeneration of the axon bundles after transection, suggesting that the glia do not participate in axon guidance (Elliot and Muller, 1982). When crayfish axon bundles are severed, the axons sprout additional processes that break down the bordering membranes of adjacent glia and engulf their nuclei. The function of this syncytium is proposed to be for large scale protein transfer necessary to support the regenerating axon (Pearce et al., 2003). Whereas some aspects of invertebrate glia regeneration are reminiscent of vertebrate processes, others appear different. Surveying more invertebrate species is necessary to determine which regenerative features are ancestral to all Bilateria and how studying them in a 32 non-mammalian organism can lead to insights into nervous system repair and regeneration in vertebrate species. 33 III. Planaria as a Model for Glial Cell Biology Planarian Regenerative Potential Overview of the model system The planarian Schmidtea mediterranea is a free-living, freshwater flatworm that has recently emerged as a model system for the study of many aspects of regeneration biology, such as axis polarity determination, tissue patterning, and stem cells. Planarians are a member of the Lophotrochozoan superphylum, an understudied branch of the Bilateria and sister group to the Ecdysozoans, which includes Drosophila and C. elegans (Adoutte et al., 2000). Lophotrochozoans and Ecdysozoans constitute the Protostomia, Together, the sister group to Deuterostomia, which includes all vertebrate model organisms. Two strains of S. mediterranea are widely used: a hermaphroditic strain that is capable of sexual reproduction by crossfertilization, and an asexual strain that derived from the sexual strain following a chromosomal translocation that prevents germ line formation (Newmark and SAnchez Alvarado, 2002). Gene expression can be easily and specifically inhibited in planarians by introducing double stranded RNA, either through injection directly into the intestinal tract or by feeding the animals bacteria expressing double stranded RNA (dsRNA) (Newmark et al., 2003; Reddien et al., 2005a; Sanchez Alvarado and Newmark, 1999). Whole-mount fluorescent RNA in situ hybridization allows the spatial localization of gene expression in the context of the entire animal, providing a powerful technique to examine changes resulting from inhibition of target genes (Pearson et al., 2009; Umesono et al., 1997). 34 Planariananatomy Although planarian anatomy is simple compared to vertebrate model systems, a number of tissue types have been identified with homologous functions and specification mechanisms to these in other model systems. Externally, planarians have an epidermis that is ciliated on the ventral surface to allow for motility. Beneath the epidermis lies a pigmented epithelium, and then a muscular layer formed from a regular network of longitudinal, circular, and diagonal muscle fibers. The animal ingests food through muscular pharynx located in the midbody that is extruded through a mouth on the ventral surface. The pharynx is connected to an intestinal tract that splits into three primary branches and many secondary branches extending into almost every region of the animal. Between the gut and the muscle layer is the parenchyma, mesenchymal tissue consisting of many cell types (Hyman, 1940). At the dorsal surface in the anterior are two light-sensing organs known as photoreceptors that inform the animal's light-avoidance behavior (Carpenter et al., 1974). The photoreceptor neurons extend axon bundles to the central nervous system (CNS). The planarian CNS consists of a pair of ventrally-localized cephalic ganglia, each comprised of a cortex of neuron cell bodies fully encompassing a neuropil of neurites (Morita and Best, 1965) (Figure 3A). Each cephalic ganglion lobe lies atop a ventral nerve cord that extends from the anterior to the poster. The majority of synapses lie within the neuropil (Morita and Best, 1966) (Figure 3B). Distributed throughout the ventral surface is a grid-like network of axon bundles known as the orthogon. Commissural axon bundles emerge perpendicularly from the nerve cords and either extend medially to connect the two ventral nerve cords or laterally towards the periphery (Reuter et al., 35 1998). Neurons are most concentrated in the cortical regions of the cephalic ganglia and nerve cords but are also found distributed throughout the body and in the pharynx. Planarianregeneration Planarians can heal almost any injury without scar formation or permanent changes to normal morphology (Morgan, 1898). Almost any piece of the animal, when separated from the body, can regenerate all missing portions and form a new individual. The only regions incapable of regenerating when isolated are the pharynx and the tip of the head, for reasons discussed below. When significant portions of the body are amputated, the remaining body must both generate new tissue to replace what was lost (epimorphosis) and reorganize old tissue to accommodate the now smaller size of the animal (morphallaxis) (Morgan, 1898). The blastema, an unpigmented outgrowth of newly formed cells emerging from the amputation site, is a common feature in other regenerative species and is useful for studying formation and differentiation of cells. Pieces of an original animal are capable of fully regenerating all missing structures in a week and retain the capacity to regenerate following subsequent wounding. Planarian Stem Cells Characterizationofplanarianstem cells Proliferative cells can be identified through RNA in situ hybridization by their expression of smedwi-1, a gene encoding an ortholog of Piwi ortholog (Reddien et al., 2005b). This marker has been confirmed as a proliferation mark by overlapping expression of S phase marker histone 36 H2B (Newmark and Sanchez Alvarado, 2000) and M phase marker anti-phosphorylated Histone H3 (Wenemoser and Reddien, 2010), as well as by expression of smedwi-J in cells with >2c DNA content isolated by FACS (Hayashi et al., 2006). When ionizing radiation is used to ablate all dividing cells, planarians can survive for up to four weeks but are unable to regenerate following amputation (Reddien and Sinchez Alvarado, 2004). These data suggest that the source of new tissue during regeneration is the population of all proliferative cells in the adult animal; this population is known as the neoblasts. Neoblasts are distributed throughout the parenchyma of the body except in the pharynx and at the tip of the head, which explains why those two regions are unable to regenerate on their own. Neoblasts are dynamic cells that are capable of differential responses to wounding. During homeostasis in unwounded animals, neoblasts undergo mitosis at a low rate to replace cells lost as a consequence of normal tissue turnover (Pellettieri et al., 2010; Sal6 and BaguftA, 1984). If the animal is injured without tissue loss, such as from a stab, a wave of mitoses is observed throughout the animal six hours after wounding. If the animal suffers an injury that results in lost tissue, the six-hour mitotic peak is followed by a second wave of mitoses localized to the injury site at 48 hours after wounding (Wenemoser and Reddien, 2010). In addition to proliferating in response to wounding, neoblasts also migrate towards sites of injury (Guedelhoefer and Sanchez Alvarado, 2012). Descendants of proliferating neoblasts can be identified for a short period of time after dividing by immunofluorescence of SMEDWI-1 protein, which perdures after smedwi-J is downregulated during differentiation (Wenemoser and Reddien, 2010). When combined with fluorescent in situ hybridization for differentiated cell markers, whether differentiation occurs can be determined (Figure 4A). 37 Neoblastpotential The term neoblast refers to all proliferative cells in the animal, which is likely a heterogeneous population. The irradiation sensitive cell population consists of stem cells as well as their immediate descendants, identified by markers prog-1 and agat-1 (Eisenhoffer et al., 2008). In the first experiment that examined neoblasts on a single cell level, qPCR of individual FACSisolated neoblasts demonstrated that subsets of the population expressed differentiated cell markers (Hayashi et al., 2010). Finding heterogeneity in the neoblast compartment has raised the question of whether there exists a pluripotent stem cell that is capable of differentiating into any cell type, or whether multiple lineage-restricted stem cell types must work in concert to power regeneration (Figure 4B). In one experiment, a large number of cells collected from a donor animal were transplanted into an irradiated host animal that lacked all endogenous neoblasts. The transplantation was able to restore regenerative abilities in the host animal, suggesting that the transplanted cells were capable of generating all differentiated tissue types in the adult animal (BagufiA et al., 1989). These studies, however, were insufficient in examining the stem cell potential of individual cells. Therefore, the question of whether a pluripotent stem cell type exists in planarians was left unanswered. 38 Evidence of Planarian Glia Ultrastructuralidentification ofglia Electron microscopy studies have provided evidence for planarian glia in the neuropil of the central nervous system. Originally described as accessory cells, these structures are distinguishable from neurons and secretory cells based on their relatively clear cytoplasm (Morita and Best, 1966). The low abundance of vesicles and granules has led some to hypothesize that the primary role of these cells is mechanical support, but the presence of glycogen granules suggests additional roles in providing nutrient support to neighboring neurons (Golubev, 1988). These studies provide no functional or molecular evidence that validate these cells as glia or allow comparisons with established glia in other organisms, but provide a foundation on which future studies of planarian glia may be conducted. Candidateglia cells by marker localization Although the expression patterns for many genes have been determined, currently only one has been shown in cells localized to the neuropil, the region where glia are hypothesized to reside. This gene, netrin 2 (net2), encodes a secreted ligand that binds to Netrin receptors expressed in neurons, and when its expression is inhibited by RNAi, causes disrupted axon guidance and defective brain patterning (CebriA and Newmark, 2005). The axon guidance cue Netrin is expressed in glia in C. elegans (Wadsworth et al., 1996), the floor plate glia of vertebrates (Serafini et al., 1994), and the midline glia of Drosophila (Mitchell et al., 1996), suggesting that the planarian net2 may be a marker for candidate glial cells. 39 Slit, another secreted axon guidance factor, is expressed in Drosophila midline glia (Rothberg et al., 1990) and the floor plate and roof plate of the vertebrate neural tube (Holmes et al., 1998), and the gene encoding the planarian ortholog is expressed in a ventral midline stripe lying between the cephalic ganglia lobes (Cebrii et al., 2007). Inhibition of slit in planarians results in midline defects including cyclopic photoreceptors and failed separation between the brain lobes (CebriA et al., 2007). The localization and function of the slit+ cells is reminiscent of the midline of both Drosophilaand vertebrates, raising the possibility that the cells within this domain are glia. Potentialglia-associatedgenes A number of genes encoding orthologs of proteins involved in glia specification in other systems has been described in planarians, but none of these genes have been examined in the context of planarian glia specification. The basic helix-loop-helix (bHLH) family includes many transcription factors involved in determining neural versus glial cell fates, including Olig2 and Hairy/Enhancer of Split (Nieto et al., 2001; Zhou and Anderson, 2002). Many members of the bHLH family in planarians are expressed in the nervous system and result in cephalic ganglia patterning defects upon inhibition (Cowles et al., 2013). Inhibition of planarian BMP4-J, which encodes an ortholog of a factor involved in OPC fate specification (Wu et al., 2012), results in ectopic photoreceptors in addition to defects in regeneration of lateral domains of the body (Reddien et al., 2007). EGFs have been implicated in reactive astrogliosis and NSC proliferation, and three genes encoding orthologs have been identified in planarians. In addition to its expression in pharynx and neoblasts, egfr-3 is 40 expressed at the outer edge of the cephalic ganglia lobes, and its inhibition results in failed differentiation of neuronal cell types in the anterior blastema (Fraguas et al., 2011). FGF is an important mitogen for maintaining stem cell characteristics and promoting proliferation in astrocytes and neural stem cells (Feng et al., 2014; Gritti et al., 1999). nou-darake (ndk), a planarian gene encoding a protein similar to FGF receptors, is expressed in the nervous system. Posterior expansion of the cephalic ganglia with ectopic photoreceptors is observed upon inhibition of ndk, suggesting that its role is to restrict the cephalic ganglia to the anterior domain (CebriA et al., 2002). The genes mentioned here encode proteins with roles in nervous system regeneration in planarians and may be involved in regulating glial cell biology as well, but without a reliable marker for glial cells, either by in situ hybridization or by immunofluorescence, those roles cannot be assessed. There remain many other signaling pathways with demonstrated roles in Drosophilaand vertebrate glia development and biology that either have not been described as yet or appear to have no role in nervous system regeneration. Notch signaling controls neuron versus glia fate choices through contact inhibition in both developing glia and oligodendrocyte progenitors (Gaiano et al., 2000; Grandbarbe et al., 2003; Wilhelmsson et al., 2012). Aside from studies of the role of the downstream bHLH transcription factor-encoding gene hesl-3 in nervous system regeneration, Notch signaling in planarians has not been described in the literature. The Hedgehog signaling pathway has known roles in planarians, but none described yet for nervous system regeneration (Rink et al., 2009) (Figure 5A). Inhibition of Hedgehog pathway components results in anterior-posterior polarity defects during regeneration (Rink et al., 2009; Yazawa et al., 2009) (Figure 5B). Interestingly, hedgehog is expressed in cells in the 41 nervous system adjacent to the neuropil, suggesting that it may have a previously unexplored role in neural or glial cell specification (Rink et al., 2009) (Figure 5C). Given that Hedgehog signaling is involved in oligodendrocyte specification (Wegner, 2008) and reactive astrocyte activation (Sirko et al., 2013) in vertebrates and posterior midline glia specification in Drosophila(Watson et al., 2011), it is an interesting pathway to investigate for potential roles in regulation of planarian glia biology. Evidence for a glial cell type in planarians would open the field to the study of glia in a highly regenerative organism. A comparison of mechanisms, both cell autonomous and system wide, between mammalian glia and planarian glia may offer insight into how and why mammalian glia evolved to limit regenerative potential. The stem cell potential of the organism offers a unique perspective of observing the complete developmental pathway of the cell in an adult animal, from naive to terminally differentiated. Additionally, continued characterization of glia in other species will contribute to our understanding of the homology between vertebrate and invertebrate glia types and also the origins of this complex and vital cell type. 42 IV. Content Overview Determining the source of specialized cell types, such as glia, during planarian regeneration is integral to understanding how they are specified and whether their differentiation has similarities to developmental mechanisms in other species. Planarian neoblasts are the source of new tissue during regeneration, but the pathways by which specified cells are determined and whether pools of progenitors exist to repopulate depleted differentiated cell types are largely unknown. For example, do planarian glia arise from a unipotent progenitor population that can be depleted, similarly to newly formed astrocytes during reactive astrogliosis, or do they stem from more potent cells that contribute to other cell types, such as all specialized cells during embryonic development? In order to gain a better understanding of how planarian glia are regenerated and contribute to regeneration, the origin of these cells must be identified. Once uncovered, questions pertaining to how new planarian glia are made throughout adulthood and whether the system for replenishing these cells is similar to mammalian mechanisms can be asked. Chapter 2 of this thesis demonstrates the existence of a pluripotent stem cell type within the neoblast population. These cells, called clonogenic neoblasts, were shown to be capable of self-renewal and differentiation using sublethal irradiation or single cell transplantation colony assays. Additionally, a single transplanted clonogenic neoblast was demonstrated to be sufficient to restore regeneration in animals lacking all endogenous neoblasts. These results suggest that, within the adult animal, all specialized cells can arise from a single cell type, which expands our understanding of how terminally differentiated cells such as glia are regenerated after injury. With this knowledge, the formation of specialized cell types can begin to be elucidated. 43 In addition to answering a long-standing question in the planarian field and providing a basis for continued study of basic regenerative mechanisms and stem cell biology, this discovery presents an opportunity to dissect the differentiation mechanisms of planarian glial cells during regeneration and compare the process to macroglia development or injury response in mammalian systems. To further investigate the function of planarian glia and their similarity to glial cells of other species, genes with known function in glia cells and pathways with known roles in glia specification in other model systems were investigated in planarians. Molecular characteristics are important for establishing cell types, deducing function, and finding homology with similar cell types in other species. With these data, arguments can be made concerning the evolutionary origins of glial cells. Furthermore, it would aid analysis to look for similar functions shared between invertebrate and vertebrate glia, especially in the context of wound response and regeneration. The Hedgehog signaling pathway is involved in both the specification of glia during development and the modulation of astrocyte activity in mammals and, therefore, is an ideal target for perturbation in order to characterize putative glial cell types. Chapter 3 of this thesis provides molecular evidence for the existence of planarian glia and demonstrates that gene expression in these cells is regulated by Hedgehog signaling from adjacent neurons. The identification of planarian glia by both marker expression and morphology builds upon ultrastructural studies done in the past as well as provide a foundation on which comparisons of planarian glia can be made with glia of other model systems. These data contribute to our understanding whether planarian glia share a common evolutionary origin with mammalian glia, and thereby whether roles during regeneration and nervous system repair 44 are likely to be similar. Additionally, the involvement of Hedgehog signaling in gene expression in these terminally differentiated cells shows surprising similarity to a mechanism by which neurons communicate with nearby astrocytes using Sonic hedgehog. This conserved role of Hedgehog points to the possibility of an ancestral function of the pathway shared between deuterostomes and protostomes. This new evidence for planarian glia creates a number of opportunities to study specification and development of this cell type, its role in regeneration of the nervous system, and the evolution of glia. 45 Figure 1 M0 IF UI Ctenophora SMC3 Placozoa ND Cnidaria NO Acoelomorpha ND Rotifera ND Platyhelminthes MDN Gastrotricha -U Bryozoa ND Entoprocta -UC Cycliophora -U Annelida DD Mollusca -U Nemertia -N0 Brachiopodia -U Phoronida DD Nematoida -U Tardigrada ND Onychophora OU Arthropoda Hemichordata Echinodermata Cephalochordata NED Urochordata -U Craniata 46 Regeneration Key No Data No Regeneration D3 Limb Segment Whole Body r0 0 OR 0 Nk m 0 N 0 (D 0 (A 0) Glia Key D: N No Data No Glia Primitive Glia Developed Glia Figure 1. Presence or absence of regeneration and glia in the Metazoa. Phylogenetic tree of the Metazoa with Bilaterian superphyla (Deuterostomia, Ecdysozoa, and Lophotrochozoa) noted on right side. Regenerative potential of each taxa (based on highest regenerative ability of a single species within the taxon) is classified based on no regeneration of body structures (red), regeneration of limbs (yellow), regeneration of segments of the primary body axes (green), and regeneration of whole bodies from small pieces (blue) (Bely and Nyberg, 2010). Presence of glia-like cells in each taxa (based on closest morphological similarity to mammalian glia of a single species within the taxon) is classified by absence of glia (red), presence of primitive non-ensheathing glia (yellow), and well-developed glia (green) (Hartline, 2011). 47 Figure 2 A Reactive Astrogliosis B Remyelination Neuron 0 0 Astrocyte 0 OL NG2 Glia * V z ~JNG2- GLAST+ GS+ EGF/FGFI 0 t TNF/SHH PDGFaR+ Myelin PDGFFGF2 ;IWo 0) E gA2+ Oligl+ Sox2+ G4G 2utamate 0 LU 0 0 EL I.. 0 a * Oli401g2+ I I - 0 0 0L 0. 0 w MRF+ _______ Collagen / Fibronectin / CSPG 48 MRF+ Figure 2. Overview of reactive astrogliosis and remyelination. (A) Astrocytes upregulate expression of GFAP and Olig2 in response release of EGF, FGF, TNF, of SHH from damaged portions of the nervous system. Activated astrocytes proliferate and secrete FGF4 to promote proliferation in nearby astrocytes. Reactive astrocytes accumulate and seal the wound site by secretion of extracellular matrix proteins collagen, fibronectin and CSPG. (B) Myelin represses proliferation and differentiation in NG2 glia. Demyelination induces response in astrocytes, which in turn secrete PDGF and FGF2 to activate Nkx2.2, Oligl and Sox2 expression in NG2 glia. After proliferation, immature oligodendrocytes express Olig2 to begin differentiation and MRF to synthesize myelin proteins. Depolarization of immature oligodendrocytes by unmyelinated axons triggers the formation of the myelin sheath. 49 Figure 3 A B Dc2 mRNA Synapsin Protein DAPI syn mRNA Synapsin Protein 50 Figure 3. Localization of cell bodies and synapses in the planarian nervous system. (A) Fluorescent in situ hybridization (FISH) of pan-neuronal marker prohormone convertase 2 (pc2) (green) (Collins et al., 2010) with DAPI for nuclei (blue). Overview image (left) shows cephalic ganglia lobes (CG) and ventral nerve cords (VNC). Detail image (right) shows cephalic ganglia with DIC. Cell bodies labeled by FISH are localized to the cortex (ctx), which encompasses the cell body-sparse neuropil (np). (B) Immunofluorescence (IF) for synapse- associated protein Synapsin (magenta) and FISH for synapsin (syn) transcript (green, right images only). Overview image (left) shows high concentration of synapses in the neuropil of the CG and VNC as well as smaller clusters throughout the ventral parenchyma. Inset (i) (top right) shows detail of cephalic ganglia, with no overlap between Synapsin transcript and protein. Inset (ii) (bottom right) shows detail of ventral nerve cords. Anterior up, ventral surface shown for all. Scale bars: I00um for A and left panel of B; 50um for right panels of B. 51 Figure 4 A 0) 4L 0) C smedwi-1+ SMEDWI 4 smedw-1+ SMEDWI-1+ C. 0 Neoblast smedwi-1+ SMEDWI 1+ SMEDWI- i SMEDWI-1+ marker+ Lineage Restricted marker+ MARKER+ Pluripotent Ii, * 0 * B Differentiated Cell I I 52 4K Figure 4. Planarian neoblast stem cell characteristics and potential. (A) Self-renewal of neoblasts can be traced by detection of either smedwi-J mRNA by in situ hybridization or SMEDWI-1 protein by immunofluorescence. Differentiation of neoblasts can be traced by detection of overlapping expression of SMEDWI- 1 protein, which perdures after smedwi-J transcription ceases, and differentiated cell markers. (B) Lineage restricted model and pluripotent model of neoblast population potential. In the lineage restricted model, the neoblast population contains multiple subpopulations, each of which can only generate specific cell types. In the pluripotent model, the neoblast population contains a pluripotent stem cell type capable of giving rise to all differentiated cell types in the adult animal. 53 Figure 5 B C control RNAI hh RNAi ptc RNAI hh chat 54 ptc RNAI Figure 5. The Hedgehog signaling pathway in planaria. (A) Simplified schematic representing major Hedgehog signaling pathway components studied in planaria. Hedgehog (Hh), a secreted ligand, binds and inhibits the transmembrane receptor Patched (Ptc). Inhibition of Ptc relieves inhibition of the transmembrane protein Smoothened (Smo). Activated Smo inhibits cytoplasmic protein Suppressor of Fused (Sufu). Inhibition of Sufu releases sequestration of Gli-1 transcription factor. (B) Images of anterior and posterior blastemas of live animals following Hedgehog signaling perturbation and six days of regeneration. Right-most image shows severe ptc RNAi phenotype. Red dotted lines delineate approximate amputation plane. Red arrowhead indicates fused photoreceptors. (C) Double FISH of hh (red) and neuronal marker choline acetyltransferase(chat) (green). Co-expression is observed in the medial cortex of the cephalic ganglia. 55 References Adoutte, A., Balavoine, G., Lartillot, N., Lespinet, 0., Prud'homme, B., and de Rosa, R. (2000). The new animal phylogeny: reliability and implications. Proc. Natl. Acad. Sci. U.S.a. 97,44534456. Altshuller, Y., Copeland, N.G., Gilbert, D.J., Jenkins, N.A., and Frohman, M.A. (1996). GcmI, a mammalian homolog of Drosophilaglial cells missing. FEBS Lett. 393, 201-204. Anderson, C.M., and Swanson, R.A. (2000). Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32, 1-14. Anson-Cartwright, L., Dawson, K., Holmyard, D., Fisher, S.J., Lazzarini, R.A., and Cross, J.C. (2000). The glial cells missing-i protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 25, 311-314. Bachoo, R.M., Kim, R.S., Ligon, K.L., Maher, E.A., Brennan, C., Billings, N., Chan, S., Li, C., Rowitch, D.H., Wong, W.H., et al. (2004). Molecular diversity of astrocytes with implications for neurological disorders. Proc. Natl. Acad. Sci. U.S.a. 101, 8384-8389. BagufiA, J., Sal6, E., and Auladell, C. (1989). Regeneration and pattern formation in planarians. III. that neoblasts are totipotent stem cells and the cells. Development (Cambridge, England) 7786. Bale, S.D., Howard, T.A., and Moffett, S.B. (2001). Neuronal and non-neuronal responses to nerve crush in a pulmonate snail, Melampus bidentatus. Invert. Neurosci. 4, 105-117. Banerjee, S., Pillai, A.M., Paik, R., Li, J., and Bhat, M.A. (2006). Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila.Journal of Neuroscience 26, 3319-3329. Barres, B.A., Hart, I.K., Coles, H.S., Burne, J.F., Voyvodic, J.T., Richardson, W.D., and Raff, M.C. (1992). Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 3146. Barreto, G.E., Sun, X., Xu, L., and Giffard, R.G. (2011). Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS ONE 6, e2788 1. Baumgartner, S., Littleton, J.T., Broadie, K., Bhat, M.A., Harbecke, R., Lengyel, J.A., ChiquetEhrismann, R., Prokop, A., and Bellen, H.J. (1996). A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87, 1059-1068. Bely, A.E., and Nyberg, K.G. (2010). Evolution of animal regeneration: re-emergence of a field. Trends Ecol. Evol. (Amst.) 25, 161-170. Bergles, D.E., Roberts, J.D., Somogyi, P., and Jahr, C.E. (2000). Glutamatergic synapses on 56 oligodendrocyte precursor cells in the hippocampus. Nature 405, 187-191. Bossing, T., and Technau, G.M. (1994). The fate of the CNS midline progenitors in Drosophila as revealed by a new method for single cell labelling. Development (Cambridge, England) 120, 1895-1906. Boyle, M.E., Berglund, E.O., Murai, K.K., Weber, L., Peles, E., and Ranscht, B. (2001). Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30, 385-397. Bradbury, E.J., Moon, L.D.F., Popat, R.J., King, V.R., Bennett, G.S., Patel, P.N., Fawcett, J.W., and McMahon, S.B. (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636-640. Brenner, M., Kisseberth, W.C., Su, Y., Besnard, F., and Messing, A. (1994). GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 14, 1030-1037. Bruni, J.E. (1998). Ependymal development, proliferation, and functions: a review. Microsc. Res. Tech. 41, 2-13. Buffo, A., Rite, I., Tripathi, P., Lepier, A., Colak, D., Horn, A.-P., Mori, T., and G~tz, M. (2008). Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proceedings of the National Academy of Sciences 105, 3581-3586. Burda, J.E., and Sofroniew, M.V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229-248. Bush, T.G., Puvanachandra, N., Homer, C.H., Polito, A., Ostenfeld, T., Svendsen, C.N., Mucke, L., Johnson, M.H., and Sofroniew, M.V. (1999). Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23, 297-308. Carldn, M., Meletis, K., G6ritz, C., Darsalia, V., Evergren, E., Tanigaki, K., Amendola, M., Barnab&Heider, F., Yeung, M.S.Y., Naldini, L., et al. (2009). Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 12, 259267. Carpenter, K.S., Morita, M., and Best, J.B. (1974). Ultrastructure of the photoreceptor of the planarian Dugesiadorotocephala.Cell Tissue Res 148. Cebri&, F., and Newmark, P.A. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development (Cambridge, England) 132, 3691-3703. Cebria, F., Guo, T., Jopek, J., and Newmark, P.A. (2007). Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Developmental Biology 307, 394-406. 57 CebriA, F., Kobayashi, C., Umesono, Y., Nakazawa, M., Mineta, K., Ikeo, K., Gojobori, T., Itoh, M., Taira, M., Sinchez Alvarado, A., et al. (2002). FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature 419, 620-624. Chari, D.M., and Blakemore, W.F. (2002). Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia 37, 307-313. Chen, Y., Miles, D.K., Hoang, T., Shi, J., Hurlock, E., Kernie, S.G., and Lu, Q.R. (2008). The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. Journal of Neuroscience 28, 10983-10989. Collins, J.J., III, Hou, X., Romanova, E.V., Lambrus, B.G., Miller, C.M., Saberi, A., Sweedler, J.V., and Newmark, P.A. (2010). Genome-Wide Analyses Reveal a Role for Peptide Hormones in Planarian Germline Development. PLoS Biol 8, e1000509. Condorelli, D.F., Dell'Albani, P., Kaczmarek, L., Messina, L., Spampinato, G., Avola, R., Messina, A., and Giuffrida Stella, A.M. (1990). Glial fibrillary acidic protein messenger RNA and glutamine synthetase activity after nervous system injury. J. Neurosci. Res. 26, 251-257. Coutinho-Budd, J., and Freeman, M.R. (2013). Probing the enigma: unraveling glial cell biology in invertebrates. Current Opinion in Neurobiology. Cowles, M.W., Brown, D.D.R., Nisperos, S.V., Stanley, B.N., Pearson, B.J., and Zayas, R.M. (2013). Genome-wide analysis of the bHLH gene family in planarians identifies factors required for adult neurogenesis and neuronal regeneration. Development (Cambridge, England) 140, 4691-4702. Craig, C.G., Tropepe, V., Morshead, C.M., Reynolds, B.A., Weiss, S., and van der Kooy, D. (1996). In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 16, 2649-2658. Cregg, J.M., DePaul, M.A., Filous, A.R., Lang, B.T., Tran, A., and Silver, J. (2014). Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197-207. Davies, S.J., Fitch, M.T., Memberg, S.P., Hall, A.K., Raisman, G., and Silver, J. (1997). Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680-683. De Biase, L.M., Kang, S.H., Baxi, E.G., Fukaya, M., Pucak, M.L., Mishina, M., Calabresi, P.A., and Bergles, D.E. (2011). NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. Journal of Neuroscience 31, 12650-12662. Derouiche, A., and Frotscher, M. (2001). Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia 36, 330-341. DeSilva, T.M., Kabakov, A.Y., Goldhoff, P.E., Volpe, J.J., and Rosenberg, P.A. (2009). 58 Regulation of glutamate transport in developing rat oligodendrocytes. Journal of Neuroscience 29, 7898-7908. Dickendesher, T.L., Baldwin, K.T., Mironova, Y.A., Koriyama, Y., Raiker, S.J., Askew, K.L., Wood, A., Geoffroy, C.G., Zheng, B., Liepmann, C.D., et al. (2012). NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 15, 703-712. Doetsch, F., Caill6, I., Lim, D.A., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703716. Doetsch, F., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046-5061. Doherty, J., Logan, M.A., Tagdemir, O.E., and Freeman, M.R. (2009). Ensheathing glia function as phagocytes in the adult Drosophilabrain. Journal of Neuroscience 29, 4768-4781. Donato, R. (1999). Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450, 191-231. Eisenhoffer, G.T., Kang, H., and Sanchez Alvarado, A. (2008). Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea.Cell Stem Cell 3, 327-339. Elliot, E.J., and Muller, K.J. (1982). Synapses between neurons regenerate accurately after destruction of ensheathing glial cells in the leech. Science 215, 1260-1262. Erber, A., Riemer, D., Bovenschulte, M., and Weber, K. (1998). Molecular Phylogeny of Metazoan Intermediate Filament Proteins. J Mol Evol 47, 751-762. Fan, G., Martinowich, K., Chin, M.H., He, F., Fouse, S.D., Hutnick, L., Hattori, D., Ge, W., Shen, Y., Wu, H., et al. (2005). DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development (Cambridge, England) 132, 3345-3356. Faulkner, J.R., Herrmann, J.E., Woo, M.J., Tansey, K.E., Doan, N.B., and Sofroniew, M.V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. Journal of Neuroscience 24, 2143-2155. Feng, G.-D., He, B.-R., Lu, F., Liu, L.-H., Zhang, L., Chen, B., He, Z.-P., Hao, D.-J., and Yang, H. (2014). Fibroblast Growth Factor 4 Is Required but not Sufficient for the Astrocyte Dedifferentiation. Mol. Neurobiol. FernAndez-HernAndez, I., Rhiner, C., and Moreno, E. (2013). Adult neurogenesis in Drosophila. Cell Rep 3, 1857-1865. 59 Flici, H., Erkosar, B., Komonyi, 0., Karatas, O.F., Laneve, P., and Giangrande, A. (2011). Gcm/Glide-dependent conversion into glia depends on neural stem cell age, but not on division, triggering a chromatin signature that is conserved in vertebrate glia. Development (Cambridge, England) 138,4167-4178. Fraguas, S., Barberin, S., and Cebrik, F. (2011). EGFR signaling regulates cell proliferation, differentiation and morphogenesis during planarian regeneration and homeostasis. Developmental Biology 354, 87-101. Freeman, M.R., and Doherty, J. (2006). Glial cell biology in Drosophilaand vertebrates. Trends Neurosci. Freeman, M.R. (2010). Specification and morphogenesis of astrocytes. Science 330, 774-778. Frost, E.E., Nielsen, J.A., Le, T.Q., and Armstrong, R.C. (2003). PDGF and FGF2 regulate oligodendrocyte progenitor responses to demyelination. J. Neurobiol. 54, 457-472. Fry, E.J., Chagnon, M.J., L6pez-Vales, R., Tremblay, M.L., and David, S. (2010). Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia 58, 423-433. Gaiano, N., Nye, J.S., and Fishell, G. (2000). Radial glial identity is promoted by Notchl signaling in the murine forebrain. Neuron 26, 395-404. Gallo, V., Zhou, J.M., McBain, C.J., Wright, P., Knutson, P.L., and Armstrong, R.C. (1996). Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J. Neurosci. 16, 2659-2670. Gibson, E.M., Purger, D., Mount, C.W., Goldstein, A.K., Lin, G.L., Wood, L.S., Inema, I., Miller, S.E., Bieri, G., Zuchero, J.B., et al. (2014). Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science. Giesen, K., Hummel, T., Stollewerk, A., Harrison, S., Travers, A., and KlAmbt, C. (1997). Glial development in the DrosophilaCNS requires concomitant activation of glial and repression of neuronal differentiation genes. Development (Cambridge, England) 124, 2307-2316. Goldstein, L.S., and Gunawardena, S. (2000). Flying through the Drosophilacytoskeletal genome. J. Cell Biol. 150, F63-F68. Golubev, A.I. (1988). Glia and neuroglia relationships in the central nervous system of the Turbellaria (Electron microscopic data). Fortschr Zool 36, 31-37. Gonzalez-Perez, 0., Romero-Rodriguez, R., Soriano-Navarro, M., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (2009). Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells 27, 2032-2043. 60 Grandbarbe, L., Bouissac, J., Rand, M., Hrab6 de Angelis, M., Artavanis-Tsakonas, S., and Mohier, E. (2003). Delta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development (Cambridge, England) 130, 1391-1402. Granderath, S., Stollewerk, A., Greig, S., Goodman, C.S., O'Kane, C.J., and KlAmbt, C. (1999). loco encodes an RGS protein required for Drosophila glial differentiation. Development (Cambridge, England) 126, 1781-1791. Greenwood, K., and Butt, A.M. (2003). Evidence that perinatal and adult NG2-glia are not conventional oligodendrocyte progenitors and do not depend on axons for their survival. Mol. Cell. Neurosci. 23, 544-558. Gritti, A., Fr6lichsthal-Schoeller, P., Galli, R., Parati, E.A., Cova, L., Pagano, S.F., Bjornson, C.R., and Vescovi, A.L. (1999). Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 19, 3287-3297. Guedelhoefer, O.C., and SAnchez Alvarado, A. (2012). Amputation induces stem cell mobilization to sites of injury during planarian regeneration. Development (Cambridge, England) 139, 3510-3520. Guo, F., Ma, J., McCauley, E., Bannerman, P., and Pleasure, D. (2009). Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. Journal of Neuroscience 29, 7256-7270. Hanisch, U.-K., and Kettenmann, H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387-1394. Hartenstein, V. (2011). Morphological diversity and development of glia in Drosophila. Glia 59, 1237-1252. Hartfuss, E., Galli, R., Heins, N., and G6tz, M. (2001). Characterization of CNS precursor subtypes and radial glia. Developmental Biology 229, 15-30. Hartline, D.K. (2011). The evolutionary origins of glia. Glia 59, 1215-1236. Hayashi, T., Asami, M., Higuchi, S., Shibata, N., and Agata, K. (2006). Isolation of planarian Xray-sensitive stem cells by fluorescence-activated cell sorting. Dev. Growth Differ. 48, 371-380. Hayashi, T., Shibata, N., Okumura, R., Kudome, T., Nishimura, 0., Tarui, H., and Agata, K. (2010). Single-cell gene profiling of planarian stem cells using fluorescent activated cell sorting and its "index sorting" function for stem cell research. Dev. Growth Differ. 52, 131-144. He, F., Ge, W., Martinowich, K., Becker-Catania, S., Coskun, V., Zhu, W., Wu, H., Castro, D., Guillemot, F., Fan, G., et al. (2005). A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat. Neurosci. 8, 616-625. 61 Hess, D.C., Abe, T., Hill, W.D., Studdard, A.M., Carothers, J., Masuya, M., Fleming, P.A., Drake, C.J., and Ogawa, M. (2004). Hematopoietic origin of microglial and perivascular cells in brain. Exp. Neurol. 186, 134-144. Hirabayashi, Y., Suzki, N., Tsuboi, M., Endo, T.A., Toyoda, T., Shinga, J., Koseki, H., Vidal, M., and Gotoh, Y. (2009). Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600-613. Holmes, G.P., Negus, K., Burridge, L., Raman, S., Algar, E., Yamada, T., and Little, M.H. (1998). Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech. Dev. 79, 57-72. Hosoya, T., Takizawa, K., Nitta, K., and Hotta,'Y. (1995). glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82, 1025-1036. Houle, J.D., and Jin, Y. (2001). Chronically injured supraspinal neurons exhibit only modest axonal dieback in response to a cervical hemisection lesion. Exp. Neurol. 169, 208-217. Howes, E.A., Smith, P.J., and Treherne, J.E. (1989). Adult insect glial culture: Activation, substrate effects and proliferation. Tissue Cell 21, 759-772. Hyman, L.H. (1940). The invertebrates: Platyhelminthes and Rhynchocoela, the acoelomate Bilateria. (McGraw-Hill). Jacques, C., Soustelle, L., Nagy, I., Diebold, C., and Giangrande, A. (2009). A novel role of the glial fate determinant glial cells missing in hematopoiesis. Int. J. Dev. Biol. 53, 1013-1022. Johansson, C.B., Momma, S., Clarke, D.L., Risling, M., Lendahl, U., and Frisdn, J. (1999). Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 2534. Juurlink, B.H., Thorburne, S.K., and Hertz, L. (1998). Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 22, 371-378. Kang, S.H., Fukaya, M., Yang, J.K., Rothstein, J.D., and Bergles, D.E. (2010). NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668-681. Kaplan, M.R., Meyer-Franke, A., Lambert, S., Bennett, V., Duncan, I.D., Levinson, S.R., and Barres, B.A. (1997). Induction of sodium channel clustering by oligodendrocytes. Nature 386, 724-728. Karalay, 0., Doberauer, K., Vadodaria, K.C., Knobloch, M., Berti, L., Miquelajauregui, A., Schwark, M., Jagasia, R., Taketo, M.M., Tarabykin, V., et al. (2011). Prospero-related homeobox 1 gene (Proxi) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proceedings of the National Academy of Sciences 108, 62 5807-5812. Kato, K., Awasaki, T., and Ito, K. (2009). Neuronal programmed cell death induces glial cell division in the adult Drosophilabrain. Development (Cambridge, England) 136, 51-59. Kato, K., Forero, M.G., Fenton, J.C., and Hidalgo, A. (2011). The glial regenerative response to central nervous system injury is enabled by pros-notch and pros-NFicB feedback. PLoS Biol 9, e1001 133. Kdrad6ttir, R., Cavelier, P., Bergersen, L.H., and Attwell, D. (2005). NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162-1166. Keirstead, H.S., and Blakemore, W.F. (1997). Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J. Neuropathol. Exp. Neurol. 56, 1191-1201. Kessaris, N., Fogarty, M., Iannarelli, P., Grist, M., Wegner, M., and Richardson, W.D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173-179. Koenning, M., Jackson, S., Hay, C.M., Faux, C., Kilpatrick, T.J., Willingham, M., and Emery, B. (2012). Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. Journal of Neuroscience 32, 12528-12542. Kotter, M.R., Li, W.-W., Zhao, C., and Franklin, R.J.M. (2006). Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. Journal of Neuroscience 26, 328-332. Kuhn, H.G., Winkler, J., Kempermann, G., Thal, L.J., and Gage, F.H. (1997). Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 17, 5820-5829. Lang, B., Liu, H.L., Liu, R., Feng, G.D., Jiao, X.Y., and Ju, G. (2004). Astrocytes in injured adult rat spinal cord may acquire the potential of neural stem cells. Neuroscience 128, 775-783. Laywell, E.D., Rakic, P., Kukekov, V.G., Holland, E.C., and Steindler, D.A. (2000). Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. U.S.a. 97, 13883-13888. Levine, J.M., and Card, J.P. (1987). Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J. Neurosci. 7, 2711-2720. Levison, S.W., and Goldman, J.E. (1993). Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 10, 201-212. 63 Levison, S.W., Chuang, C., Abramson, B.J., and Goldman, J.E. (1993). The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development (Cambridge, England) 119,611-622. Li, L., Harms, K.M., Ventura, P.B., Lagace, D.C., Eisch, A.J., and Cunningham, L.A. (2010). Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58, 1610-1619. Li, Y., and Raisman, G. (1995). Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp. Neurol. 134, 102-111. Lois, C., and Alvarez-Buylla, A. (1993). Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. U.S.a. 90, 2074-2077. Malatesta, P., Hartfuss, E., and G6tz, M. (2000). Isolation of radial glial cells by fluorescentactivated cell sorting reveals a neuronal lineage. Development (Cambridge, England) 127, 52535263. Manev, H., Favaron, M., Guidotti, A., and Costa, E. (1989). Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 36, 106-112. Marshall, C.A.G., Novitch, B.G., and Goldman, J.E. (2005). Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. Journal of Neuroscience 25, 7289-7298. Mason, J.L., Toews, A., Hostettler, J.D., Morell, P., Suzuki, K., Goldman, J.E., and Matsushima, G.K. (2004). Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am. J. Pathol. 164, 1673-1682. McKeon, R.J., Schreiber, R.C., Rudge, J.S., and Silver, J. (1991). Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 11, 3398-3411. McTigue, D.M., and Tripathi, R.B. (2008). The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 107, 1-19. Menn, B., Garcia-Verdugo, J.M., Yaschine, C., Gonzalez-Perez, 0., Rowitch, D., and AlvarezBuylla, A. (2006). Origin of oligodendrocytes in the subventricular zone of the adult brain. Journal of Neuroscience 26, 7907-7918. Menne, T.V., and Klimbt, C. (1994). The formation of commissures in the Drosophila CNS depends on the midline cells and on the Notch gene. Development (Cambridge, England) 120, 123-133. Merkle, F.T., Tramontin, A.D., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (2004). Radial 64 glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. U.S.a. 101, 17528-17532. Miller, R.H., and Raff, M.C. (1984). Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J. Neurosci. 4, 585-592. Mitchell, K.J., Doyle, J.L., Serafini, T., Kennedy, T.E., Tessier-Lavigne, M., Goodman, C.S., and Dickson, B.J. (1996). Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203-215. Moon, L.D., Asher, R.A., Rhodes, K.E., and Fawcett, J.W. (2001). Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci. 4,465-466. Morgan, T.H. (1898). Experimental studies of the regeneration of Planariamaculata. Dev. Genes Evol. 7, 364-397. Morita, M., and Best, J.B. (1965). Electron microscopic studies on Planaria. II. Fine structure of the neurosecretory system in the planarian Dugesia dorotocephala.J. Ultrastruct. Res. 13, 396408. Morita, M., and Best, J.B. (1966). Electron microscopic studies of planaria. III. Some observations on the fine structure of planarian nervous tissue. J. Exp. Zool. 161, 391-411. Nait-Oumesmar, B., Picard-Riera, N., Kerninon, C., Decker, L., Seilhean, D., H6glinger, G.U., Hirsch, E.C., Reynolds, R., and Baron-Van Evercooren, A. (2007). Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc. Natl. Acad. Sci. U.S.a. 104,4694-4699. Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S.-I., Hatano, 0., Kawahara, N., Tamura, A., Kirino, T., and Nakafuku, M. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429-441. Namihira, M., Kohyama, J., Semi, K., Sanosaka, T., Deneen, B., Taga, T., and Nakashima, K. (2009). Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell 16, 245-255. Newmark, P.A., and Sanchez Alvarado, A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental Biology 220, 142-153. Newmark, P.A., and SAnchez Alvarado, A. (2002). Not your father's planarian: a classic model enters the era of functional genomics. Nat Rev Genet 3, 210-219. Newmark, P.A., Reddien, P.W., Cebrii, F., and S~nchez Alvarado, A. (2003). Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc. Natl. Acad. Sci. U.S.a. 100 Suppl 1, 11861-11865. 65 Nieto, M., Schuurmans, C., Britz, 0., and Guillemot, F. (2001). Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron 29, 401-413. Nishiyama, A. (2007). Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist 13, 62-76. Noctor, S.C., Flint, A.C., Weissman, T.A., Dammerman, R.S., and Kriegstein, A.R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714-720. Noctor, S.C., Martinez-Cerdeflo, V., Ivic, L., and Kriegstein, A.R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136-144. Oikonomou, G., and Shaham, S. (2011). The glia of Caenorhabditiselegans. Glia 59, 12531263. Oyallon, J., Apitz, H., Miguel-Aliaga, I., Timofeev, K., Ferreira, L., and Salecker, I. (2012). Regulation of locomotion and motoneuron trajectory selection and targeting by the Drosophila homolog of Olig family transcription factors. Developmental Biology 369, 261-276. Pearce, J., Lnenicka, G.A., and Govind, C.K. (2003). Regenerating crayfish motor axons assimilate glial cells and sprout in cultured explants. J. Comp. Neurol. 464, 449-462. Pearson, B.J., Eisenhoffer, G.T., Gurley, K.A., Rink, J.C., Miller, D.E., and Sinchez Alvarado, A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev. Dyn. 238,443-450. Pellettieri, J., Fitzgerald, P., Watanabe, S., Mancuso, J., Green, D.R., and SAnchez Alvarado, A. (2010). Cell death and tissue remodeling in planarian regeneration. Developmental Biology 338, 76-85. Penderis, J., Shields, S.A., and Franklin, R.J.M. (2003). Impaired remyelination and depletion of oligodendrocyte progenitors does not occur following repeated episodes of focal demyelination in the rat central nervous system. Brain 126, 1382-1391. Qi, Y., Cai, J., Wu, Y., Wu, R., Lee, J., Fu, H., Rao, M., Sussel, L., Rubenstein, J., and Qiu, M. (2001). Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development (Cambridge, England) 128, 2723-2733. Reddien, P.W., and SAnchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725-757. Reddien, P.W., Bermange, A.L., Kicza, A.M., and SAnchez Alvarado, A. (2007). BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development (Cambridge, England) 134, 4043-4051. 66 Reddien, P.W., Bermange, A.L., Murfitt, K.J., Jennings, J.R., and Sinchez Alvarado, A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635-649. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and Sanchez Alvarado, A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. Redwine, J.M., and Armstrong, R.C. (1998). In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J. Neurobiol. 37, 413-428. Reuter, M., Mntyl1, K., and Gustafsson, M.K.S. (1998). Organization of the orthogon - main and minor nerve cords - Springer. Hydrobiologia 383, 175-182. Reynolds, R., and Hardy, R. (1997). Oligodendroglial progenitors labeled with the 04 antibody persist in the adult rat cerebral cortex in vivo. J. Neurosci. Res. 47, 455-470. Rink, J.C., Gurley, K.A., Elliott, S.A., and Sanchez Alvarado, A. (2009). Planarian Hh Signaling Regulates Regeneration Polarity and Links Hh Pathway Evolution to Cilia. Science 326, 14061410. Rival, T., Soustelle, L., Strambi, C., Besson, M.-T., Ichd, M., and Birman, S. (2004). Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophilabrain. Curr. Biol. 14, 599-605. Rivers, L.E., Young, K.M., Rizzi, M., Jamen, F., Psachoulia, K., Wade, A., Kessaris, N., and Richardson, W.D. (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392-1401. Robel, S., Berninger, B., and G6tz, M. (2011). The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 12, 88-104. Rolls, A., Shechter, R., and Schwartz, M. (2009). The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10, 235-241. Rothberg, J.M., Jacobs, J.R., Goodman, C.S., and Artavanis-Tsakonas, S. (1990). slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 4, 2169-2187. Rudge, J.S., and Silver, J. (1990). Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 10, 3594-3603. Saito, A., Kanemoto, S., Kawasaki, N., Asada, R., Iwamoto, H., Oki, M., Miyagi, H., Izumi, S., Sanosaka, T., Nakashima, K., et al. (2012). Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat Commun 3, 967. Sal6, E., and Bagufia, J. (1984). Regeneration and pattern formation in planarians. I. The pattern 67 of mitosis in anterior and posterior regeneration in Dugesia (G) tigrina, and a new proposal for blastema formation. J Embryol Exp Morphol 83, 63-80. Sanchez Alvarado, A., and Newmark, P.A. (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. U.S.a. 96, 5049-5054. Scholz, H., Sadlowski, E., Klaes, A., and KlAmbt, C. (1997). Control of midline glia development in the embryonic DrosophilaCNS. Mech. Dev. 62, 79-91. Selmaj, K.W., Farooq, M., Norton, W.T., Raine, C.S., and Brosnan, C.F. (1990). Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J. Immunol. 144, 129-135. Serafini, T., Kennedy, T.E., Galko, M.J., Mirzayan, C., Jessell, T.M., and Tessier-Lavigne, M. (1994). The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78,409-424. Shafer, O.T., Chen, A., Kumar, S.M., Muller, K.J., and Sahley, C.L. (1998). Injury-induced expression of endothelial nitric oxide synthase by glial and microglial cells in the leech central nervous system within minutes after injury. Proc. Biol. Sci. 265, 2171-2175. Shen, S., Sandoval, J., Swiss, V.A., Li, J., Dupree, J., Franklin, R.J.M., and Casaccia-Bonnefil, P. (2008). Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11, 1024-1034. Shen, Y., Tenney, A.P., Busch, S.A., Horn, K.P., Cuascut, F.X., Liu, K., He, Z., Silver, J., and Flanagan, J.G. (2009). PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592-596. Shibata, T., Yamada, K., Watanabe, M., Ikenaka, K., Wada, K., Tanaka, K., and Inoue, Y. (1997). Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J. Neurosci. 17, 9212-9219. Silver, J., and Miller, J.H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146-156. Sim, F.J., Zhao, C., Penderis, J., and Franklin, R.J.M. (2002). The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. Journal of Neuroscience 22, 2451-2459. Sirko, S., Behrendt, G., Johansson, P.A., Tripathi, P., Costa, M., Bek, S., Heinrich, C., Tiedt, S., Colak, D., Dichgans, M., et al. (2013). Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell 12, 426-439. Sirko, S., Neitz, A., Mittmann, T., Horvat-Br6cker, A., Holst, von, A., Eysel, U.T., and Faissner, A. (2009). Focal laser-lesions activate an endogenous population of neural stem/progenitor cells 68 in the adult visual cortex. Brain 132, 2252-2264. Smith, K.M., Ohkubo, Y., Maragnoli, M.E., Rasin, M.-R., Schwartz, M.L., Sestan, N., and Vaccarino, F.M. (2006). Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat. Neurosci. 9, 787-797. Smith, P.J., Howes, E.A., and Treherne, J.E. (1987). Mechanisms of glial regeneration in an insect central nervous system. J. Exp. Biol. 132, 59-78. Smith, P.J., Howes, E.A., and Treherne, J.E. (1990). Cell proliferation in the repairing adult insect central nervous system: incorporation of the thymidine analogue 5-bromo-2-deoxyuridine in vivo. J. Cell. Sci. 95 (Pt 4), 599-604. Sofroniew, M.V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638-647. Sofroniew, M.V., and Vinters, H.V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119, 7-35. Song, H., Stevens, C.F., and Gage, F.H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39-44. Spassky, N., Merkle, F.T., Flames, N., Tramontin, A.D., Garcia-Verdugo, J.M., and AlvarezBuylla, A. (2005). Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. Journal of Neuroscience 25, 10-18. Stork, T., Engelen, D., Krudewig, A., Silies, M., Bainton, R.J., and Klambt, C. (2008). Organization and function of the blood-brain barrier in Drosophila.Journal of Neuroscience 28, 587-597. Sun, Y., Nadal-Vicens, M., Misono, S., Lin, M.Z., Zubiaga, A., Hua, X., Fan, G., and Greenberg, M.E. (2001). Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104, 365-376. Swiss, V.A., Nguyen, T., Dugas, J., Ibrahim, A., Barres, B., Androulakis, I.P., and Casaccia, P. (2011). Identification of a gene regulatory network necessary for the initiation of oligodendrocyte differentiation. PLoS ONE 6, e18088. Talbott, J.F., Loy, D.N., Liu, Y., Qiu, M.S., Bunge, M.B., Rao, M.S., and Whittemore, S.R. (2005). Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp. Neurol. 192, 11-24. Treherne, J.E., Harrison, J.B., Treherne, J.M., and Lane, N.J. (1984). Glial repair in an insect central nervous system: effects of surgical lesioning. J. Neurosci. 4, 2689-2697. Umesono, Y., Watanabe, K., and Agata, K. (1997). A planarian orthopedia homolog is 69 specifically expressed in the branch region of both the mature and regenerating brain. Dev. Growth Differ. 39, 723-727. Unhavaithaya, Y., and Orr-Weaver, T.L. (2012). Polyploidization of glia in neural development links tissue growth to blood-brain barrier integrity. Genes Dev. 26, 31-36. Vaccarino, F.M., Fagel, D.M., Ganat, Y., Maragnoli, M.E., Ment, L.R., Ohkubo, Y., Schwartz, M.L., Silbereis, J., and Smith, K.M. (2007). Astroglial cells in development, regeneration, and repair. Neuroscientist 13, 173-185. Voskuhl, R.R., Peterson, R.S., Song, B., Ao, Y., Morales, L.B.J., Tiwari-Woodruff, S., and Sofroniew, M.V. (2009). Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. Journal of Neuroscience 29, 11511-11522. Wadsworth, W.G., Bhatt, H., and Hedgecock, E.M. (1996). Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16, 35-46. Wake, H., Lee, P.R., and Fields, R.D. (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647-1651. Watanabe, M., Toyama, Y., and Nishiyama, A. (2002). Differentiation of proliferated NG2positive glial progenitor cells in a remyelinating lesion. J. Neurosci. Res. 69, 826-836. Watson, J.D., Wheeler, S.R., Stagg, S.B., and Crews, S.T. (2011). Drosophilahedgehog signaling and engrailed-runt mutual repression direct midline glia to alternative ensheathing and non-ensheathing fates. Development (Cambridge, England) 138, 1285-1295. Wegner, M. (2008). A matter of identity: transcriptional control in oligodendrocytes. J. Mol. Neurosci. 35, 3-12. Wenemoser, D., and Reddien, P.W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental Biology 344, 979-991. Wilhelmsson, U., Faiz, M., de Pablo, Y., Sjdqvist, M., Andersson, D., Widestrand, A., Potokar, M., Stenovec, M., Smith, P.L.P., Shinjyo, N., et al. (2012). Astrocytes negatively regulate neurogenesis through the JaggedI-mediated Notch pathway. Stem Cells 30, 2320-2329. Wu, M., Hernandez, M., Shen, S., Sabo, J.K., Kelkar, D., Wang, J., O'Leary, R., Phillips, G.R., Cate, H.S., and Casaccia, P. (2012). Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation. Journal of Neuroscience 32, 6651-6664. Xin, M., Yue, T., Ma, Z., Wu, F.-F., Gow, A., and Lu, Q.R. (2005). Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in OligI-null mice. Journal of Neuroscience 25, 1354-1365. 70 Yang, H., Ling, W., Vitale, A., Olivera, C., Min, Y., and You, S. (2011). ErbB2 activation contributes to de-differentiation of astrocytes into radial glial cells following induction of scratch-insulted astrocyte conditioned medium. Neurochemistry International 59, 1010-1018. Yazawa, S., Umesono, Y., Hayashi, T., Tarui, H., and Agata, K. (2009). Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proceedings of the National Academy of Sciences 106, 22329-22334. Yoon, K., Nery, S., Rutlin, M.L., Radtke, F., Fishell, G., and Gaiano, N. (2004). Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notchi signaling in telencephalic progenitors. Journal of Neuroscience 24, 9497-9506. Young, K.M., Psachoulia, K., Tripathi, R.B., Dunn, S.-J., Cossell, L., Attwell, D., Tohyama, K., and Richardson, W.D. (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873-885. Yu, Y., Chen, Y., Kim, B., Wang, H., Zhao, C., He, X., Liu, L., Liu, W., Wu, L.M.N., Mao, M., et al. (2013). Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248-261. Zamanian, J.L., Xu, L., Foo, L.C., Nouri, N., Zhou, L., Giffard, R.G., and Barres, B.A. (2012). Genomic analysis of reactive astrogliosis. Journal of Neuroscience 32, 6391-6410. Zhang, R.L., Zhang, Z.G., Zhang, L., and Chopp, M. (2001). Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105, 33-41. Zhou, Q., and Anderson, D.J. (2002). The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61-73. Zhu, X., Bergles, D.E., and Nishiyama, A. (2008). NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development (Cambridge, England) 135, 145-157. Zhu, X., Hill, R.A., Dietrich, D., Komitova, M., Suzuki, R., and Nishiyama, A. (2011). Agedependent fate and lineage restriction of single NG2 cells. Development (Cambridge, England) 138, 745-753. Zhu, X., Zuo, H., Maher, B.J., Serwanski, D.R., LoTurco, J.J., Lu, Q.R., and Nishiyama, A. (2012). Olig2-dependent developmental fate switch of NG2 cells. Development (Cambridge, England) 139, 2299-2307. Ziskin, J.L., Nishiyama, A., Rubio, M., Fukaya, M., and Bergles, D.E. (2007). Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 10, 321-330. Zou, J., Wang, Y.-X., Dou, F.-F., L, H.-Z., Ma, Z.-W., Lu, P.-H., and Xu, X.-M. (2010). Glutamine synthetase down-regulation reduces astrocyte protection against glutamate 71 excitotoxicity to neurons. Neurochemistry International 56, 577-5 84. 72 Chapter 2 Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration Daniel E. Wagner', Irving E. Wang', and Peter W. Reddien 'These authors contributed equally Experiments shown in Figures 1-3, Supplemental Figures S1-S16, and Supplemental Table 1 were performed by DEW. Experiments shown in Figures 4-6 and Supplemental Figures S17S19 were performed by IEW in collaboration with DEW. All authors contributed to the design of experiments and editing of the manuscript. Published as: Wagner, D.E., Wang, I.E., and Reddien, P.W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816 73 Abstract Pluripotent cells in the embryo can generate all cell types, but lineage-restricted cells are generally thought to replenish adult tissues. Planarians are flatworms and regenerate from tiny body fragments, a process requiring a population of proliferating cells (neoblasts). Whether regeneration is accomplished by pluripotent cells or by the collective activity of multiple lineagerestricted cell types is unknown. Using ionizing radiation and single-cell transplantation, we identified neoblasts that can form large descendant-cell colonies in vivo. These clonogenic neoblasts (cNeoblasts) produce cells that differentiate into neuronal, intestinal, and other known post-mitotic cell types and are distributed throughout the body. Single transplanted cNeoblasts restored regeneration in lethally irradiated hosts. We conclude that broadly distributed, adult pluripotent stem cells underlie the remarkable regenerative abilities of planarians. 74 Introduction Pluripotent cells, such as embryonic blastomeres, differentiate into mature cell types spanning three germ layers (Evans, 1972; Evans and Kaufman, 1981; Martin, 1981). Although essential for development, pluripotent cells are generally not known to be present in adult animals (Wagers and Weissman, 2004; Wagers et al., 2002). Adult tissues, by contrast, are typically maintained by specialized, tissue-specific adult stem cells (Barker et al., 2007; Blanpain et al., 2004; Ohlstein and Spradling, 2006; Spangrude et al., 1988; Uchida et al., 2000; Wagers and Weissman, 2004; Weissman, 2000). Planarians are flatworms well known for the ability to regenerate whole animals from small pieces of tissue (Morgan, 1898). Planarian regeneration requires a population of proliferative cells, historically known as neoblasts, that exist throughout the body and collectively produce all known differentiated cell types (Keller, 1894; Reddien and Sanchez Alvarado, 2004). Neoblasts have great potential for molecular genetic studies in Schmidtea mediterranea where a sequenced genome and molecular tools (including RNAi technology) enable identification and study of genes controlling regeneration(Reddien and Sanchez Alvarado, 2004; Reddien et al., 2005a). To date, however, neoblast properties have only been studied at the level of a population (BagufiA et al., 1989; Eisenhoffer et al., 2008; Lange and Gilbert, 1968; Newmark and Sanchez Alvarado, 2000; Reddien and Sanchez Alvarado, 2004; Salvetti et al., 2009; Wolff and Dubois, 1948). The cell population known as neoblasts, therefore, could either contain only lineage-restricted cells that together allow regeneration, or could contain, within the population, stem cells that are pluripotent at the single- 75 cell level. A fundamental issue to address for understanding planarian regeneration, therefore, is the in vivo potential of individual proliferating planarian cells. Results and Discussion Colonies are generatedfromsingle smedwi-J+ cellsfollowing irradiation We developed an in vivo method, utilizing ionizing radiation, that permits study of rare, individual proliferating cells and their descendants. Irradiation eliminates dividing cells and is a classic strategy for studying stem cells (Becker et al., 1963; Till and McCulloch, 1961). All dividing cells in adult planarians express the smedwi-J gene (Guo et al., 2006) (Fig. IA); these cells are specifically, rapidly, and completely depleted following exposure to high irradiation doses (e.g., 6,000 rads) (Eisenhoffer et al., 2008; Guo et al., 2006; Reddien et al., 2005b). Low irradiation doses (i.e., 500 rads) eliminate some proliferating cells, leaving a large number spread ventrally throughout the animal (Salvetti et al., 2009). We identified an irradiation dose (1,750 rads) that eliminated all smedwi-J+ cells from most (78%) animals (Fig. 1B). However, seven days after 1,750 rad exposure, rare smedwi-J+ cells were present in the minority of animals (22%) as sparse "clusters" (Fig. lB-C). Clusters consistently displayed compact, isolated, colony-like morphology and originated ventrally throughout the body (Fig. ID, S lA-C) but were not associated with specific known tissues (Fig. S1D-E). These clusters, if resulting from clonal growth of single smedwi-J+cells, provide the opportunity to study the developmental potential of individual planarian cells. 76 Numerous smedwi-J+ cluster attributes indicate they result from clonal growth. smedwi1J clusters were preceded by isolated smedwi-J+ cells present 3-4 days following irradiation, and typically displayed 3-10 cells after one week (Fig. 1E-F). Based on the low proportion of animals with smedwi-J+ cells in close proximity 3 days post-irradiation with 1,750 rads (Fig. S2), it is improbable that clusters arose from multiple adjacent smedwi-J' cells (P = 0.0138, 2-tailed Fisher's Exact Test). Cluster size increased dramatically over time, suggesting exponential growth, ultimately yielding hundreds of smedwi-J+ cells 14-18 days post-irradiation (Fig. 1E-F). Consistent with clusters originating from pre-existing smedwi-J+ cells that survived irradiation, a cluster location scatterplot resembled the normal smedwi-J]-expression pattern (Fig. ID), and cluster frequency decreased with increasing irradiation doses (see below, Fig. 3A). BrdU delivery labels smedwi-J-expressing cells (Guo et al., 2006) (Fig. S3A), followed by a rapid decline in incorporation within 24-48 hours (Eisenhoffer et al., 2008) (Fig. S3B-D) demonstrating unincorporated BrdU does not perdure long term. A BrdU pulse followed by irradiation resulted in clusters consisting entirely of BrdU+; smedwi-J1 cells (Fig. S3E), indicating smedwi-I+-clusterexpansion results from division of existing smedwi-J+ cells (i.e., by clonal growth). If some other process, such as dedifferentiation, produced smedwi-J+ cluster cells, these cells should have been BrdU~. Following irradiation, every proliferative cell detected by an 8-hour BrdU pulse (Fig. 1G), or by using probes for the conserved proliferation marker genes histone h2b (Smed-h2b) (Hewitson et al., 2006), pcna (Smed-pcna) (Bravo et al., 1987), or ribonucleotide reductase (Smed-RRM2-1) (Eisenhoffer et al., 2008; Eriksson et al., 1984) (Fig. S3F-H), existed in clusters and expressed smedwi-J (n=815). Therefore, no other source (non-smedwi-J*) for proliferating 77 cells exists outside of smedwi-J clusters in irradiated animals. Furthermore, if additional sources for smedwi-1] cells (other than clonal growth) existed, cluster number would be expected to increase with time and small, newly formed clusters might be present at late timepoints following irradiation. Neither of these possibilities was observed (Fig. IF). New cluster production was also not observed following amputation or feeding (Fig. S4), which elicit proliferative responses (BagufiA, 1976; Wenemoser and Reddien, 2010). These data indicate that clonal expansion (producing colonies) represents the source of new smedwi-J+ dividing cells during cluster formation and growth. Not all proliferating cells (neoblasts) necessarily have the capacity to form colonies. We term cells displaying this capacity clonogenic neoblasts (cNeoblasts); these cells express smedwi-1, have a body-wide (head-to-tail) distribution (Fig. ID), and generate large, expanding colonies of smedwi-J+ cells. The ability of small numbers of colonies ultimately to restore both smedwi-J+ cells and mitotic activity to normal levels (Fig. S5) suggests a stem cell-like capacity for self-renewal. To investigate the potential of individual cNeoblasts, we analyzed smedwi-J+ colonies using three well-established differentiation assays involving a SMEDWI-1 antibody (Guo et al., 2006; Scimone et al., 2010; Wenemoser and Reddien, 2010), BrdU pulse-chase (Eisenhoffer et al., 2008; Newmark and Sanchez Alvarado, 2000; Scimone et al., 2010), and post-mitotic cell type markers (Eisenhoffer et al., 2008; Pearson and Sanchez Alvarado, 2010; Scimone et al., 2010; Wenemoser and Reddien, 2010). SMEDWI-1 protein is present in smedwi-I mRNA+ dividing cells and temporarily remains detectable in post-mitotic descendant cells (Guo et al., 2006). Differentiating cells therefore transit through a SMEDWI-1(protein)+; smedwi-J(mRNA)- 78 state (Scimone et al., 2010; Wenemoser and Reddien, 2010). All colonies examined (12/12) contained SMEDWI-l+; smedwi-1- cells (Fig. 1H). Independently, BrdU can label cells that divide and exit the smedwi-J' state. All colonies analyzed by 4-day BrdU pulse-chase (31/31) contained BrdU+; smedwi-1- cells (Fig. 11) and no BrdU+ cells existed in worms lacking smedwi1J colonies (25/25 animals), indicating that colonies produce, and are the only source for, cells exiting the smedwi-J+ undifferentiated state. SMEDWI-l+ or BrdU+ colony cells can thus be assessed for lineage-specific marker expression to determine the developmental potential of individual cNeoblasts. cNeoblasts display broaddifferentiation capacity Described adult stem cells typically only produce differentiated cells corresponding to their germ layer and tissue of origin (Wagers and Weissman, 2004). To address whether cNeoblasts, by contrast, could produce cell types derived from multiple germ layers, we identified and characterized markers for neuronal (ectoderm-derived) and intestinal (endoderm-derived) lineages. In untreated animals, some SMEDWI-1+ descendant cells expressed a choline acetyltransferase ortholog, Smed-chat (Fig. S6); chat expression is widely conserved in cholinergic neurons (Nishimura et al., 2010). SMEDWI-1*; chati cells were enriched in brain regions, had neuronal morphology, and chate cells co-expressed additional neuronal markers (Fig. S6), indicating that SMEDWI- 1; chat7 cells are differentiating neurons. Smed-gata4/5/6 and Smedhnf4, orthologs of endoderm-promoting GATA4/5/6 and HNF4 transcription factors, respectively (Morrisey et al., 1998), were expressed in intestinal cells and also in interspersed cells surrounding the intestine (Fig. S7-10). Many of these interspersed cells were irradiation- 79 sensitive and SMEDWI-1*, indicating they represent differentiating endodermal cells (Fig. S8, S 10). A third endoderm marker gene used, Smed-mat, was expressed in intestinal branches (Fig. S8, S10). Finally, additional differentiation marker genes used (Smed-AGA T-1, NB.21.11E, Smed-MCP-1, Smed-ODC-1, Smed-CYPJAI-1, and NB.52.12F) are expressed in partially overlapping mesenchymal populations of post-mitotic cells (Fig. SIlA-F) (Eisenhoffer et al., 2008). These populations have unknown lineage relationships but turnover rapidly, and are consequently depleted following irradiation (Eisenhoffer et al., 2008) (Fig. S IIG-L). Using SMEDWI-1 to label colony cell descendants, individual colonies were examined for the presence of both gata4/5/6+ and chat*, both gata4/5/6' and AGAT-1], or both AGAT-1 and chati differentiating cells. In nearly all cases (n=20/22), individual colonies contained newly produced cells of both lineages tested (Fig. 2A-C, S12). The 1,750 rad dose yields rare, well-separated colonies (See Fig. 1C); animals fixed 7 days after irradiation contained single colonies (12/28 animals), no colonies (12/28), and only rarely, more than one colony (4/28). Given the high frequency of colonies producing multiple lineages (20/22), it is improbable that all such cases were the result of multiple colonies merging (P<0.0001, Fisher's Exact Test). In addition, using a 4-day BrdU pulse chase as an independent method, we identified several colonies containing both BrdU+; chat+ (neuronal) and BrdU+; mat+ (intestinal) cells (Fig. 2D). Nearly all colonies examined, using the SMEDWI-1 antibody or BrdU, produced differentiated cells for any single lineage analyzed (n=61/64) (Fig. S13). These colonies were distributed throughout the body and not restricted to specific anatomical regions (Fig. 2E). Finally, nearly every smedwi-J+ colony examined had associated cells expressing every additional differentiation marker tested (NB.21.1 lE, MCP-1, ODC-1, CYPJAJ-1, NB.52.12F) (n1 10/115, 80 Fig. S14A-E); descendant cell clusters, furthermore, were never observed in regions lacking smedwi-J+ colonies (Fig. S 11G-L). In addition, even early colonies (7 days post-irradiation) had associated differentiated cells (Fig. S14F). Together, these data indicate that broad multipotency and a body-wide distribution are fundamental attributes of individual cNeoblasts. Small numbers of cNeoblasts restore regenerativeability Irradiated planarians cannot regenerate (Bardeen and Baetjer, 1904) and suffer massive tissue loss because of failed replacement of aged differentiated cells (Bardeen and Baetjer, 1904; Dubois, 1949). However, transplantation of large numbers of cells (Bagufti et al., 1989) or tissue fragments can restore regenerative ability to irradiated hosts and change sexual behavior to that of a donor (BaguA et al., 1989; Lange and Gilbert, 1968; Lender and Garcia, 1965; Wolff and Dubois, 1948). We sought to determine whether small numbers of cNeoblasts would restore regenerative ability to irradiated animals. Following irradiation, some animals were fixed and colony numbers determined; remaining animals were followed to assess survival and regeneration frequencies. Increasing irradiation doses resulted in decreasing colony numbers (Fig. 3A-B) and survival rates (Fig. S15A). Regeneration, which involves production of diverse cell types (Fig. S16), was initially impeded in animals cut 4 days post-irradiation (Fig. S15B-C); however, many animals both survived and ultimately regenerated at doses that produced sparse, measurable colony numbers (Fig. 3A-D, Table S1). These animals regenerated heads containing neurons (ectoderm), muscle (mesoderm), and intestine (endoderm) (Fig. 3D-E). The minimum number of cNeoblasts initially present in irradiation survivors can be estimated by comparing the number of colonies present (in fixed animals) to observed regeneration 81 frequencies (see Table Sl). Our data indicate as few as three (P=0.0478), four (P=0.0017), or five colonies (P<0.0001, Fisher's Exact Test) can be sufficient to restore regenerative ability to entire animals. Transplantationof individualcNeoblasts To determine whether a single cNeoblast can generate all essential adult cells, we developed a method for isolating and transplanting individual cNeoblasts into lethally irradiated hosts. Previous flow cytometry studies identified an irradiation-sensitive cell population with a high percentage of smedwi-J+ cells (Hayashi et al., 2006; Reddien et al., 2005b). However, the Hoechst 33342 DNA dye used in this method is cytotoxic. Therefore, size and complexity properties of cells within the Xl fraction were used to define a gate for sorting unlabeled cells, which we refer to as the X1(FS) fraction (Fig. 4A-B). X1(FS) cells are heterogeneous; however, cells with a similar morphology to Xl cells can be identified microscopically (Fig. 4C). Single selected cells were loaded into needles and transplanted post-pharyngeally into lethally irradiated hosts (Fig. 4D). To confirm that only single cells were transplanted, needles were loaded and the contents expelled into media. In all cases, only a single cell was observed exiting the needle (n=136/136). Furthermore, some animals were fixed immediately following transplant and labeled with a smedwi-J RNA probe. All injected animals had either one (n=20/60) or zero (n=40/60) smedwi-J+cells (Fig. 4E-F). If a transplanted cell was a cNeoblast capable of engraftment, then clonal growth of progeny cells would be expected. Indeed, animals examined 6 days after single cell transplantation displayed clusters with 1 to 13 smedwi-J+ cells (n=23/100) (Fig. 4G). 82 Furthermore, selecting for XI(FS) cells that were approximately 10pm in diameter and that had blebs and/or cytoplasmic processes increased engraftment rates, ranging from 12% (n=17) to 75% (n=20) (Fig. S17). Cells with properties of cNeoblasts, therefore, are present in the Xl(FS) fraction and can be successfully transplanted. If cNeoblasts are pluripotent stem cells capable of self-renewal, then a single cNeoblast should, in principle, be capable of restoring tissue turnover and regenerative capacity to lethally irradiated hosts. However, for this to occur the irradiated host must survive long enough for the cNeoblast to repopulate the smedwi-1] population and replenish dying tissue. We therefore used a sexual S. mediterranea strain (S2F1L3F2) that can survive longer than the asexual strain (CIW4) after a 6,000 rad irradiation dose (Fig. S18). Sexual hosts transplanted with single asexual cells had colonies consisting of large numbers of SMEDWI-1+ cells 30 days after transplantation (n=4/17) (Fig. 4H). Every colony examined contained SMEDWI-1*; Smed- gata4/5/6 double-positive cells (n=4/4) and most of these colonies also contained SMEDWI- 1; Smed-chat' double-positive cells (n=3/4) (Fig. 4H). Transplant data thus independently confirm attributes of colonies described post 1,750 rads, further indicating that clonal growth and multipotency are important features of individual cNeoblasts. Entire animals and strains regeneratedfrom a single transplantedcNeoblast Two weeks after irradiation, lesions appeared at sexual animal head tips, followed by progressive anterior to posterior tissue regression with 100% penetrant animal death after approximately six weeks (n=78) (Fig. 5A). Remarkably, several transplant recipients lived past seven weeks and eventually developed blastemas at the site of tissue regression (n=7/130) (Fig. 5A). Animals that 83 developed blastemas regenerated anterior and midbody structures, such as photoreceptors and pharynges (Fig. 5A), and regained feeding behavior by eight weeks after irradiation. Of the seven rescued animals, three were expanded into strains (RI, R2, and R3) by serial amputation and regeneration (Fig. S19). These animals exhibited normal blastema formation and the capacity to regenerate photoreceptors and intestine following amputation (Fig. 5B). The ability to produce multiple regenerating animals from a single transplanted cell indicates a selfrenewing capability of cNeoblasts. Rescued strains did not display sexual features, such as large size and a gonopore; by contrast, animals in all three strains reproduced by binary fission, an asexual behavior seen very rarely in sexual animals (Fig. S 19). To confirm that all new tissue in rescued strains resulted from clonal division from the donor cNeoblast, we genotyped the animals using SNPs identified between the asexual strain and the sexual strain (see Materials and Methods). Genomic DNA was isolated from strain Ri, R2, and R3 animals following two rounds of regeneration; growth and regeneration should replace host tissues with donor-derived cells (Fig. 6A). If, on the other hand, host cells continue to replenish tissues after irradiation, host SNPs in the collected genomic DNA would be expected. PCR-RFLP analysis of two loci (RFLP 00310 and RFLP 00463) revealed that the rescued strains have the asexual strain RFLPs, indicating that the majority of cells in these animals were donorderived (Fig. 6B). Sequencing of three independent homozygous haplotypes (00163, 00463, and 02716), each containing six SNPs that distinguish asexual CIW4 and sexual S2F1L3F2 strains, confirmed that the rescue strains possessed the donor, rather than host, genotype (Fig. 6C). These data indicate that descendants of a single cNeoblast ultimately transformed the recipient into a genetic clone of the donor by replacing all cells present in the original host. We conclude 84 that cNeoblasts are pluripotent stem cells with a broad, body-wide distribution and that persistence into adulthood of a pluripotent stem cell enables the remarkable regenerative feats of planarians. 85 Figure 1 1I EV J1 aw JAI Rads B A D 1 00 93.7% (468/499) 80 "' 60 40 A 20 4 Ime chumbm 111Cm 12cjm 0 1 2 3 4 >5 # Clusters I Worm F - 4) 1000 size 6 4 100 2 Cr 4) 4 7 10 1I Frequency 0 0 5 10 15 20 Days after Irradiation 86 D Figure 1. Expanding colonies are generated from isolated smedwi-1+ cells following irradiation (A-B) Proliferating cells were detected by smedwi-J expression using whole-mount in situ hybridization (ISH). Anterior, up. Ventral surface shown. (B) Representative images 7 days after 1,750-rad treatment show clusters (arrowheads) of smedwi-J+ cells (individual purple dots). (C) Histogram of cluster frequencies following I 750 rads. (D) Clusters observed by smedwi-J ISH 7 days post-1,750 rads displayed in a scatterplot. phx, pharynx. (E-F) Animals fixed in a timecourse after 1,750-rad treatment analyzed by smedwi-) fluorescence in situ hybridization (FISH). (F) Mean cluster frequency (#clusters/worm) and size (#smedwi-1J cells/cluster) are plotted. Error bars, standard deviation (n=17-22 animals/timepoint). (G) IF (BrdU) and FISH (smedwi-1). 234/234 BrdU+ cells (8-hour BrdU-pulse in seven-day-irradiated worms) were smedwi-J+. (H) IF (SMEDWI-1) and FISH (smedwi-1); 12/12 colonies contained SMEDWI-1*; smedwi-1~ cells (arrowheads) 7 days post-1,750 rads. (I) IF (BrdU) and FISH (smedwi-1). 31/31 colonies (with BrdU pulse days 7-11 post-1,750 rads) contained BrdU+; smedwi-~ cells. Scale bars, 200pm (A-B), 20pm (E, G-I). 87 Figure 2 E Colony D ALLocations t- - 88 Figure 2. Clonogenic neoblasts display broad differentiation capacity (A-C) Triple-labeling of individual colonies 22 days after irradiation. Shown are projections through optical sections from irradiated animals. Left, tiled images (images from overlapping regions assembled) of representative animals with individual colonies are shown (anterior, up). Circles indicate approximate location of region imaged at high magnification for middle panels; middle images are optical sections with anterior to the right. Example differentiating cells from individual colonies labeled by IF (SMEDWI-1) and double FISH for gata4/5/6 and chat (A), gata4/S/6 and AGAT-1 (B), or AGAT-1 and chat (C) are shown. Proportions of colonies displaying multiple differentiating cell types are indicated. Roman numerals indicate doublepositive cells, with individual channels shown in columns to the right. Additional double-positive cells are indicated with arrowheads. See also Figure S12. (D) IF (BrdU) and double FISH (Smedchat; Smed-mat) worms with BrdU-pulse days 14-18 after irradiation. Single colonies (n=7) contained both BrdU+; chat- (neuronal) and BrdU+; mat* (intestinal) descendants. Boxes indicate zoomed regions. (E) Scatterplots showing locations of individual colonies producing differentiated cell types (see also Figure S 13). Colony cell differentiation was assessed by labeling with SMEDWI-1 (circles) or BrdU (diamonds). Scale bars, (A-C) left, 100im; middle 20ptm; right 5p[m; (D) top image, 20tm; others 5Rm. 89 Figure 3 smedwi*1 C B - .4 100 . >121 . A ---- so C with 26 colonies moo animis 10, 0w E with a3 colonies LM 60- +4.-4---.--84 ------ *4 * 40 - -1,260 Rads --------... % animals 1,500 Reds 0 C, Ana 40II 44 -4--i---00 ** .4 + - 0 -......-- C 6, 4, ++ 4W 4 2, 6,000 Rads 01 --- --- - - - 0 t-- ---- - 1,750 Rads 2-- - 20 Irradiation Dose (Rads) Irradiation Dose (Rads) 90 Figure 3. Small numbers of cNeoblasts can restore tissue turnover and regenerative ability (A-B) Animals were irradiated at different doses. Some of these animals were fixed 7 days postirradiation (1,000 rads, 25 animals; between 1,125 and 1,875 rads, >38 animals/dose; for 6,000 rads, 26 animals) and labeled by smedwi-J+ ISH. (A) Representative smedwi-1] ISH images. Anterior, left. (B) Colony numbers/worm are plotted (each dot represents one animal). (C) The percentage of animals with restored regeneration following irradiation (>98 worms/dose were examined; animals were from the same irradiated cohort as in A-B). Data indicate 3 or more cNeoblasts were sufficient to restore regeneration (see also Table S1). (D) Normal head regeneration in 97/99 worms amputated 39 or 40 days after 1,250 rads. (E) Heads regenerated after irradiation contained differentiated neuronal (chat), intestinal (mat-]+), and muscle (mhc1) cells (41/41 worms, 1,250 rads; 15/15 worms, 1,500 rads). SMEDWI-1* cells were also restored (n=9/9 worms, 1,250 rads). Dotted lines, approximate amputation plane. Arrowheads, photoreceptors. Scale bars, 200pm (A), 20Rm (D-E). 91 Figure 4 BIE i Hoechst Red G | Day 9 0% -F FS a er 6,000 23% WSo 231100 1 UnInjected Il Injecled 92 p Figure 4. Single transplanted cNeoblasts display properties of clonal growth and multipotency Irradiation-sensitive cells (polygonal gate) were identified by Hoechst 333342 labeling (A) and back-gated to set the X1(FS) gate (oval) based on size (FS) and complexity (SS) parameters (B). The X I(FS) fraction is heterogeneous and contains some cells approximately 1 Otm in diameter with processes (arrowhead) (C). (D) Individual cells were loaded into needles (one needle used per injection) and transplanted into the medial, post-pharyngeal, parenchymal space of hosts. (EF) FISH (smedwi-1) of a host immediately after transplantation. Anterior, up. Ventral surface shown. Zero (n=40/60) or one (n=20/60) smedwi-1] cells were observed in all cases, with expected size and morphology. (F) is a zoomed image of(E). (G) Colony formation 9 days after irradiation, 6 days after transplant. Anterior, up. Ventral surface shown. Colonies of smedwi-J+ cells (arrowhead) appeared in transplant recipients (n=23/100) but not in untreated animals (n=5). (H) IF (SMEDWI-1) and double FISH (Smed-gata4/5/6; Smed-chat) 33 days after irradiation, 30 days after transplant. Single colonies were observed (n=4/17); example differentiating cells from displayed colony are shown. Scale bars, 10pim (C), 50tm (E), 5pm (F) and zoomed images in (H), 20ptm (H), 200tim (G). 93 FIgure 5 A DaV 15 < Day 54 Dy3 Dy3 ppx B ay 0 Day 8 Dy1+ px I.. 94 Dy 6+ Figure 5. Restoration of regeneration in lethally irradiated hosts by single transplanted cNeoblasts (A) Representative images of transplant hosts. Tissue regression (asterisks) began anterior to photoreceptors (arrowheads) and progressed from anterior to posterior (px, pharynges). Rescued animals developed blastemas (arrow) at regression site (dotted line) after seven weeks and fully regenerated after eight weeks. Anterior, up. Dorsal surface shown. (B) Representative images of rescue strains undergoing regeneration following amputation. Blastemas formed at approximate amputation plane (dotted line). Intestine (labeled with red food coloring) and photoreceptors (arrowheads) were observed in blastemas after 12 days of regeneration. Anterior, up. Dorsal surface shown. Scale bars, 1mm (A), 50O0m (B). 95 Figure 6 A Number of Reads C Asexual HaDlotvye I Sexual Haplotvye Asexual (Donor) Sexual (Host) Rescue Strain 1 94 Rescue Strain 2 94 Co Rescue Strain 3 Locus 0E00163 0 00463 96 Figure 6. Genotype conversion by single transplanted cNeoblasts (A) Schematic showing replacement of host tissue by transplanted donor cells (blue); animals for genotyping were amputated (dotted line) and allowed to regenerate twice. (B) PCR-RFLP analysis of rescued strains. Locus 00310 was cut by Hpal in sexual animals (S) but not asexual animals (A) or the rescued strains (1, 2, 3). Locus 00463 was cut by Sca in asexual animals and the rescued strains, but not in sexual animals. (C) Haplotype sequencing. Stacked histogram representing number of sequencing reads from each locus for each strain. Bars extend left for number of reads corresponding to the asexual haplotype and right for number of reads corresponding to the sexual haplotype. Bar absence indicates no reads. 97 Figure SI C B 0 | Sing le Clusters, 7 da s after 1,750 Rads 0 Untreated 98 Figure S1. smedwi-1+ clusters originate in ventral positions throughout the body (A-B) Transverse tissue sections shown, dorsal up. Control worms display smedwi-)+ cells distributed in parenchyma throughout the dorsal-ventral axis (A). Representative image of a smedwi-J+ cluster (arrowhead) seven days post-1,750 rad treatment (B). (C) Representative images of animals displaying single smedwi-1+ clusters seven days post-1,750 rad treatment. Whole animals, anterior up. Individual clusters were scattered throughout body, but consistently displayed compact, isolated, colony-like morphology. (D-E) Triple FISH for synapsin (central nervous system), mat (intestine), and smedwi-) seven days after 1,750 rads. Nuclei are labeled with Hoechst. Shown are projections through Apotome optical sections. Individual smedwi-J' clusters were distributed throughout the ventral regions and were not invariantly associated with specific organs or anatomical features. Some clusters had cells adjacent (within -1 cell diameter) to the ventral nerve cords (28/44); some clusters were located large distances from the nerve cords (16/44). Scale bars 20mm (A-B, D-E), 200mm (C). 99 Figure S2 100 Figure S2. Following irradiation, clusters are preceded by isolated smedwi-1] cells (A-E) Representative images of smedwi-J (FISH) after 1,750 rad exposure. Whole animal, anterior up. Magnified regions are indicated by boxes. Arrowheads denote individual smedwi-J' cells. Three days after irradiation, the majority of animals (16/20) displayed isolated smedwi-J+ cells (A). Animals with >1 smedwi-J+ cell in close proximity (within 50mm) were rare (2/20) (B). Remaining animals at this condition (2/20) displayed no smedwi-JI cells (C). Animals fixed 8 days post-irradiation displayed either small smedwi-J+ clusters (9/19) (D) or were devoid of smedwi-J+ cells (E). Based on these proportions, the possibility that all 8-day clusters arose from multiple cells can be excluded (P = 0.0138, 2-tailed Fisher's Exact Test). Scale bars 200mm (ae), 20mm (zoomed boxes). 101 Figure S3 cesny, S pmSt. 11 C.Ifly ,w poa W D * sne*4-1 Ssmdwf-1* ; BrdU+ ~75 * BrdU* 25 60 0 Hour pos4kdU C 12hr befor Irradiation ; BrdU C - 7 atr1,70 Radb 102 Figure S3. BrdU incorporation and proliferation of smedwi-1] colony cells (A) Immunofluorescence (BrdU) and FISH (smedwi-J). All dividing cells (571/571) detected in unirradiated, intact worms by a 12-hour BrdU pulse expressed smedwi-J+ (see also, Guo et al, 2006). Thus, smedwi-J+ cells are the sole source for BrdU-labeled cells in these animals. (B-D) BrdU incorporation and dilution kinetics in expanding clusters of smedwi-J+ cells. Animals were injected with BrdU 7 days after 1,750 rads and fixed at various timepoints. Representative immunofluorescence (BrdU) and FISH (smedwi-1) images are shown (B-C). Percentage of smedwi-1, BrdU+, and smedwi-1J; BrdU+ (double-positive) cells are indicated for each timepoint (n> 154 cells analyzed/timepoint) (D). BrdU is rapidly incorporated into a majority of smedwi-J+ cells within the first 8 hours following labeling. Following a brief 48-hour chase period, the first signs of BrdU dilution from the continuously dividing smedwi-1 population are evident. At 24 hours post-BrdU, 123/133 (92.5%) smedwi-1 cells were BrdU+, compared with 91/119 (76.5%) at 48 hours post injection. Fisher's Exact Test indicates that this difference is significant (P=0.0006, 2-tailed). Unincorporated BrdU, therefore, is unlikely to remain within injected animals for longer than 48 hours post-injection (see also, Eisenhoffer et al, 2008). (E) Animals were irradiated (1,750 rads) 12 hours after BrdU injection and fixed 5-6 days later. Shown is a representative cluster following IF detection of BrdU+ cells and FISH (smedwi-1). Several colonies of 3-12 smedwi-J+ cells were identified (n=9), and nearly all of these cells (47/48) contained BrdU+ nuclei (BrdU signal is anticipated to be absent from some cells after many cell divisions). (F-H) Double FISH seven days after 1,750-rad irradiation. All proliferating cells expressing Smed-h2b (F, 304/304), Smed-RRM2 (G, 111/111), or Smed-pcna (H, 166/166) were smedwi-] +-colony cells. Scale bars 200mm (A-E), 20mm (zoomed images, F-H). 103 Figure S4 Irradiation Only Amputation after Irradiation C , A 25/54 Colonies I 29/54 D 31/55 24/55 Colonies No Colonies I I | No Colonies Irradiation Only I 104 I Figure S4. Amputation and feeding fail to stimulate new colony formation following irradiation (A-D) Animals were exposed to 1,750 rads and fixed 12 days later for smedwi-1 ISH. Shown are whole animals (anterior up) amputated into fragments five days after irradiation (A-B), or left intact (C-D). Dotted lines indicate amputation plane. Amputated fragments from individual worms were cultured, fixed, and stained independently. The proportion of amputated worms displaying colonies (25/54) was not significantly altered from that of intact worms (24/55), indicating that amputation did not stimulate formation of additional colonies (P=0.8483, Fisher's Exact Test, 2-tailed). (E-H) Animals were exposed to 1,750 rads and fixed on day 12 for smedwi-] FISH. Animals were either fed four days after irradiation (E-F), or left untreated (GH). The proportion of fed worms displaying colonies (18/49) was not significantly different from that of control worms (14/54), indicating that feeding failed to stimulate formation of additional colonies (P=0.2885, Fisher's Exact Test, 2-tailed). Scale bars, 200 mm. 105 Figure S5 Irradiated 1500 Rads 250- ........ ft ..... mm :1:0-0-0-oooel m -m .......... .......... 200 ..... .... ft E - G ...ft..mmm ...... 150 cjn 0 100 50 C 5 15 iO Days After 1,500 Rads El 7 10 |[ 106 Figure S5. smedwi-1+ cells and mitotic activity are restored to normal levels following irradiation with 1,500 rads (A-F) Whole-mount smedwi-J ISH (A-C) and IF detection of Histone-H3 (phospho-serine-10)+ mitotic cells (D-F). Representative images demonstrate that the number and body-wide distribution of both smedwi-1 cells and mitotic activity are gradually restored following irradiation at 1,500 rads. (G) A timecourse illustrates that mitotic numbers (normalized by worm area) are almost completely depleted 7 days after irradiation. Successive timepoints display increased levels of mitotic activity. Furthermore, the increase in mitotic activity slowed between days 17 and 21, suggesting that colony expansion is a regulated (rather than neoplastic) process. Shown are means and standard deviations for each data point. Mean and standard deviation mitotic levels for untreated worms (184.6 22.4 mitoses / mm2 tissue) are represented by solid and dotted lines, respectively. Scale bars, 200mm. 107 Figure S6 108 Figure S6. A subset of SMEDWI-1 cells undergo neuronal differentiation Double-labeling analysis of cephalic ganglia in intact, unirradiated planarians. Anterior, up. (A) IF (SMEDWI-1) and FISH (Smed-chat) identifies SMEDWI-1I cells co-expressing Smed-chat, a marker of differentiated neurons (double-positive cells are indicated by arrowheads). Zoomed regions are indicated by boxes. These cells were enriched in brain regions and often adopted a non-neoblast cell morphology that included long axon-like cytoplasmic processes, suggesting differentiation into neurons. (B-C) Double FISH in intact, unirradiated planarians indicates that Smed-chat+ cells exist in the planarian CNS and co-express Smed-synapsin (b) and Smedproprotein- convertase-2 (Smed-PC2) (C), two independent markers of the planarian nervous system. (D) A representative FISH image shows that Smed-chat mRNA is localized within or in close proximity to the nucleus. Scale bars, 20mm (A-C), 5mm (zoomed images, D). 109 Figure S7 MmGata3 HsGata3 DrGata3 821802 HsGata2 MmGata2 DrGata2 TcGatu123 GATA 112/3 AgGata 23 AmGatal23 DpGata123 73 1 735 DmGraln 871 939 NvGata HsGatal 92/941 MmGatal DrGatal 601761 MmGata4 HsGata4 921 930 DrGata6 MmGata6 57/330 HsGataG MmGata6 HsGata5 DrGata5 AmGata46Sbba GA l A 415/6 AgGata456bba TcGata466bba 87/845 DmPannler 521/ 504 - 5DpGata46bb DpGatata456ba AgG456ba TcGata456bn ~ ~AmGata466ba $71939 mrpn DpGaui456a TcGata456a 41/556 80 L-=64' 694 Ag Smed-Gata4/5/6 i 801 DmGatae AmGata456a 110 Figure S7. Phylogenetic analysis of the Smed-gata4/5/6 gene Maximum likelihood and neighbor-joining analysis provide strong support for the Schmidtea mediterraneagene Smed-gata4/S/6 falling within the GATA4/5/6 clade with known protostome members of this family. Genes used in this analysis are well-established representatives of the GATAl/2/3 or GATA4/5/6 gene families (Gillis et al., 2008). Accession numbers for genes listed in this tree can be found in ref (Gillis et al., 2008). Mm, Mus musculus; Hs, Homo sapiens; Dr, Danio rerio; Tc, Tribolium castaneum; Ag, Anopheles gambiae; Am, Apis mellhfera; Dp, Daphnia pulex; Dm, Drosophila melanogaster; Nv, Nematostella vectensis; Smed, Schmidtea mediterranea. 111 Figure S8 112 Figure S8. A subset of SMEDWI-1* cells express Smed-gata4/5/6 Double-labeling analysis of posterior intestinal branches in untreated or lethally- irradiated planarians. (A-B) Double FISH on untreated (A) or 5 day, lethally irradiated (B) planarians indicate that Smed-gata4/S/6 is expressed in fully differentiated Smed- mat+ cells of the planarian intestine. Smed-gata4/S/6 is also expressed in isolated, irradiation-sensitive cells associated with the intestine (arrowheads). (C) Many isolated Smed-gata-4/5/6+ cells were co-labeled by SMEDWI-1 (IF). (D) A representative FISH image shows subcellular localization of Smedgata4/S/6 mRNA. Scale bars, 50mm (A- C), 5mm (zoomed images, D). 113 Figure S9 C.NHR2 5 I NR5 NR3 100 100 DERR ER HSERRa 02DmDHR4 99/ NR6 7CNHR91 7 NvNR1 811433 DmDHR38 681 984 HsNGFIB 981976 CNHR6 78) 435 I NR4 HsRORa 100 11001 CoNHR23 S100 / 1000 * DmDHR3 DmUSP - =99/ 1000 I HsRXRa NR2-B DmDHR78 CNHR41 0 1971814 HsTR2 811433 831659 66 NR2-E DmDSF 10 691I963 -- 59 NvNR10 100 11000 HsCOUPTFa 941I998 DmSVP NR2-F CoNHR49 Smed-HNF4 641N923 81 DmHNF4NRNvNR4 sNF4I 114 NR2-C I NRI Figure S9. Phylogenetic analysis of the Smed-hnf4 gene Maximum likelihood and neighbor-joining analysis provide strong support for the Schmidtea mediterraneagene Smed-hnf4 falling within the NR2A/HNF4 family of nuclear receptors. Genes used in this analysis are well-established representatives of the six families of nuclear receptor genes NRI-6. Sequences for Nematostella genes used in this trte can be found in ref (Reitzel and Tarrant, 2009). Hs, Homo sapiens; Dim, Drosophilamelanogaster;Nv, Nematostella vectensis; Ce, Caenorhabditiselegans; Smed, Schmidtea mediterranea. 115 Figure SIO 116 Figure S10. A subset of SMEDWI-1+ cells express Smed-hnf4 Double-labeling analysis of posterior intestinal branches in untreated or lethally irradiated planarians. (A-B) Double FISH on untreated (A) or 5 day, lethally irradiated (B) planarians indicate that similarly to Smed-gata4/5/6, Smed-hnf4 is expressed in filly differentiated Smedmat' cells of the planarian intestine. Smed-hnf4 is also expressed in isolated, irradiation-sensitive cells associated with the intestine (arrowheads). (C) Many isolated Smed-hnf4+ cells were colabeled by SMEDWI-l (IF). (D) A representative FISH image shows subcellular localization of Smed-hnf4 mRNA. Scale bars, 50mm (A-C), 5mm (zoomed images, D). 117 Figure S11 118 Figure S11. Characterization of post-mitotic cell populations (A-F) Double FISH analysis of post-mitotic cell types in unirradiated, intact planarians (see also, Eisenhoffer et. al., 2008). Shown are ventral, prepharyngeal regions of the animal. A previously reported panel of marker genes labels multiple populations of cells. "Category 3" gene SmedAGAT-1 only partially overlapped in expression with other category 3 genes Smed-MCP-1 (A), Smed-ODC-1 (B), Smed-CYPJAJ-J (C), and NB.52.12F (D). However, cells expressing SmedRas-related, another category 3 gene, showed extensive overlap with the Smed-AGAT-J+ population (E). Similarly, "category 2" markers NB.21.11 E and NB.32.1G were expressed by the same population of cells (F). A revised panel of six markers NB.21.11E, Smed-AGAT-1, Smed-MCP-1, Smed-ODC-1, Smed-CYPJA1-1, and NB.52.12F therefore encompasses a heterogeneous set of cell populations with minimal redundancy. (G-L) FISH showing presence of NB.21.1 lE, Smed-AGAT-1, Smed-MCP-1, Smed-ODC-1, Smed-CYPJAJ-1, and NB.52.12Fexpressing cells. Shown are ventral, anterior regions from untreated or day 19-irradiated worms lacking smedwi-]+ colonies. All six cell types were depleted after 1,750 rads. 119 Figure S12 120 Figure S12. Clonogenic neoblasts display broad differentiation capacity Triple labeling of individual colonies 22 days after irradiation. Shown are additional cells from the same colonies depicted in Fig. 2. Each row of panels shows individual channel images for a single cell. (A-B) Additional examples of colony cells positive for chat (A) and gata4/S/6 (B) expression from the same colony shown in Fig. 2A. (C-D) Additional examples of colony cells positive for AGAT-J (C) and gata4/S/6 (D) expression from the same colony shown in Fig. 2B. (E-F) Additional examples of colony cells positive for chat (E) and AGA T-J (F) expression from the same colony shown in Fig. 2C. Scale bars, 5pm. 121 Figure S13 122 Figure S13. A high percentage of cNeoblast colonies produce differentiated cell types Double-labeling analysis of individual colonies 15-20 days after irradiation. Shown are representative optical sections from irradiated animals. Boxes indicate zoomed regions within the same colony that contain double-positive cells. Locations of all colonies in these analyses are shown in a scatterplot in Figure 2E. (A-B) Two representative colonies labeled by IF (SMEDWI1) and FISH (Smed-chat) from worms 15-19 days after 1,750-1,800 rads. 29/30 such colonies contained differentiating neurons (SMEDWI+; Smed-chat+ double-positive cells). (C) Representative colony labeled by IF (BrdU) and double FISH (Smed-chat; smedwi-1) from 1,750 rad-treated animals. Worms with growing colonies were injected with a pulse of BrdU 14 days after irradiation and examined 4 or 5 days later to detect differentiating cells. Every such colony examined (n=8/8) contained Smed-chat*; BrdU+ double-positive cells. (D-E) Two representative colonies labeled by IF (SMEDWI-1) and FISH (Smed-gata4/5/6) from worms 19 days after 1,750 rads. 14/15 such colonies contained differentiating intestinal cells SMEDWI-1*; Smedgata4/5/6+ cells. (F-G) Two representative colonies labeled by IF (SMEDWI-1) and FISH (Smed-hnf4) from worms 19 days after 1,750 rad exposure. 10/11 such colonies contained SMEDWI-1; Smed-hnf4+ cells. Scale bars, 20mm (5mm, zoomed images). 123 Figure S14 D 1750 E +7 124 Figure S14. Nearly all colonies locally produce post-mitotic cell types (A-E) smedwi-J' colonies 20 days after 1,750 rads contained post-mitotic cells expressing NB.21.l lE (A, 16/16 colonies), NB.52.12F (B, 13/15 colonies), CYPA1-1 (C, 15/17), MCP-1 (D, 20/20 colonies), and ODC-1 (E, 20/20 colonies). (F) Representative colony labeled by double FISH (smedwi-1 and NB.21.1 lE) from worms 7 days after exposure to 1,750 rads. Nearly all individual colonies analyzed (14/15) examined differentiating cells. Scale bars, 20mm. 125 at this early timepoint displayed Figure S15 A 10 ----------- -- --- 0 --..... 40 ------------------ - - -- 20 ------------------ - ---- - - ----....... ---...... ......... -. -......-----.. 0 ---.................... 0 0 10 20 30 40 50 60 Days After Irradiation -1,000 -1,125 -1,250 -1,375 rads rads rads rads 1,500 -1,750 -1,875 -6,000 rads rads rads rads 126 Figure S15. Effects of irradiation on planarian survival and regeneration (A) Survival curves of irradiated worms irradiated at various doses of irradiation. Animals are from the same experiment shown in Figure 3. Viability decreased sharply above 1,500 rads (n>98 worms/sample). (B-C) Head regeneration was initially impeded by even low doses of irradiation. Shown are head regions from worms 8 days post- amputation. (B) Control worm. (C) Worm amputated 4 days after exposure to 1,000 rads (49/49 animals). Similar results were obtained after 1,250 rads (50/50 worms), or 1,500 rads (50/50 worms). Scale bars, 20mm. 127 Figure S16 128 Figure S16. Diverse types of differentiating cells are produced during head regeneration Planarians were injected with BrdU 18 hours prior to decapitation. IF (BrdU) together with FISH shows 5-day-regenerating heads containing newly formed (BrdU+) differentiated cells. These cells include (A) excretory, (B) muscle, (C) intestinal, and (D) neuronal lineages as determined by expression of Smed-carbonic-anhydrase (Smed-CA), Smed-myosin-heavy-chain-J (Smedmhc-1), Smed-methionine-adenosyltransferase(Smed-mat), and Smed-choline-acetyl-transferase (Smed-chat), respectively. Scale bars, 100mm. Anterior, up. Dotted line, approximate amputation plane. 129 Figure S17 i I 10% 2120 130 Figure S17. Morphological characteristics and transplant frequencies of different X1(FS) cells (A-E) Representative images of X1(FS) cells. Nuclei were labeled with Hoechst 33342. (F-J) Representative images of animals 9 days after irradiation, 6 days after transplant. Anterior, up. Ventral surface shown. Transplantation of cells 10-14mm in diameter with low cytoplasmic granularity (A) resulted in few smedwi-]+ clusters (n=2/20) (F). Transplantation of cells 8-10mm in diameter with low cytoplasmic granularity (B) resulted in zero smedwi-J+ clusters (n=0/20) (G) Transplantation of cells 10-12mm in diameter with low cytoplasmic granularity and processes (C) resulted in many formed smedwi-1* clusters (n=15/20) (H). Transplantation of cells 10-14mm in diameter with high cytoplasmic granularity (D) resulted in few formed smedwi-J+ clusters (n=5/20) (I). Transplantation of cells 8-10mm in diameter with high cytoplasmic granularity (E) resulted in few formed smedwi-J+ clusters (n=1/20) (J). Scale bars, 10mm (A-E). 131 Figure S18 120- . 100- E 0 M Asexual Strain Sexual Strain 6040- 20 0 10 20 30 40 Days Post Irradiation 132 50 60 70 Figure S18. Survival of asexual and sexual strain animals following lethal irradiation Exact survival times vary between experiments. In this particular experiment, asexual CIW4 animals exposed to a 6,000 rad dose of radiation had a median survival period of 17 days and a longest survival period of 21 days (n=105). Sexual S2F1L3F2 strain animals exposed to identical conditions within the same experiment had a median survival period of 39 days and a longest survival period of 63 days (n=97). 133 Figure S19 Irradiation Transplantation Day 50 Day 100 Day 200 Day 150 I _ _ _ _ Rescue Strain 1 Rescue Strain 2 a 0 Rescue Strain 3 T--O -4 Survivor 4 m - Survivor 7 - Survivor 5 Survivor 6 134 1111 Figure S19. Timeline of rescued transplant hosts Trees detailing survival of rescued animals, with branches indicating expansion of a single animal into multiple individuals by amputation or fissioning. Red end points indicate natural death. White end points indicate sacrifice of the individual for experimental purposes. Green intersections indicate amputations to expand the population. Blue intersections indicate fissioning events. Black arrowheads indicate which individuals are still alive at the time of writing. 135 100 100 1 (1) 0 1 (1) 0 40 26 2(5) 0 1(3) 0 0 0 0 0 0 0 1875 6000 99 3(3) 4(4) 38 9(24) 5(13) 3(8) 2(5) 0.0017 0.0032 colonies 5 or more 0.0478 4 or more colonies and restore regenerative ability. as few as three (P=0.0478), four (P=0.001 7), or five colonies (P<0.0001) colonies are sufficient to rescue entire animals frequencies of smedwi-1 colonies observed from in situ data, a set of predictions can be generated based on a hypothetical minimum number of colonies required for restoring regeneration. Fisher's Exact test (2-tailed, a = 0.05) facilitates a direct comparison between the number of colonies present and number of worms regenerated. P-values for tests in which significantly more animals regenerated than predicted are shown in bold. Together these data indicate that and regenerated are shown. Nearly all worms that survived also displayed normal head regeneration. Given the Planarians were exposed to a range of irradiation doses. A portion of these animals were fixed 7 days later and colonies visualized by smedwi-1 ISH. The number and percentage (in parentheses) of animals displaying various numbers of colonies are shown. N indicates the total number of animals analyzed. Remaining animals were followed for several weeks and decapitated 39-40 days after irradiation. Number and percentage of total worms that survived or both survived 1750 1(3) 28 (28) 30 (30) 57 24 (42) 16 (28) 2 (4) 1500 11(19) 100 4(7) 0.0302 <0.0001 100 80(80) 82(82) 51 47 (92) 43 (84) 34 (67) 32 (63) 1375 39 (76) 0.0002 99 97(98) 98(99) 52 52 (100) 47 (90) 44(85) 41(79) 1250 52 (100) 100 98 25 100(100) 47 98(100) 25(100) 47(100) 25(100) 47(100) 25(100) 47(100) 25(100) 46(98) 25(100) 46(98) 1000 1125 Survived N 3 or more colonies 4 or more colonies 5 or more colonies 6 or more colonies Dose (Rads) 2 or more colonies 100(100) 98(100) 6 or more colonies Fisher's Exact Test (2-tailed) N # (%) worms recovered (d54-55) Survived & Regenerated # (%) worms with smedwi-1* colonies (d7) Table S1. A small number of smedwl-r* colonies can rescue entire animals from irradiation and restore regenerative ability Materials and Methods PlanariaCulture and Irradiation Schmidtea mediterraneaasexual strain C1W4 was maintained as described (SAnchez Alvarado et al., 2002). Animals were starved in the presence of Gentamicin (Gibco) for ten days prior to irradiation experiments. Individual irradiation experiments used size-matched animals with identical feeding and culturing histories. Irradiation was delivered to animals at 79-82 rads/min using dual Gammacell-40 '"Cesium sources (i.e., sources positioned both above and below the specimen). For survival experiments, irradiated animals were maintained in 6 cm Petri dishes (10 worms/dish) in the dark; water and dishes were changed every 3-4 days. In situ Hybridizationand Tissue Sectioning Whole-mount in situ hybridizations (ISH) and fluorescent in situ hybridizations (FISH) were performed and RNA probes prepared as described (Pearson et al., 2009). Tyramide-conjugated fluorophores were generated from AMCA, Fluorescein, Rhodamine (Pierce), and Cy5 (GE Healthcare) NHS esters as previously reported (Hopman et al., 1998). For double/triple labeling, HRP- inactivation was performed between labelings in 4% formaldehyde, 45 min. Tissue sectioning was performed as previously described (Reddien et al., 2005b). BrdUlabeling and Immunofluorescence Animals were fed or injected with 5 mg/mL BrdU (Fluka) as previously described (Newmark and Sanchez Alvarado, 2000). Specimens were fixed in 4% formaldehyde as described (Pearson 137 et al., 2009) and antibody labelings performed as reported (Reddien et al., 2005b) using rat antiBrdU (1:100, Oxford Biotech), rabbit anti-H3P (1:100, Millipore), or rabbit anti-SMEDWI-1 (Guo et al., 2006) (1:2000). Microscopy Microscopy images were captured with an AxioCam HRm on a Zeiss Stereo Lumar V12 or an Axio Imager ZI using Zeiss Axiovision software. Double-positive cells were scored in optical sections obtained with an Apotome (Zeiss). Additional images were collected on a Zeiss LSM 700 confocal microscope using Zen software. PhylogeneticAnalysis Putative members of gata4/S/6 and hnf4 gene families were identified in the Schmidtea mediterranea genome. Peptide sequences were aligned with well-known members of these families using ClustalW with default settings (Thompson et al., 1994). Alignments were trimmed using GBlocks (Castresana, 2000). Neighbor-joining trees were generated using ClustalW using default settings and 1,000 bootstrap replicates. Maximum likelihood analyses using 100 bootstrap replicates were run on each alignment using PhyML (Guindon and Gascuel, 2003) with WAG model of amino acid substitution, four substitution rate categories, and the proportion of invariable sites estimated from the dataset. Maximum likelihood trees are shown in Supplemental Figures 7 and 9. Maximum likelihood bootstrap values greater or equal to 50 (50%) and neighbor-joining bootstrap values greater than 500 (50%) are indicated in bold and italics, respectively. 138 XJ(FS) Cell Collection Animals were starved for at least seven days prior to harvesting. For control cells, animals were macerated in 1.0 mg/ml collagenase (Sigma) and 0.3 mM N-acetyl-L- cysteine (Sigma) for 1 hour and labeled in 0.4mg/ml Hoechst 33342 (Invitrogen) for 45 minutes. For transplant cells, animals were macerated in 1.0 mg/ml collagenase and 0.3 mM N-acetyl-L-cysteine for 20 minutes. The X1 population from Hoechst-labeled control cells was used to define the forward scatter/side scatter gate. Cells were sorted with a Dako Cytomation MoFlo sorter. Single Cell Transplantation Animals to receive transplants were starved in the presence of Gentamicin for at least seven days prior to onset of experiments. Three days prior to transplantation, irradiation was delivered to animals at 79-82 rads/min for 76 minutes. Cells collected by flow cytometry were loaded at low density onto glass cover slips treated with 2% dimethyldichlorosilane (Sigma) in chloroform. Individual cells were selected based on morphology with lOx magnification and loaded by mouth pipetting into the tip of pulled borosilicate glass microcapillaries (Sutter) treated with 0.1% polyvinylpyrrolidone (Sigma). Loaded cells were injected into the post-pharyngeal midline of cold- immobilized animals at 1.5-2.5 psi (Eppendorf FemtoJet). For survival experiments, transplant recipients were maintained in 6 cm Petri dishes (3 worms/dish) in the dark; water and dishes were changed every 3 days. 139 SNP Discovery Short (36 bp) sequencing reads from both asexual Clone 4 and sexual S2FlL3F2 strain expressed sequences were obtained by mRNA-Seq (Illumina). Reads were mapped to an assembly of planarian expressed sequences and Single Nucleotide Polymorphisms (SNPs) were identified with MAQ using default settings (Li et al., 2008). Only SNPs based on at least 10X read depth for both strains were considered. Candidate loci were selected based on presence of multiple homozygous SNPs. SNP-containing loci were validated by PCR and Sanger sequencing (see SNP sequencing below). Genomic DNA Isolation Animals used for genotyping were subjected to two rounds of regeneration and starved for at least five days. DNA was isolated from intact regenerated animals using Easy- DNA kit (Invitrogen). PCR-RFLPAnalysis Restriction Fragment Length Polymorphism (RFLP) loci were amplified from genomic DNA samples (Finnzymes Phusion Polymerase). PCR product was purified and digested with restriction enzymes (HpaI or ScaI-HF, New England Biolabs) for two hours. Digested DNA was purified by phenol-chloroform extraction and run on a 1.4% agarose gel. SNP Sequencing SNP loci were amplified from genomic DNA. PCR products were gel-purified, A-tailed (Roche 140 Taq Polymerase), and ligated into pGEM-T Easy vector (Promega). 94 bacterial colonies from each locus for each strain were Sanger sequenced with M13F primer. For genotyping, reads were counted if at least four SNPs corresponding to a single haplotype were present. RFLP loci sequences > RFLP 00310 (S2FlL3F2) TCGGATACAGTAAATCACCTGATACTATTGCTACGGGCTATTCTGGTGAT GCTCCCCCGTCTATAACTGCTGCACAATTACAACTGAGTCCTGGTCAAGC GGACACGGGATACGTATCATTGACGTGGAATATACTGACCCAGTCCGACA TTGCCACGAATGTGAACGGATTTTTCCGTGGATATCGAATTGAATGGTGC TTGGCAAACCTGATTGATGCGGAATGTGATGCATCAACTCAATATCAGGT AAATAGGTAATTAGACGTTTTATGTTTAATATTATAAGGATGTGATTCTC GCAACACAAAACCTCCCGGTTCTTTATGGAAATAAGCGCCGTAAAAGATC AGTACAAGATGATGATGAAGACACACAGACGGATGAAGGAAGATTTAAAT ATGATACTAAATATCGCCAGGTCATACCAGACAACCCAGCAACGTCAGTT AATTTTCAAGTCTTAAGTCGTAGAAAACGAGCTGCATTAAAAAATCCTGA TGATTGGAATTATGGAAAAAATATCACTGTAAAATTGACGATGATTCCAG GCAATACTTGGATCAAGGTTTGGCTGAGAGTTTTGAAT > RFLP 00310 (CIW4) TCGGATACAGTAAATCACCTGATACTATTGCTACGGGCTATTCTGGTGAT GCTCCCCCGTCTATAACTGCTGCACAATTACAACTAGGTCCTGGTCAAGC GGACACGGGCTACGTATCATTGACGTGGAATATACTGACCCAGTCCGACA TTGCCACGAATGTTAACGGATTTTTCCGTGGATATCGAATTGAATGGTGC TTGGCAAACCTGATTGATGCAGAATGTGATGCATCAACTCAATATCAGGT AAATAGGTAATTAGACGTTTTATGTTTAATATTATAAGGATGTGATTCTC GCAACACAAAACCTCCCGGTTCTTTATGGAAATAAGCGCCGTAAAAGATC AGTACAAGATGATGATGAAGACACACAGACGGATGAAGGAAGATTTAAAT ATGATACTAAATATCGCCAGGTCATACCAGACAACCCAGCAACGTCAGTT AATTTTCAAGTCTTAAGTCGTAGAAAACGAGCTGCATTAAAAAATCCTGA TGATTGGAATTATGGAAAAAATATCACTGTAAAATTGACGATGATTCCAG GCAATACTTGGATCAAGGTTTGGCTGAGAGTTTTGAAT > RFLP 00463 (S2FlL3F2) ATCGGATCACCTATCAATATTTGCCTCCGGCTGCATTCAACATTGAACTC GTTCCGCAATCTTCATCAGCCAATAACAGCAGCAAAACATCTTCGGATTG CCACAGGAATTCAGATGGCAGCCGGAAATTGAGATCGCATACTCTCCCAG GCGACAAAATCGCTCCTGTTGTCATTGGCAATGCGCCCGCTCAACAGTCG GCCTCCACAGCAGATTCGCCTATCATGGCAACGAGAAACCTTCGCGGATG GATTGTAATACTCAAGGAGTACTTTGGATTCGTGGAAACGGCCGATCACA 141 ACGCGCTATACAAGTTCAGCCCGTTCACAATCAAGAAGAGCAAATTGGGA GTGGAATTGAAGGTTGGCTCGGCGATTGAATTTCTGGCGGTCCCGAGCTC TGGCAGTCGGCCTCGTCGCATCATTGAGCAGTTCCTGAAGGTCCTCACCG AGCCGTTATCCAATGAG > RFLP 00463 (CIW4) ATCGGATCACCTATCAATATTTGCCTCCGGCTGCATTCAACATTGAACTC GTTCCGCAATCTTCATCAGCCAATAACAGCAGCAAAACATCTTCGGATTG CCACAGGAATTCAGATGGCAGCCGGAAATTGAGATCGCATACTCTCCCAG GCGACAAAATCGCTCCTGTTGTCATTGGCAATGCGCCCGCTCAACAGTCG GCCTCCACAGCAGATTCGCCTATCATGGCAACGAGAAACCTTCGCGGATG GATTGTAATACTCAAGGAGTATTTTGGATTCGTGGAAACGGCCGATCACA ACGCGCTATACAAGTTCAGCCCGTTCACAATCAAGAAGAGCAAATTGGGA GTGGAATTGAAGGTTGGCTCGGCGATTGAATTTCTGGCGGTCCCGAGCTC TGGCAGTCGGCCTCGTCGCATCATTGAGCAGTTCCTGAAGGTCCTCACCG AGCCGTTATCCAATGAG Sequences of SNP loci > SNP 00163 (S2F1L3F2) CCCAGTGAAAAACCCAAACATGGAAATGAAGATTGTTTCAATACATTTTT CAGTGAGACTGGAAATGGAAAATATGTTCCTCGGGCTCTTTTTGTCGATT TGGAACCAAGTGTAATTGGTAAGTTTTAGAATTTTGTGTTTTATATTTTT AATGCTCTCCTGCTTTAGGTGAAGTGAGAAATGGGGCTTATAGACAACTG TTCCATCCGGAACAACTTATTAGTGGTAAAGAAGATGCAGCTAATAATTA CGCAAGAGGACATTATACAGTGGGTAAAGAATTGATCGATCAAGTTTTAG ATAGAATTAGAAAGGTTGCTGATAATTGTACCGGTTTGCAAGGGTTTCTA ATGTTTCATTCATTTGGTGGTGGAACTGGTTCCGGGTTTACTTCTCTGTT AATGGAACGGTTAAGTGTTGATTATGGTAAAAAATCCAAGTTAGAGTTTG CTGTTTATCCTGCTCCACAAATCG > SNP 00163 (CIW4) CCCAGTGAAAAACCCAAACATGGAAATGAAGATTGTTTCAATACATTTTT CAGTGAGACTGGAAATGGAAAATATGTTCCTCGGGCTCTTTTTGTCGATT TGGAACCAAGTGTAATTGGTAAGTTTTAGATTTTTGTGTTTTCTATTTTT AATGCTCTCCTGCTTTAGGTGAAGTGAGAAATGGGGCTTATAGACAACTG TTCCATCCGGAACAACTTATTAGTGGTAAAGAAGATGCAGCTAATAATTA CGCAAGAGGACATTATACAGTTGGGAAAGAATTGATCGATCAAGTTTTAG ATAGGATAAGAAAGGTTGCTGATAATTGTACCGGTTTGCAAGGGTTTCTA ATGTTTCATTCATTTGGTGGTGGAACTGGTTCCGGGTTTACTTCTCTGTT AATGGAACGGTTAAGTGTTGATTATGGTAAAAAATCCAAGTTAGAGTTTG CTGTTTATCCTGCTCCACAAATCG > SNP 00463 (S2F1L3F2) GACGATATTGCCGGATTGATTGAGCCGGATTTATCGGTAAAGGGTGATGA 142 GGACATGCTGGTAGCGTTCAAGGCCAAGGAATGGCTACCAAGCGATGAGA AACTGACAAAGTTTGCGGTAGTTTCCTACACGCCGTTGCAAGTGTCCAGG AACGTCGGAGGCCACCAGAAGCTGACGACAACCGCGCTGCGCATCATGCC ATCAAAGGAGAGGGACAAAATGACCCACCTGAAGCTGTATCCGGCCGGCC AGTTACGGGGAGTTGTCAATACTGCAGTTCACAATCCCGATGATCTCGGC ATCATTTTCTGTCAGTTTCCCAATTCATCTACCAAACGAACGGTCGCATT TACCAGTGAGGATCTTGTCAACTGCAAGCCCAAGGT > SNP 00463 (CIW4) GACGATATTGCCGGATTGATTGAGCCGGATTTATCGGTAAAGGGCGATGA GGACATGCTGGTAGCGTTCAAGGCCAAGGAATGGTTGCCAAGCGATGAGA AACTGACAAAGTTTGCGGTAGTTTCCTACACGCCGTTGCAAGTGTCCAGG AACGTCGGAGGCCATCAGAAGCTGACGACAACCGCGCTGCGCATCATGCC ATCAAAGGAGAGGGACAAAATGACCCACCTGAAGCTGTATCCGGCCGGCC AGTTGCGGGGAGTTGTCAATACTGCAGTTCACAATCCCGATGATCTCGGC ATCATTTTCTGTCAGTTTCCCAATTCATCTACCAAACGGACGGTCGCATT TACCAGTGAGGATCTTGTCAACTGCAAGCCCAAGGT > SNP 02716 (S2F1L3F2) GCTTTCGTCTATATGTTAGAGCGTTTCATCAATACTTCACTGACTTCGGT AAATAAATGGTGTGCTATAACATTTTTTGTTTACCTATTTACATAGGATC AAGTACAAAATCATTTGTTAGATCTTTGCCAAATAATTTCTTCACCGAAA AAAGAAAAAATTATCGATTTCTTGAAAAAAGAAGGACCTAAATTGATTCC CCGAATACTTAAAAAAGACTGTCCAATAAAGATTTGTCAAATGGAAAACT TTTGTCACAAAATCGAAGTTATATTCGAAAATGATGATAAGGTTCCAAGT AATTTAATTTACTCTAAGGGATTTTCGAATATATCTTCTAATTAGATCCA TCAAAATATTGTTCGATCTGCCAATCGG > SNP 02716 (CIW4) GCTTTCGTCTATATGTTAGAGCGTTTCATCAATACTTCACTGACTTCAGT AAATAAATGGTGTGCTATAACATTTTTTGTTTACCTATTTACATAGGATC AAGTACAAAATCATTTGTTAGATCTTTGCCAAATAATTTCTTCACCGAAA AAAGAAAAAATTATCGATTTCTTGAAAAAAGAAGGACCTAAATTGATTCC ACGAATACTTAAAAAAGACTGTCCAATGAAGATTTGTCAAATGGAAAACT GTTGTCACAAAATCGAAGTTATATTCAAAAATGATGATAAGGTTCCAAGT AATTTAATTTACTCTAAGGGATTTTCGAATATATCTTCTACTTAGATCCA TCAAAATATTGTTCGATCTGCCAATCGG Primers for PCR-RFLP analysis. RFLP_00310_F1 RFLP_00310_R2 RFLP_00463_F1 5'-TCGGATACAGTAAATCACCTGATAC-3' 5'-ATTCAAAACTCTCAGCCAAACC-3' 5'-ATCGGATCACCTATCAATATTTGC-3' RFLP_00463_Ri 5'-GATAACGGCTCGGTGAGGAC-3' Primers for SNP loci sequencing. 143 SNP_00163_Fl SNP 00163 F2 SNP 00163_Ri SNP 00163 R2 SNP 00463_Fl SNP 00463_F2 SNP_00463_Ri SNP 00463 R2 SNP 02716 Fl SNP 02716 F2 SNP_02716_Ri SNP_02716_R2 5'-GTCTTGAACATGGTATTCAACAAGA-3' 5' -CCCAGTGAAAAACCCAAACA-3' 5'-ATGGTTCAACAACCGCTGTA-3' 5'-CGATTTGTGGAGCAGGATAAA-3' 5'-CTCGACATATCGGAGTTGTGAA-3' 5'-GACGATATTGCCGGATTGA-3' 5'-CAACTAACTGACAGGCAGCAAC-3' 5'-ACCTTGGGCTTGCAGTTG-3' 5'-TCACGATGGAAACCAAAAAG-3' 5'-GCTTTCGTCTATATGTTAGAGCGTTTC-3' 5'-TTTTCTAAGGCTACCCAGCTGAT-3' 5'-ACCGATTGGCAGATCGAA-3' 144 Acknowledgements We thank D. Kim for manuscript comments, S. Lapan for intestinal markers, M. Srivastava for phylogenetics advice, D. Wenemoser for SMEDWI-l antibody purification, J. Owen for Illumina data, M. Griffin for flow cytometry assistance, as well as P. Hsu, G. Bell, R. Young, and all members of the Reddien Lab for extensive comments and discussion. P.W.R. is an early career scientist of the Howard Hughes Medical Institute. R01GM080639 and Keck Foundation support. 145 We acknowledge support by NIH References BagufiA, J., Sal6, E., and Auladell, C. (1989). Regeneration and pattern formation in planarians. III. that neoblasts are totipotent stem cells and the cells. Development (Cambridge, England) 7786. BagufliA, J. (1976). Mitosis in the intact and regenerating planarian Dugesiamediterranean.sp. I. Mitotic studies during growth, feeding and starvation. J. Exp. Zool. 195, 53-64. Bardeen, C.R., and Baetjer, F.H. (1904). The inhibitive action of the Roentgen rays on regeneration in planarians. J. Exp. Zool. 1, 191-195. Barker, N., van Es, J.H., Kuipers, J., Kujala, P., van den Born, M., Cozijnsen, M., Haegebarth, A., Korving, J., Begthel, H., Peters, P.J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003-1007. Becker, A.J., McCulloch, E.A., and Till, J.E. (1963). Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197, 452-454. Blanpain, C., Lowry, W.E., Geoghegan, A., Polak, L., and Fuchs, E. (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635-648. Bravo, R., Frank, R., Blundell, P.A., and Macdonald-Bravo, H. (1987). Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature 326, 515-517. Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540-552. Dubois, F. (1949). Contribution A 1 'etude de la migration des cellules de regeneration chez les Planaires dulcicoles. Bull. Biol. Fr. Belg. 83, 213-283. Eisenhoffer, G.T., Kang, H., and SAnchez Alvarado, A. (2008). Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell 3, 327-339. Eriksson, S., GrAslund, A., Skog, S., Thelander, L., and Tribukait, B. (1984). Cell cycledependent regulation of mammalian ribonucleotide reductase. The S phase-correlated increase in subunit M2 is regulated by de novo protein synthesis. J. Biol. Chem. 259, 11695-11700. Evans, M.J. (1972). The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morphol 28, 163-176. Evans, M.J., and Kaufman, M.H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154-156. 146 Gillis, W.Q., Bowerman, B.A., and Schneider, S.Q. (2008). The evolution of protostome GATA factors: molecular phylogenetics, synteny, and intron/exon structure reveal orthologous relationships. BMC Evol. Biol. 8, 112. Guindon, S., and Gascuel, 0. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696-704. Guo, T., Peters, A.H.F.M., and Newmark, P.A. (2006). A Bruno-like gene is required for stem cell maintenance in planarians. Dev. Cell 11, 159-169. Hayashi, T., Asami, M., Higuchi, S., Shibata, N., and Agata, K. (2006). Isolation of planarian Xray-sensitive stem cells by fluorescence-activated cell sorting. Dev. Growth Differ. 48, 371-380. Hewitson, T.D., Kelynack, K.J., and Darby, I.A. (2006). Histochemical localization of cell proliferation using in situ hybridization for histone mRNA. Methods Mol. Biol. 326, 219-226. Hopman, A.H., Ramaekers, F.C., and Speel, E.J. (1998). Rapid synthesis of biotin-, digoxigenin, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for In situ hybridization using CARD amplification. J. Histochem. Cytochem. 46, 771-777. Keller, J. (1894). Die ungeschlechtliche Fortpflanzungder Stlsswasser-Turbellarien. Jen Zeit Naturw 28, 370-407. Lange, C.S., and Gilbert, C.W. (1968). Studies on the cellular basis of radiation lethality. 3. The measurement of stem-cell repopulation probability. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 14, 373-388. Lender, T., and Garcia, A. (1965). Les ndoblasts marqu6s par l"uridine tritide migrent et 6difient le blasteme des planaires d"eau douce. C. R. Acad. Sci. Paris 160, 4095-4097. Li, H., Ruan, J., and Durbin, R. (2008). Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 18, 1851-1858. Martin, G.R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.a. 78, 7634-7638. Morgan, T.H. (1898). Experimental studies of the regeneration of Planariamaculata. Dev. Genes Evol. 7, 364-397. Morrisey, E.E., Tang, Z., Sigrist, K., Lu, M.M., Jiang, F., Ip, H.S., and Parmacek, M.S. (1998). GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 12, 3579-3590. Newmark, P.A., and Sanchez Alvarado, A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental Biology 220, 142-153. 147 Nishimura, K., Kitamura, Y., Taniguchi, T., and Agata, K. (2010). Analysis of motor function modulated by cholinergic neurons in planarian Dugesiajaponica. Neuroscience 168, 18-30. Ohlstein, B., and Spradling, A. (2006). The adult Drosophilaposterior midgut is maintained by pluripotent stem cells. Nature 439, 470-474. Pearson, B.J., and Stnchez Alvarado, A. (2010). A planarian p53 homolog regulates proliferation and self-renewal in adult stem cell lineages. Development (Cambridge, England) 137, 213-221. Pearson, B.J., Eisenhoffer, G.T., Gurley, K.A., Rink, J.C., Miller, D.E., and Sanchez Alvarado, A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev. Dyn. 238,443-450. Reddien, P.W., and SAnchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725-757. Reddien, P.W., Bermange, A.L., Murfitt, K.J., Jennings, J.R., and SAnchez Alvarado, A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635-649. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and SAnchez Alvarado, A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. Reitzel, A.M., and Tarrant, A.M. (2009). Nuclear receptor complement of the cnidarian Nematostella vectensis: phylogenetic relationships and developmental expression patterns. BMC Evol. Biol. 9,230. Salvetti, A., Rossi, L., Bonuccelli, L., Lena, A., Pugliesi, C., Rainaldi, G., Evangelista, M., and Gremigni, V. (2009). Adult stem cell plasticity: neoblast repopulation in non-lethally irradiated planarians. Developmental Biology 328, 305-314. SAnchez Alvarado, A., Newmark, P.A., Robb, S.M., and Juste, R. (2002). The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development (Cambridge, England) 129, 5659-5665. Scimone, M.L., Meisel, J., and Reddien, P.W. (2010). The Mi-2-like Smed-CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea.Development (Cambridge, England) 137, 1231-1241. Spangrude, G.J., Heimfeld, S., and Weissman, I.L. (1988). Purification and characterization of mouse hematopoietic stem cells. Science 241, 58-62. Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionspecific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673-4680. 148 Till, J.E., and McCulloch, E.A. (1961). A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14, 213-222. Uchida, N., Buck, D.W., He, D., Reitsma, M.J., Masek, M., Phan, T.V., Tsukamoto, A.S., Gage, F.H., and Weissman, I.L. (2000). Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. U.S.a. 97, 14720-14725. Wagers, A.J., and Weissman, I.L. (2004). Plasticity of adult stem cells. Cell 116, 639-648. Wagers, A.J., Sherwood, R.I., Christensen, J.L., and Weissman, I.L. (2002). Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256-2259. Weissman, I.L. (2000). Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157-168. Wenemoser, D., and Reddien, P.W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental Biology 344, 979-991. Wolff, E., and Dubois, F. (1948). Sur la migration des cellules de r6g6ndration chez les planaires. Rev Suisse Zool 55, 218-227. 149 150 Chapter 3 Hedgehog signaling regulates gene expression in planarian glia Irving E. Wang', Sylvain W. Lapani, and Peter W. Reddien 'These authors contributed equally Experiments shown in Figure 1-2 and Supplemental Figure Si, S3, S5 were performed by SWL and IEW. Experiments shown in Figures 3-6 and Supplemental Figures S2, S4, S6-S8 were performed by IEW. All authors contributed to the design of experiments and editing of the manuscript. 151 Abstract Hedgehog signaling has important roles in central nervous system (CNS) development in vertebrates and Drosophila, yet it specifies different CNS cell types along distinct axes in these disparate animals. Exploring the role of Hedgehog signaling in the nervous systems of a broader range of animal phyla is critical for understanding which functions of Hedgehog in the CNS are ancestral to the Bilateria, in contrast to those that represent clade-specific adaptations. Hedgehog ligand is expressed in a population of medially-located neurons in the planarian brain. To investigate the function of Hedgehog signaling in the brain, we performed RNA-Sequencing on brain tissue samples from the planarian Schmidtea mediterranea following inhibition of hedgehog (hh) and the gene encoding its receptor, patched (ptc), by RNAi. No significant differences were observed in the expression of transcription factors that specify neurogenic domains, but transcripts for two genes expressed in the central nervous system, intermediate filament-i (f-1) and protocadherin-19 (pcdh-19), were significantly misregulated. if-1 and pcdh-19 were expressed in cells within the neuropil of the planarian CNS and adjacent to hh+ neurons. Expression was not detected following hh RNAi and was present in ectopic locations outside the CNS following ptc RNAi. if-J/pcdh-J9- cells did not express neural genes but did express planarian orthologs of astrocyte markers, such as glutamine synthetase (gs) and excitatory amino acid transporter-2/glt-J (eaat2). IF-1 processes associate closely with axons and encapsulate regions of high synapse density. We conclude that if-1J/pcdh-19* cells represent previously unidentified planarian glia. Inhibition of hh or ptc did not block expression of astrocyte markers, suggesting that certain aspects of gene expression, but not cell specification or 152 survival, was regulated by constitutive signaling from hh+ neurons. Hedgehog signaling has been implicated in astrocyte response to brain injury in vertebrates and posterior midline glia specification in Drosophila. We conclude that Hedgehog signaling confers the if-1*/pcdh-J9* identity to planarian glia within the CNS and hypothesize the control of glial identity by neighboring neuronal populations is an ancestral role for the pathway in the nervous system. Furthermore, the identification of planarian glia presents a novel tractable system for dissection of glia biology. 153 Introduction The Hedgehog signaling pathway has been implicated in numerous developmental processes across the Metazoa, including limb and midline development in vertebrates and segmentation in Drosophila (Ingham et al., 2011). Little is known, however, about the role of Hedgehog signaling in the Lophotrochozoa, one of the three superphyla that comprise the Bilateria. Further study and comparison with the other two Bilaterian superphyla, the Deuterostomes and the Ecdysozoa, is required to understand the evolution of this signaling pathway and its roles in Metazoan biology. One member of the lophotrochozoa, the planarian Schmidtea mediterranea, has recently become a model system for the study of stem cell biology, wound responses, and tissue patterning as a consequence of the development of powerful techniques such as RNA interference (Reddien et al., 2005a) and fluorescent RNA in situ hybridization (Pearson et al., 2009). Planarians are free-living platyheminthes capable of regenerating almost any lost tissue through the continued expression of developmental factors and the maintenance of a pluripotent stem cell population throughout adulthood (Morgan, 1898; Reddien and SAnchez Alvarado, 2004; Wagner et al., 2011). Previous studies showed that perturbation of the Hedgehog signaling pathway causes an anterior-posterior polarity defect during regeneration. Inhibition of the planarian hedgehog ortholog (hh) resulted in bifurcated or absent tail formation and inhibition of the patched ortholog (ptc) resulted in anterior tails in place of heads. These defects are attributed to hh-mediated modulation of Wnt signaling during regeneration (Rink et al., 2009; Yazawa et al., 2009). 154 The planarian central nervous system consists of a pair of cephalic ganglia and ventral nerve cords, each structure comprised of a cortex of cell bodies encompassing a neuropil (Morita and Best, 1965). hh is expressed in cells present in the medial cortex of the cephalic ganglia (Rink et al., 2009; Yazawa et al., 2009), a similar location for a protostome to the vertebrate neural tube floor plate (Dessaud et al., 2008). However, direct effects of Hedgehog signaling on planarian nervous system regeneration have not been described, despite a wealth of information of its involvement in the CNS of other systems. The vertebrate ortholog Sonic hedgehog (SHH) is secreted from the floor plate and forms a ventral-to-dorsal morphogenetic gradient that establishes distinct domains of transcription factor expression in the ventral neural tube (Dessaud et al., 2008). The Drosophila neurectoderm has a similar ventral-to-dorsal distribution of orthologous transcription factors, but Hedgehog signaling is not required to establish these domains (Cornell and Ohlen, 2000). One neural tube domain specified by SHH generates oligodendrocytes, the glial cells responsible for myelination (Rowitch, 2004). Additional roles for Hedgehog signaling in glial cell biology include inducing reactive astrogliosis in response to brain injury in adult mammals (Sirko et al., 2013) and specifying subtypes of midline glia during Drosophila development (Watson et al., 2011). Examining the role of Hedgehog signaling in planarian brain regeneration presents an opportunity to determine whether the pathway has ancestral roles in the differentiation and regulation of CNS cell types. Here we show that although the cephalic ganglion displays similarity in transcription factor domain distribution to the vertebrate neural tube, perturbation of Hedgehog signaling does not disrupt these expression domains. Through a tissue-specific mRNA-Sequencing approach, we identified two CNS-associated genes, intermediatefilament-i (If-1) and protocadherin-19 155 (pcdh-19), with expression levels impacted by inhibition of either hh orptc. Expression of these genes is found in cells with molecular and morphological characteristics of glia. Hedgehog signaling is not required for the formation or maintenance of glia, despite having a strong ability to modulate gene expression in these cells. This discovery presents an opportunity to not only study the regulation and function of glia in a highly regenerative organism, but also gain insight into ancestral roles of Hedgehog signaling in CNS development. 156 Results Transcriptionfactor domain specification in the planarian cephalic ganglia occurs normally following Hedgehog signalingpathway perturbation Cells in the medial cortex of cephalic ganglia express hh continually throughout the life of adult animals. This expression pattern is reminiscent of the vertebrate neural tube floor plate. SHH is secreted from cells in the floor plate, establishing a ventral-to-dorsal gradient that results in the formation of distinct domains of transcription factor expression. Near the floor plate, two homeodomain transcription factors, Nkx6.1 and Nkx2.2, are expressed and give rise to the pV3 progenitors. The pMN domain immediately dorsal to pV3 does not express Nkx2.2 but does express low levels of the transcription factor Pax6. The pV2 domain has increased Pax6 levels and continued expression of Nkx6.1 when compared to pMN, and the dorsal two domains of the ventral neural tube, pVI and pVO, express Pax6 only (Dessaud et al., 2008). Planarian orthologs of Nkx2.2, Nkx6.1, and Pax6 were identified as Smed-nkx2 (nkx2), Smed-nkx6 (nkx6), and Smed-pax6b (pax6b), respectively. nkx2 was expressed in the medial cortex and part of ventral cortical region lateral to the medial cortex (Figure IA). nkx6 was expressed more broadly, with expression concentrated most highly in the medial cortex but extending more laterally than nkx2, reaching and extending slightly beyond the outer lobe of the cephalic ganglia (Figure IB). pax6b had a complementary expression domain to nkx2. The domain extends from the outer lobe to the chemosensory branches (Figure 1 C). These mediallateral expression domains were similar to the ventral-dorsal domains observed in the vertebrate 157 neural tube, with nhx2 being closest to the source of hh ligand, pax6b being furthest, no overlap between nkx2 and pax6b, and overlap between nkx6 and both nkx2 and pax6b (Figure ID). Loss of SHH expression in the vertebrate floor plate results in failed domain formation and loss of the entire ventral neural tube (Ruiz i Altaba et al., 2003). To determine whether the planarian CNS requires Hedgehog signaling to establish its transcription factor domains, hh and ptc were targeted for inhibition by RNAi. Animals were fed a mixture of liver and bacteria expressing dsRNA for a control gene not present in the planarian genome, hh, orptc a total of six times over twenty days. The effects were examined under unamputated homeostatic conditions as ptc(RNAi) animals are not expected to regenerate heads (Rink et al., 2009; Yazawa et al., 2009). In both hh(RNAi) and ptc(RNAi) animals, no changes in the expression pattern of nkx2, nkx6, and pax6b compared to control were observed in any animals (Figure 1E). Therefore, under pathway perturbation conditions sufficient to obtain polarity defects, no role for Hedgehog signaling in maintaining transcription factor expression domains in the planarian CNS was detected. mRNA-Seq identifies a set ofCNS-enrichedgenes affected by inhibitionof hedgehog To identify nervous system defects resulting from perturbation of Hedgehog signaling, we examined changes in gene expression within the CNS following inhibition of hh or ptc. Because Hedgehog signaling is likely to have roles in homeostasis of other tissue types, we developed a method to isolate CNS tissue for RNA collection and sequencing. Amputated head fragments collected from CIW4 asexual strain animals after six RNAi feedings were used as reference samples (Figure IF). For CNS-specific sample collection, large (>2cm) sexual animals were 158 dissected following a brief acid-based fixation that permitted the cephalic ganglia to be separated from surrounding tissue and collected for RNA isolation (Figure 1 G). mRNA purified from both head and cephalic ganglia samples from control, hh(RNAi), and ptc(RNAi) animals was used in expression profiling by quantitative RNA-Seq. The transcriptomes of the head and cephalic ganglia samples from control animals were compared to determine whether the dissection technique enriched for neural transcripts. Whereas the expression level of the housekeeping gene gapdh was similar between the two samples, known neuronal transcripts synapsin (syn) (Nakazawa et al., 2003), prohormone convertase 2 (pc2) (Collins et al., 2010), and choline acetyltransferase (chat) (Nishimura et al., 2010) were between 2.6 to 7 fold increased in CNS-specific samples. The prohormone-encoding gene 1020HH (Collins et al., 2010), which is expressed in cells in the cephalic ganglia and ventral nerve cords, was enriched 81 fold. The relatively lower enrichment levels for a subset of these genes can be explained by their expression in peripheral nervous system (PNS), cells of which were present in the head fragment samples but not the cephalic ganglia sample. All examined genes with expression known to be restricted from the nervous system were, indeed, greatly diminished in the CNS-specific sample (Figure 1H). hh, which is expressed in medial cortical neurons, was enriched by 57-fold in CNS samples whereas ptc, which is expressed in many tissues in planarians, showed little difference in expression (Figure 1 H, Supplemental Table 1). The enrichment for neural transcripts indicates that analysis of these data can reveal effects of hh and ptc inhibition on the central nervous system. A comparison of hh(RNAi) and ptc(RNAi) animals to control animals showed insignificant differences in transcript levels of the housekeeping gene gapdh and neural genes 159 syn and pc2. Expression of hh in hh(RNAi) animals was, as expected, significantly reduced (pdaj < 0.05). ptc expression was decreased in ptc(RNAi) animals as well as in hh(RNAi) animals. Patched protein is a negative regulator of Hedgehog signaling and acts as a repressor of ptc transcription (Varjosalo and Taipale, 2008); increased Patched activity in animals lacking Hedgehog ligand is likely responsible for the reduction of ptc transcript levels in hh(RNAi) animals. Additionally, confirming our previous results, the expression levels of nkx2, nkx6, and pax6b were not significantly changed in the hh(RNAi) and ptc(RNAi) animals versus controls (Figure 11). We selected a set of genes that fit the criteria of at least 2 fold enrichment or depletion in hh(RNAi) or ptc(RNAi) samples and at least 1,000 reads per kilobase per million reads (RPKM) to account for minor discrepancies when harvesting tissue (Supplemental Table 2). A wholemount in situ hybridization (WISH) screen for these genes identified two that were expressed in the central nervous system (Supplemental Figure SOl). The first gene, Smed-intermediate filament-i (f-1), is an ortholog of cytoplasmic intermediate filament genes (Supplemental Figure S02) (Kuo and Weisblat, 2011). Intermediate filaments are cytoskeletal proteins that provide structural support and mechanical stress resistance in a variety of cell types (Herrmann et al., 2007). The second gene, Smed-protocadherin-19 (pcdh-19), although lacking identifiable cadherin domains, has BLAST homology to protocadherin-19, a calcium-dependent cell adhesion molecule that has been implicated in nervous system development and synapse formation (Frank and Kemler, 2002). 160 Expression and localization of f andpcdh-19 is alteredby Hedgehogpathwayperturbation Fluorescent in situ hybridization (FISH) revealed that if-] and pcdh-19 are co-expressed primarily in cells in the neuropil, the cell body sparse region within the neuron-dense cortex (6.5% if-+, 4.0% pcdh-19+, 89.5% ifJ+/pcdh-19+)(Figure 2A, 2B). mRNA signal was detected in processes extending from the cell body, a characteristic not commonly observed by FISH, indicating that the morphology of the cells is likely to be highly branched (Supplemental Figure SO3B). Small numbers of cells were also observed outside the neuropil, in locations such as within the cortex of the cephalic ganglia, in between the two lobes of the cephalic ganglia, and sparsely throughout the ventral parenchyma (Supplemental Figure S03A, SO3B). if-J/pcdh-19+ cells both inside and outside the neuropil express ptc, indicating that these cells may be responsive to Hedgehog signaling (Figure 2C). While the majority of ptc+ cells within the neuropil were if-J*/pcdh-19+, expression of if-] and pcdh-19 occurred in only a small subset of ptc+ cells in the CNS cortex and parenchymal space. Additionally, if-]J/pcdh-19+neuropil cells were adjacent to the hh+ neurons in the medial cortex, suggesting that these cells were not only competent to respond to hh but also in close proximity to a source of the ligand (Figure 2D). To characterize the effect of Hedgehog signaling on the neuropil cells, we performed FISH for if-] and pcdh-19 following inhibition of hh or ptc and quantified the density of cells in two regions of the head, inside the neuropil and outside the neuropil. Cells expressing one or both of the two markers were found inside the neuropil of the cephalic ganglia at a density of about 2200 cells/mm 2 of cephalic ganglion and outside the neuropil at a density of about 65 cells/mm 2 of head (Figure 2E, 2F). Upon RNAi of hh, the density of if-1/pcdh-19+ cells decreased 92% in the neuropil and 98% outside the neuropil (Figure 2E, 2F). 161 As ptc is a negative regulator of Hedgehog signaling (Varjosalo and Taipale, 2008), RNAi of ptc is expected to result in an increase in if-1 and pcdh-19 expression. Accordingly, the density of cells expressing either or both if-1 and pcdh-19 in ptc(RNAi) animals increased 57% in the neuropil and 615% outside the neuropil (Figure 2E, 2F). A portion of the increased cell count consisted of cells expressing only one of the two markers, with if-1-/pcdh-19* comprising 38.2% of the population (Figure 2H). Expression at ectopic locations outside the neuropil was localized to the parenchyma near the ventral surface of the animal, with concentration of expression at the rim of the head where presumptive chemosensory neurons reside (Supplemental Figure SO3A, SO3C). To ensure that ablation of if-1 and pcdh-19 signal resulted from loss of Hedgehog signaling, the Gli transcription factors, which are the downstream effectors of the pathway, were also targeted for inhibition. gli-i and gli-2, encoding activating transcription factors, and gli-3, encoding a repressing transcription factor, have been found in the S. mediterranea genome. Inhibition of gli-i results in a similar defective tail regeneration phenotype as inhibition of hh (Rink et al., 2009). We found that RNAi of gli-i also resulted in loss of if-1 and pcdh-19 signal whereas RNAi of gli-2 and gli-3 did not have any discernable effect on expression of if-1 and pcdh-19 (Supplemental Figure SO4A). These data indicate that the Hedgehog signaling pathway regulates the biology of specific cell type in the axon rich neuropil of the planarian nervous system. During regeneration, if-1*/pcdh-19+ cells accumulate in the blastema, a protruding mass of newly formed tissue that replaces missing portions of the body. As expected, no if-1+/pcdh19* cells were observed in hh(RNAi) animals, both in the blastema and throughout the preexisting tissue. Inhibition of plc results in defective head regeneration; the cephalic ganglia in 162 the anterior blastema appear as masses of cells without any discernable neuropil region. An increase in the total number of ifI/pcdh-19* cells, however, was observed in ptc(RNAi) anterior blastemas despite impaired head formation (Supplemental Figure SO4B). if1/pcdh-19' cells are not neurons To understand the role of Hedgehog signaling in the planarian nervous system, we sought to identify the if-J+/pcdh-19+ cell type. Given the localization of these cells within the central nervous system, we first tested for co-expression of neural markers. Both pc2 and syn were expressed in a few cells in the neuropil region and but were not in any ifJ]/pcdh-19+cells inside or outside the neuropil (Figure 2G, 2H, Supplemental Figure S05A, S05B). The planarian genome contains a number of voltage-gated ion channels, some of which are expressed in a CNS-like pattern. Three genes, one encoding a potassium channel, one encoding a sodium channel, and one encoding a calcium channel, were examined but did not show co-expression with if-1 or pcdh-19 (Figure 21). A gene encoding a sodium and potassium co-transporter involved in maintaining ion concentration gradients in neurons also was not expressed in ifJ+/pcdh-19+ cells (Figure 21). Furthermore, choline acetyltransferase (chat), which encodes an acetylcholine synthesis enzyme, glutamate decarboxylase (gad), which encodes a GABA synthesis enzyme, tyrosine hydroxylase (th), which encodes a dopamine synthesis pathway protein (Fraguas et al., 2011), tryptophan hydroxylase (tph), which encodes a serotonin synthesis enzyme (Fraguas et al., 2011), were all not found in if-J+/pcdh-19+ cells even though their expression was readily detected in other CNS cells (Figure 21). Finally, we examined genes encoding orthologs of proteins involved in synapse function. Genes encoding orthologs of three 163 Synaptotagmin synaptic vesicle docking and fusion proteins, the synaptic vesicle associated protein Synaptogyrin 2, synaptic vesicle fusion proteins SNAP25 and Unc-13, and the vesicular neurotransmitter transporters VAchT and VGluT (Mairz et al., 2013) were not expressed in f 1*/pcdh-19+ cells (Figure 21). Additional genes encoding synaptic proteins, neurotransmitter biosynthesis enzymes, and transcription factors were also tested and showed no overlapping expression with if1 and pcdh-19 (Supplemental Figure S05C). Netrin 2, a marker previously described to be expressed in cells in the neuropil (CebriA and Newmark, 2005), also was not expressed in if1/pcdh-19+ cells (Supplemental Figure SO5C). We conclude that, despite the localization within the central nervous system and the presence of cytoplasmic extensions, the f I/pcdh-J9' cells are not neurons. if1J/pcdh-19* cells express neurotransmitterreuptake and metabolism genes In addition to neurons, the other predominant cell type in the central nervous systems of various animals is glia. Glia act as neuronal support cells by providing trophic support, axon insulation, environmental maintenance, blood-brain barrier, and synapse pruning (Pfeiffer et al., 1993; Sofroniew and Vinters, 2010). Invertebrate glia have been extensively characterized in Drosophila(Hartenstein, 2011) and C. elegans (Oikonomou and Shaham, 2011), and have been identified in annelids (Deitmer et al., 1999) and molluscs (Reinecke, 1976). Electron microscopy performed on transverse sections of the planarian Dugesia tigrina revealed cells distributed throughout the ventral nerve cords that lack neuronal characteristics; these have been described as candidate planarian glial cells (Golubev, 1988; Morita and Best, 1966). We hypothesized that the if 1/pcdh-19+ cells might be glia, and therefore we performed a candidate 164 screen for expression of known glial markers from vertebrates and Drosophila in the planarian neuropil. Four astrocyte-associated genes were found to be co-expressed in if-J*/pcdh-19+ cells. Smed-gs (gs) encodes an ortholog of Glutamine Synthetase, which converts the neurotransmitter glutamate to glutamine (Anderson and Swanson, 2000), and was expressed in cells in the neuropil as well as cells in the ventral parenchyma and the intestine (Supplemental Figure S06A). The majority of if-1/pcdh-19+ cells expressed gs both inside the neuropil (97%) and outside the neuropil (72%), although gs*/if-1~/pcdh-19" cells were abundant outside the neuropil (Figure 3A). Similar to if-1 and pcdh-19, gs transcripts were observed in cytoplasmic branches extending from the cell body (Supplemental Figure S06B). Smed-gat-1 (gat-]) is predicted to encode a protein with high similarity to GABA transporters (Featherstone, 2011) and was expressed almost exclusively in cells within the neuropil (Supplemental Figure S06C). if-]J/pcdh-19+ cells showed almost complete overlap with gat-] both inside the neuropil (95%) and outside the neuropil (99%) (Figure 3B). Unlike gs expression, few gat-I+ cells outside the neuropil did not express if-1 or pcdh-19 (10%), and the transcript appeared to be restricted to the cell body close to the nucleus (Supplemental Figure S06D). Smed-eaat2 (eaat2), which encodes a protein similar to the vertebrate glutamate transporter and glial cell marker GLT-1/EAAT2 (Featherstone, 2011), was expressed most abundantly in the neuropil but was also expressed in cells throughout the ventral parenchyma with some concentration along the rim of the entire animal and in the pharynx (Supplemental Figure S06E). FISH for eaat2 indicated that the gene is expressed in the majority of if-1+/pcdh- 165 19+ cells in the neuropil and to a lesser degree outside the neuropil (Figure 3C). Branches were also observed in eaat2' cells (Supplemental Figure S06F). Smed-eaat3 (eaat3) encodes a protein similar to a neuronally-expressed vertebrate glutamate transporter, EAAC1/EAAT3. eaat3 expression was punctate and dispersed throughout the entire neuropil (Supplemental Figure S06G, S06H). Whereas overlapping expression with if-1 and pcdh-19 was confirmed, it was difficult to determine whether other cell types within the cephalic ganglia also expressed this gene (Figure 3D). Because if-*/pcdh-19+ cells express genes encoding proteins that reuptake extracellular glutamate (eaat2 and eaat3) and metabolize glutamate (gs), we propose that these cells participate in maintaining the extracellular environment, a role typically performed in vertebrates by astrocytes. Together with the lack of neuronal marker expression, these results are consistent with the possibility that if-1]/pcdh-19+ cells are planarian glia. The similar expression of gs+ cells and ectopic if-l/pcdh-19+ cells in the ventral parenchyma of ptc(RNAi) animals led us to further characterize marker expression outside the neuropil. gs+ cells outside the neuropil were observed to express ptc at low rates, suggesting that at least a subset of the cells might respond to Hedgehog signaling (Figure 3E). ptc was also coexpressed with gat-], eaat2, and eaat3 in a few cells outside the neuropil (Supplemental Figure S061, S06J, S06K). Cells expressing gs, gat-1, eaat2, or eaat3 did not express the pc2, suggesting that they were not neurons (Figure 3I, Supplemental Figure S06L, S06M, S06N). In the gs+ cell population outside the neuropil, some cells expressed eaat2 (Figure 3F) and very few expressed gat-1 and eaat3 (Figure 3E, 3G), presumably because of the scarcity of those markers in cells outside the neuropil. To ensure RNA probe specificity for its target molecule, WISH was 166 performed on animals following gs, gat-1, eaat2, or eaat3 RNAi. Detection by in situ hybridization of the target molecules was reduced in animals following inhibition of the same molecule by RNAi (Supplemental Figure S060). Co-expression of the astrocyte markers and lack of neuronal markers in these cells outside the neuropil raise the possibility that other glial cell types with differing expression profiles may exist in the planarian peripheral nervous system. IF-] protein localizes to cytoplasmic extensions that closely associatewith neurons To examine the morphology of cells that express if-1, we raised a polyclonal antibody against a peptide corresponding to a segment of the IF-1 protein primary structure. Whole-mount immunofluorescence revealed an extensive network of IF-i+ branches concentrated in the neuropil and appearing in the parenchyma near the ventral surface (Figure 4A). RNAi of if-1 resulted in complete loss of IF-i antibody immunolabeling, confirming that labeling did not result from cross-reactivity with other planarian proteins (Supplemental Figure S07A). In the cephalic ganglia, the IF-I+ processes formed a mesh that congregated into tracts leading out of the neuropil between the chemosensory branches to the periphery of the head (Figure 4B). The IF-1 cytoplasmic extensions were also observed forming hollow columns oriented along the dorsal-ventral axis (Figure 4C). In the periphery, IF-i+ processes ran along tracts that were mostly devoid of cell bodies (Figure 4D). These peripheral branches varied between animals in extent, number, and location along the AP axis. In the ventral nerve cords, the processes ran parallel to one another in a tight bundle in the dorsal domain of the VNC and formed lacunae in the ventral portion of the VNC (Figure 4E, Supplemental Figure S07B). IF-i appeared to be 167 localized mainly to the processes of if-1 cells given the minimal overlap between the protein and if-] transcripts (Supplemental Figure S07C). To determine the association of the IF-i+ processes with neurons, if any, we performed immunofluorescence in conjunction with antibodies against a-tubulin and synapsin. Antibodies against a-tubulin label axons of both the central and peripheral nervous system in planarians (Sanchez Alvarado and Newmark, 1999). Axons run parallel to the AP axis through the neuropil and regularly exit the ventral nerve cords to form orthogonal commissures that extend from the VNC to the edge of the body. The IF-1 processes emerging from the cephalic ganglion neuropil appeared to follow the same tracts as the a-tubulin+ axon bundles (Figure 4F). A similar effect was observed in the orthogonal branches throughout the parenchyma (Figure 4G). The IF-1I processes were embedded within the nerve bundles and did not appear to fully enclose the commissural axon fascicle. An anti-Synapsin antibody labels large clusters of synapses within the neuropil and in nerve plexuses in the grid-like network of commissural axon bundles called the Orthogon (Adell et al., 2009; Reuter et al., 1998). Accumulations of synapses formed discrete, regularly spaced structures in the ventral nerve cord that strongly resembled synaptic glomeruli described in insect species (Boeckh and Tolbert, 1993; Leise, 1990). Immunofluoresence with both the anti-IF-i antibody and the anti-Synapsin antibody showed IF-1 branches weaving through and filling gaps within the synapse-dense cephalic ganglion neuropil (Figure 4H). Interestingly, the IF-1I cytoplasmic extensions also appeared to fully encapsulate the synaptic glomeruli of ventral nerve cords (Figure 41). The highly branched morphology of the if-1/pcdh-19* cells and their close contact with both axons and areas of high synaptic density support our prediction that these cells 168 are acting in a similar fashion to astrocytes. We conclude that if-1/pcdh-19Y cells represent planarian glia. Perturbationof Hedgehog signalingaffects expression and localizationof IF-] protein To determine the effect of Hedgehog signaling on intermediate filament protein expression and localization, we performed IF-I immunofluorescence following RNAi of hh and ptc. Inhibition of hh resulted in complete ablation of IF-i immunofluorescence signal and no change in expression or localization of Synapsin protein, whereas inhibition of ptc caused increase of IF-I protein throughout the animal (Figure 4J). The increase observed in ptc(RNAi) animals manifested primarily as an increase in the number of orthogonal commissures that came in contact with an IF-I + process. Normally 15.1% of orthogonal axon bundles are associated with IF-I + processes, whereas the percentage decreased to 2.1% following hh inhibition and increased to 61.4% following ptc inhibition (Figure 4K). Because no peripheral branches in ptc(RNAi) animals were observed deviating from the orthogonal axon network, and because Synapsin localization was unchanged under ptc inhibition, IF- I+ processes appear strictly limited to axon bundles even when ectopically formed (Supplemental Figure S07D). Accumulation of IF-I protein in ptc(RNAi) animals was also observed at the rim of the head where small Synapsin* clusters are normally seen with minimal IF protein in control animals (Supplemental Figure S07E). The observation that IF-1 processes necessarily associate with axon bundles led us to hypothesize that newly formed planarian glia use axon tracks to guide growing processes. To determine whether IF-1 branches extend along a pre-existing neuronal network, we examined 169 the regeneration of these structures in the anterior blastema. Heads of wild type animals were amputated and trunks were fixed at two-day intervals. Synapsin protein was detected in the blastema after four days of regeneration, indicating the terminally differentiated neurons have reformed an active network at that time point. IF-I protein was first detected in the blastema at low levels at six days post-amputation and more substantially at eight days post-amputation. IF1+ branches in the blastema were always found associating with the Synapsin* clusters that formed earlier, suggesting that the glia cells extend their branches through pre-existing neuronal networks (Figure 4L). Inhibitionof hedgehog does not ablate glial cells To determine whether inhibition of hh results in ablation of planarian glia, expression of gs, gat1, eaat2, and eaat3 was examined in hh(RNAi) and ptc(RNAi) animals. We found that in hh(RNAi) the expression of if-1 and pcdh-19 was eliminated, but expression of the other glia markers was still observed throughout the neuropil and indistinguishable from control animals. Similarly, inhibition of ptc had no effect on the expression or localization of gs, gat-1, eaat2, or eaat3 (Figure 5A). To confirm these results, we examined the differential expression of each gene in the RNA-Seq data described above. The expression level differences between RNAi samples were statistically significant for none of the four glia markers, and most had only log2 fold changes of less than 0.5 between RNAi conditions (Figure 5B). gat-] expression levels displayed a similar trend to that seen for if-] and pcdh-19 transcript levels. Inhibition of hh resulted in depletion of gat-1 transcripts in both cephalic ganglia and whole head samples, and 170 inhibition of ptc resulted in slight increase. Expression of gat-] may show a delayed or slower response to inhibition of hh or ptc if it is an indirect target of Hedgehog signaling. We quantified numbers of gs+ and gat-JX cells in animals following inhibition of hh or ptc. eaat2 and eaat3 were omitted from this analysis due to their wider expression pattern in the neuropil. We observed no significant change in the total number of gs+ cells within the neuropil in hh(RNAi) and ptc(RNAi) (Figure 5C). Similar results were obtained when examining expression of gat-] in RNAi conditions (Figure 5D). However, we do observe a shift in the ratio of gs+ or gat-JX cells that express if-1 and pcdh-19 to those that do not (Figure 5C, 5D). The shift in ratio suggests that upon loss of Hedgehog signaling, gs+/gat-1+/if-1+/pcdh-19'cells stop expression of if I and pcdh-19, or that the gs+/gat-1+/if-1+/pcdh-19+cells die and are replaced by gs+/gat-JI/'f-JI/pcdh-19~cells. In an effort to ablate the glial cells, we performed RNAi on candidate transcription factors with known roles in glia specification in other systems. We have identified two homologs of the Drosophilagene glial cells missing, gcm1-1 and gcm1-2, which regulates the formation of glia during development (Hosoya et al., 1995). We also extended our analysis to two other genes, olig2 and nkx2, that encode orthologs of transcription factors necessary for specification of oligodendrocytes in the vertebrate neural tube (Qi et al., 2001; Zhou and Anderson, 2002). Following RNAi, animals were assayed for expression of if-1 and pcdh-19 by WISH and were found to have no observable difference with control animals, both in homeostasis and regeneration conditions (Supplemental Figure S08). Therefore, these homologs of glial transcription factors do not appear to have an effect on planarian glia. We note that 171 vertebrates and flies do not share any known common glia specification factors, indicating that gene regulatory networks for these cells may be highly divergent. Hedgehogsignalingpromotes expression of if-1 andpcdh-19 in existing glia-like cells To determine whether neuropil cells that lack if-1 and pcdh-19 expression in hh(RNAi) animals are viable, we examined whether they are still specified during regeneration when the Hedgehog pathway is perturbed. Following RNAi, heads and tails of animals were amputated and the trunk blastemas were examined after six days of regeneration. Blastemas of control animals contained cells expressing planarian glia markers, both inside the forming neuropil and outside (Figure 6A). Similar results were observed in ptc(RNAi) animals (Figure 6A). Because ptc(RNAi) animals have defective anterior blastema formation, comparison of number of cells found within the blastema could be unreliable. In hh(RNAi) animals, expression of if-1 and pcdh-19 was eliminated, but cells expressing gat-1, eaat2, and eaat3 were observed throughout the blastema (Figure 6A). The presence of these markers in newly formed cells of the blastema suggests that the animal is capable of regenerating this cell type in the absence of positive Hedgehog signaling. There are two possible mechanisms for loss of if-1 and pcdh-19 in the neuropil glia population. The first possibility is that Hedgehog signaling promotes expression of these specific genes and that loss of the ligand results in inactivation of those genes. The second possibility is that Hedgehog signaling is responsible for differentiation of an if-1*/pcdh-19+ subtype of glia from a pool of existing gs*/gat-J+/eaat2+/eaat3+glia. If this were the case, then we would expect continued expression of if-1 and pcdh-19 in neuropil glia following hh RNAi 172 until they were replaced during normal tissue turnover. We distinguished between these two possibilities by examining whether IF-1 protein persists longer than if-1 transcript following hh RNAi. If if-1 expression is no longer detected due to cell death, then the mRNA should be ablated along with the protein, but if, by contrast, the cell inactivates transcription of if-1, then the protein is expected to perdure longer than the mRNA transcripts. We performed a time course where subsets of animals were examined after each RNAi treatment interval for presence of if-1 and pcdh-19 transcripts as well as IF-I protein. In animals treated with hh RNAi for 12 days, the number of cells expressing if-1 and pcdh-19 transcripts was greatly reduced compared to control, but the level of protein detection was similar, suggesting that there existed cells (Figure 6B). -1~/IF-1+ These data support the model that Hedgehog signaling is required for continued expression of certain genes in neuropil glia, but not for the survival of these cells. Similarly, the ectopic expression of if-1 and pcdh-19 observed in ptc(RNAi) animals may be due to formation of new cells or to promoted expression of the two genes in pre-existing cells. To determine whether perturbation of Hedgehog signaling affects gene expression in existing cells or formation of new ifJ1/pcdh-19* cells, we examined the effects of hh and ptc RNAi in animals where all neoblasts have been ablated by irradiation (Wolff and Dubois, 1948). After irradiation, animals are capable of surviving for a short duration before their tissues begin to regress. We exposed animals to 6,000 rads of ionizing radiation and immediately afterwards began RNAi. If ectopic expression resulting from inhibition ofptc requires new cell production, then we would not expect to see an increase in numbers of if-1]/pcdh-19+ cells outside the neuropil in irradiated animals. Otherwise, it would suggest that differentiated cells that existed prior to irradiation are induced to express if-] and pcdh-19. 173 Indeed, we observed greater numbers of if-i+/pcdh-i9f cells in animals where ptc was inhibited after irradiation compared to control (Figure 6C). This result is consistent with a role of Hedgehog signaling in controlling gene expression rather than specification of a cell type. Based on these and previously discussed data, we believe that Hedgehog ligand constitutively secreted from hh+ neurons in the medial cortex activates transcription of if-1 and pcdh-19 in neuropil glia. Loss of hedgehog ligand results in inactivation of if-1 andpcdh-19 but does not affect the viability of the cell, judging from continued expression of gs, gat-i, eaa2, and eaat3. Cells competent to respond to Hedgehog signaling exist outside the neuropil and will transcribe if-1 and pcdh-19 ectopically when repression of gli-i is relieved by RNAi ofptc. 174 Discussion Planarianglial cell biology is controlled by Hedgehog signaling We have described two principle findings in this work: molecular evidence for a planarian glial cell type and a specific role for Hedgehog signaling in the planarian nervous system. Previous studies using electron microscopy have identified neuronally-associated cells with branching morphology and sparse cytoplasm in the planarian ventral nerve cord. Given their localization, association with neurons, and morphological similarity to other invertebrate glia, these cells were classified as candidate planarian glia (Golubev, 1988; Morita and Best, 1966). These studies, however, did not provide any molecular evidence, which is important not only for providing markers for continued study but also for discerning function, specification, and evolution of the cell type. We have described here the first molecular markers for a planarian glial cell type, as well as a morphological characterization of these cells and a mechanism to control gene expression within the cell. One of the functions of planarian glia can be inferred from our data. First, the greatest accumulation of planarian glia is in the neuropil, a region filled with axons (based on a-tubulin immunofluorescence) and synapses (based on Synapsin immunofluorescence). Second, the cells have long processes that are closely associated with neurons. These processes extend through the synapse-rich regions of the neuropil, travel along orthogonal commissures of the peripheral nervous system, and encapsulate synaptic glomeruli. Third, three neurotransmitter transporters are expressed in planarian glia. Orthologs of the proteins encoded by eaat2 and eaat3 have known roles in the transport of glutamate from the extracellular environment into the cytoplasm 175 where it is metabolized by orthologs of the enzyme encoded by glutamine synthetase (Anderson and Swanson, 2000), another gene expressed in planarian glia. Glutamate released from the presynaptic neuron can continue to activate glutamate receptors on the post-synaptic neuron, resulting in high intracellular levels of calcium and activation of pathways that lead to cellular damage (Manev et al., 1989). We hypothesize, given these data and by analogy to the function of astrocytes in other animals, that planarian glia uptake the excitotoxic neurotransmitter glutamate from areas near synapses to prevent damage to the nervous system. Constitutive expression of hh is required for expression of if1 and pcdh-19 in planarian glial cells during homeostasis. Upon inhibition of hh expression, cells cease transcription of if-1 and pcdh-19, with loss of detectable levels of ifI transcript within two weeks of RNAi followed by loss of detectable levels of IF-I protein by four weeks of RNAi. Inhibition of ptc results in transcription of if-1 and pcdh-19 in cells distributed throughout the ventral parenchyma, likely from derepression of the Gli-1 transcription factor. This indicates that cells competent to respond to hedgehog ligand normally exist outside the central nervous system. Additionally, the accumulation of if1 and pcdh-19 in cells outside the neuropil in ptc(RNAi) animals following ablation of new cell formation indicates that Hedgehog signaling induces expression of the two genes in existing competent cells. Our data support the possibility of at least one other glial cell type. Both gs and eaat2, genes abundantly expressed in fly glia (Freeman et al., 2003) and vertebrate glia (Cahoy et al., 2008), are co-expressed in cells outside the neuropil. These cells show a branched morphology by in situ hybridization similar to that found in cells expressing if and pcdh-19. Their concentration at the rim of the head where chemosensory neuron processes accumulate suggest 176 that if these cells are indeed glia, they may be a type specifically associated with the peripheral nervous system. Interestingly, in ptc(RNAi) animals we observed an increase in the number of gs+/eaat2+/if-1+/pedh-19F cells throughout the ventral parenchyma of the animal without a concomitant increase in the number of all cells expressing both eaat2 and gs. While these data lead us to hypothesize that if-1 and pcdh-19 are induced in cells already expressing eaat2 and gs upon inhibition of ptc, lineage tracing techniques that would allow us to definitively determine the origin of ectopic if-J*/pcdh-19F cells outside the neuropil are currently unavailable. The ability of Hedgehog signaling to modulate function in already existing glia has been described in vertebrates; Sonic hedgehog secreted from neurons has been proposed to regulate distinct subpopulations of astrocytes in adult brains (Garcia et al., 2010). The functions of if-1 and pcdh-19 are currently unknown. Inhibition of these genes has resulted in no observable behavioral or morphological defect. If if-1 and pcdh-19 confer specialized functions to planarian glia cells, then it is possible that the phenotype cannot be detected with our current assays. In reactive astrogliosis, the mammalian CNS response to injury, Sonic hedgehog is one of the inductive signals that induces expression of the gene encoding the intermediate filament GFAP (Sirko et al., 2013). Increased levels of GFAP protein result in an increase in cell size, which is necessary for the formation of an astrocytic scar at the wound site (Wilhelmsson et al., 2004). While there is currently no evidence that planarian glia are involved in wound response or that a glial scar is formed, this role of Hedgehog signaling in reactive astrogliosis raises a number of possible functions for if-1 that can be explored. 177 Ancestral roles of Hedgehog signalingin central nervous system development In central nervous system development, Hedgehog signaling plays a critical role in vertebrates but a seemingly less direct role in Drosophila. Sonic hedgehog expression in the vertebrate floor plate establishes distinct domains of transcription factor expression in the ventral neural tube. These domains first give rise to neurons and then, at later stages of development, glia (Dessaud et al., 2008; Yu et al., 2013). The dorsal-ventral distribution of homologous transcription factors in the developing Drosophilacentral nervous system bears a resemblance to the distribution seen in vertebrates (Cornell and Ohlen, 2000). Hedgehog, however, appears to play a role in the anterior-posterior patterning of neuroblasts rather than dorsal-ventral patterning (Bhat, 1999). Hedgehog signaling, while crucial to vertebrate neural tube patterning, has not been shown to be involved in specifying similar progenitor domains in Drosophila or planarians. The most parsimonious explanation is that while hedgehog was expressed in or near the midline of developing nervous systems of the common bilaterian ancestor, only in vertebrates was the pathway co-opted into dorsal-ventral patterning. The floor plate, which is induced by Sonic hedgehog secreted from the notochord, serves as a moderator of axonal midline crossing through the secretion of axon guidance cues (Colamarino and Tessier-Lavigne, 1995). Sonic hedgehog continues its involvement in neural patterning by acting as a chemoattractant and by mediating cellular responses to other guidance cues (Parra and Zou, 2010). The Drosophila midline glia are considered to be an analogous structure to the vertebrate floor plate due similar gene expression and roles in controlling midline crossing (Evans and Bashaw, 2010). Hedgehog is required for the decision to form posterior midline glia, whose function is still not fully understood, instead of anterior midline glia, which 178 develop into ensheathing glia in the Drosophila neuropil (Watson et al., 2011). A shared function of Hedgehog signaling among Deuterostomes, Ecdysozoans, and Lophotrochozoans appears to be in the control of glia near the midline. Thus, it will be interesting to see if planarian neuropil glia participate in axon guidance during regeneration, suggesting a possible ancestral role of Hedgehog signaling to all Bilaterians. Implications ofmolecular evidencefor planarianglial cells Planarians are an ideal model for the study of regeneration due to their unrivaled regenerative ability and the molecular tools developed for the system. The role of glia in regeneration in other systems has been described in vertebrates, where glia proliferate in response to brain injury, and in insects, where surface glia can reform the blood-brain barrier (Sofroniew, 2009; Treherne et al., 1984). Interestingly, astrocytic scars appear to counteract neural regeneration by blocking the extension of axons into the damaged region (Silver and Miller, 2004). Glial scars are unlikely to form in planarians, given that regeneration results in re-establishment of IF-i protein patterns seen in unwounded animals. Whether glia actively participate in repatterning the nervous system after injury is an interesting topic to explore, possibly leading to studies on both mechanisms of glia-neuron interaction and glial roles in neural network connectivity. If, on the other hand, glia passively extend their processes into existing neural architecture, then the mechanisms that guide glial cell development and migration may be studied instead. Several lines of evidence support the second hypothesis. IF-i+ processes are not seen deviating from axonal tracts, inhibition of hh affects glial processes but overall disruption to the neural network 179 has not been observed, and synapses are formed prior to IF-1 processes in the regeneration blastema. The work we present here opens the field to a number of opportunities for continued research. Glia are now gaining recognition as an active player in nervous system development, function, and regeneration, and not just as a passive cell type that glues neurons together (Freeman and Rowitch, 2013; Perea and Araque, 2010; Robel et al., 2011). Planarians are a tractable model organism that will be amenable to the study of glia in a highly regenerative system and member of the understudied Lophotrochozoan superphylum. Additionally, further characterization of planarian glia, especially in its developmental pathway, will surely provide more insight into the long-standing question of whether invertebrate and vertebrate glia share a common origin (Hartline, 2011). 180 181 00 U z z I * U "3 gapdh pc2 synapsin chat 1020HH Smedwi-I agat-1 * *mag ca *mat * collagen *1 mhc6 hh ptc smo sufu *m gli-I gli-2 gli-3 * * Log 2 Fold Change - Log 2 Fold Change x U 0 I U synapsin * nkx6 r* pcdh-19 if-I pax6b nkx2 - ptc hh pc2 * gapdh U -Z) Clb I pax6b jesjop nkx6 0A .eje nkx2 e. r 14 m z z 0 0 Eu Eu 11z I 1 W- .9 'n Figure 1. Hedgehog signaling in the planarian cephalic ganglia (A-C) Expression of neural transcription factors by fluorescent in situ hybridization (FISH). (A) Expression of nkx2 in medial domain and outer edge of cephalic ganglia lobes. (B) Expression of nlx6 in medial and lateral domains of lobes. (C) Expression of pax6b in lateral and outer edge of lobes. (D) Comparison of transcription factor domains and neuron subtype distribution between planarian cephalic ganglia (top) and vertebrate neural tube (bottom). Transverse view for both. Planarian cephalic ganglia (one lobe shown) oriented dorsal up, medial left, lateral right; neural tube oriented dorsal right. (E) Inhibition of hh (center column) or ptc (right column) shows no change in expression pattern of nkx2 (top row), nkx6 (middle row), or pax6b (bottom row) from controls (left column). (F) Head amputation for control Illumina libraries. Circle indicates portion of animal taken for RNA isolation. (G) Cephalic ganglia dissection was performed by creating an incision (inc) to peel away the dorsal epidermis, followed by removal of intestinal tissue (gut) and the pharynx (phx), revealing the cephalic ganglia (cg) and ventral nerve cords (vnc). (H) Bar graph depicting log2 fold change of selected markers between head fragment reads and cephalic ganglia reads. Statistically significant log2 fold change (pdj < 0.05) For a list of all analyzed genes, please see Table 1. (I) Bar graph indicated by asterisks. depicting log2 fold change of selected markers between cephalic ganglia reads from hh(RNAi) and ptc(RNAi) animals. observed for hh (pj = Statistically significant log2 fold change, indicated by asterisks, 6.9e-5), if-] (p4 = 0.04), and pcdh-19 (paj = 1.6e-24) in hh(RNAi) animals. For a list of all affected genes, please see Table 2. Anterior up, ventral surface shown for A-C and E. Anterior up, dorsal surface shown for F and G. 183 Figure 2 E ptc RNAi hh RNA Control RNAi F M if-1+ [3 if-1+/pcdh-19+ M pcdh-19+ - Control RNAi 4' K - hh RNAi I - ptc RNAi 0 I I I I U- 1000 2000 3000 4000 Cells / mm 2 inside neuropil 0 200 2 400 600 Cells mm outside neuropil if-I /ipcdh-19 184 Figure 2. Expression of if-1 and pcdh-19 in neuropil cells is dependent on Hedgehog signaling (A) Double FISH for if1 and pcdh-19 in wild type animals. DAPI-labeled nuclei (blue) enriched in cortical region surrounding cell-body sparse neuropil. (B) Detail of if-1 and pcdh-19 expression in the cephalic ganglia neuropil. (C) Double FISH for if1/pcdh-19 and ptc indicates co-expression. (D) Double FISH for if1/pcdh-J9 and hh indicates lack of co-expression. (E) Double FISH for if-1 and pcdh-19 in animals following inhibition of control gene, hh, or ptc. (F) Quantification of results from (E), with White dotted line delineates edge of animal. distribution of if1I only cells (green), pcdh-19+ only cells (magenta), and if1+/pcdh-19* cells (white). Within the neuropil, cells that express one or both markers are present at 2135.6 265.8 cells/mm2 in control RNAi conditions (n = 5 animals), 169.3 118.6 cells/mm 2 in hh RNAi conditions (n = 4 animals), and 3354.0+249.5 cells/mm2 inptc RNAi conditions (n = 5 animals). Differences were significant in both hh RNAi (p = 2.7e-6, two-tailed t test) and ptc RNAi (p = 7.1 e-5, two-tailed t test). In the head not including the neuropil region, cells that express one or both markers are present at 64.4+16.6 cells/mm 2 in control RNAi conditions (n = 5 animals), 1.5 2.9 cells/mm2 in hh RNAi conditions (n RNAi conditions (n = = 4 animals), and 465.4 68.7 cells/mm2 in ptc 5 animals). Differences were significant in both hh RNAi (p = 1.5e-5, two-tailed t test) and ptc RNAi (p = 1.4e-6, two-tailed t test). (G) Double FISH for if-1/pcdh-19 and pc2 indicates no co-expression. (H) Double FISH for if-1/pcdh-19 and syn indicates no coexpression. (I) Double FISH for if-1/pcdh-19 and panel of neural markers indicates no co- expression. For a list of all neuronal markers tested, please see Table 3. Anterior up, ventral surface shown for all. Scale bars: I00um for all. 185 Figure 3 U 186 Figure 3. if-1]/pcdh-19+ cells express neurotransmitter reuptake and metabolism genes (A-D) Double FISH showing overlapping expression of if-J'/pcdh-19+ neuropil cells with (A) glutamine synthetase, (B) GABA transporter gat-], (C) glutamate transporter eaat2, and (D) glutamate transporter eaat3. Detail of one representative cell included with each cephalic ganglia overview image, split into single channels. (E-I) Schematic indicates portion of animal shown. Images show one hemisphere of the cephalic ganglia and the lateral parenchymal space. White dotted line delineates edge of animal. Arrowheads denote double positive cells. (E) Double FISH for ptc and gs showing significant overlap within the neuropil and rare overlap outside the neuropil (F) Double FISH for ptc and gs showing no overlap in expression. (G-I) Double FISH indicating that gs is co-expressed with (G) eaat2, (H) eaat3, and (I) gat-1 in the majority of cells in the neuropil and in few cells outside the CNS. Anterior up, ventral surface shown for all. Scale bars: I00um for overviews; lOum for insets for A-D; 50um for E-I. 187 Figure 4 ptc RNAi Control RNAi 80 - J 60 - K0 - c08e 40 0>> 20 - (U (0 - 0 4 ** UN WC L Day 0 Day 2 Day 6 Day 4 C rC 188 Day 8 Figure 4. if-1]Ipcdh-19+ cells have long processes that closely associate with neurons (A) Whole-mount immunofluorescence for IF-1 protein in wild type untreated animals. Top dotted box refers to panel (B). Middle dotted box refers to panel (D). Bottom dotted box refers to panel (E). (B) Collapsed stack of IF-I immunofluorescence in the cephalic ganglia. Dotted box refers to panel (C). (C-E) Detail of IF-i immunofluorescence in (C) the cephalic ganglia neuropil, (D) the lateral ventral parenchyma of the trunk, and (E) the ventral nerve cord. (F-G) Immunofluorescence of IF-I (magenta) and a-tubulin (green) in (F) the head and (G) the lateral ventral parenchyma of wild type untreated animals. (H-1) Immunofluorescence of IF-I (magenta) and Synapsin (green) in (H) the head and (I) the ventral nerve cord of wild type untreated animals. (J) Immunofluorescence of IF-i (magenta) and Synapsin (green) following inhibition of hh, ptc, or a control gene. (K) Quantification of hh and ptc RNAi phenotype based on percentage of orthogonal axon bundles in contact with IF-I+ processes. In control RNAi animals, 15.1 5.1% of orthogonal axon bundles contained IF-lI processes (n = 5 animals). In hh RNAi animals, 2.1 2.8% of orthogonal axon bundles contained IF-1+ processes (n = 5 animals). In ptc RNAi animals, 61.4 7.8% of orthogonal axon bundles contained IF-lI processes (n = 4 animals). Difference between control and hh(RNAi) (p = 1.Oe-3, two-tailed t test) and control and ptc(RNAi) (p = 1.3e-5, two-tailed t test) were statistically significant. (L) Immunofluorescence for IF-i (magenta) and Synapsin (green) in anterior regeneration time course. White dotted line delineates edge of animal. Yellow dotted line delineates approximate amputation plane. Anterior right, ventral surface shown. Scale bars: 1 00um for A, B, F, H, and J; 5Oum for L; lOum for C, D, E, G, and I. 189 0 Q"0 0 0+0 0/ 00 0 0I 0 01 0) U 0U 1111 (11 CA N) 0 0 0 Cells /mm +0 0 0 0 -.L elsim 0 0U 10 2 2 0 L"1 0 0 wi 0 0 0 0 -- '1 -I (~u W z z ;0 0 0) C" 0 ptc Head hh Head ptc CG hh CG Log 2 Fold Change Log 2 Fold Change Log 2 Fold Change Log 2 Fold Change -L gs gat-I if-I Ipcdh-19 gs eaat2 if-I /pcdh-19 gat-I eaat3 if-1I/pcdh-19 eaat3 if-1I/pcdh-19 eaat2 Figure 5. hh inhibition does not ablate glial cells (A) Double FISH for if-1/pcdh-19 with gs (first row), gat-] (second row), eaat2 (third row), and eaat3 (fourth row) following inhibition of a control gene (first column), hh (second column), or ptc (third column). (B) Comparison of gene expression levels from cephalic ganglia (CG) or head fragment (Head) tissue samples following inhibition of hh or ptc. Expression level changes for each gene are not statistically significant. (C-D) Stacked bar graph of depicting number of cells expressing gs (C) or gat-] (D) per square millimeter following inhibition of a control gene, hh, or ptc. Bar sections denote ratio of if-1]/pcdh-19+ subpopulation (white) to if-1-/pcdh-19~ subpopulation (green). Differences between RNAi conditions not statistically significant (twotailed t test). Anterior up, ventral surface shown for all. Scale bars: I00um for A. 191 t0J if-I pcdh-19 Lethal Irradiation Reduced RNAi if-1 pcdh-19 IF-1 W Anterior Blastema (d6) if-1 pcdh-19 eaat2 eaat3 if-1 pcdh-19 gat-1 Anterior Blastema (d6) z z z 0 :3 l> ;> 0) C -n Figure 6. Hedgehog signaling is required for if-1 and pcdh-19 expression in planarian glia (A) Double FISH Qf-1/pcdh-19; gat-]) or triple FISH (f-1/pcdh-19; eaat2; eaat3) in d6 anterior blastemas of trunks following inhibition of control gene, hh, or ptc. White dotted line delineates edge of animal. Yellow dotted line delineates approximate amputation plane. (B) FISH of ifJ/pcdh-19 and immunofluorescence of IF-1 in animals following reduced RNAi treatment (fed dO, d4, d8, fixed d12) of control gene, hh, orptc. (C) FISH of if-/pcdh-19 in animals following lethal irradiation and subsequent RNAi treatment (irradiated dO, fed dO, d4, d8, fixed dl1). Anterior up, ventral surface shown for all. Scale bars: I00um for all. 193 Supplemental Figure S1 7131 3461 9961 6605 433 12264 648 69 821 1206 16532 122 13510 284 2627 331 2649 57 1530 348 1242 I 750 1636 2169 I 628 9223 13594 447 194 Supplemental Figure S1. Whole mount in situ hybridization (WISH) screen for genes with significant differential expression levels following inhibition of hh or ptc. Labels correspond to contig ID numbers listed in Supplemental Table S2. Anterior up, dorsal surface shown on left, ventral surface shown on right. Scale bars: 500um for all. 195 I 543 543 294 126 --'= 234 go 35 878~C 9998 |9 sapiens H. sapienslammn AJC 1411' .sain Oegns mu"- 963 C. slogans dfa-3 i2 82 H. robustaIamin2 H. robustalamiri1 famin ertn1 Ii"fi chan 7340 poei 861 C. intestinells protein 2 943 9396 23084 C. elegans mn-i L.iate ro>1 042 C. angns4gan ane protein H.po 1 8atspoen24 protn384 cyloplnmmucin 00L.Me .al Laplcantarmproteltila23084 itate filament 8 10. L. D. molanopaster lamin C C. voctonsis protein 170348 sapiens lammn 81 motanogastor tannin N. H moic CCslogans ibb-1 Ob-an C.ean iai dilfmentt1 ntMd 938 H. r.busto ChaustC etlme nntrmd 0lgn 1000 robusta1000 gigantoe protein 284411 H, 543 76 H. robust&a o otuuayt cytoplmamiedtlem C. I. pfganlsm prate n 179001 D. u383 H. modicirialsmacrfli L.. 34900 1O0O0 921~~H H, tobusaactoplasmc mntermediate Ifiaent 5 H8 m.mdcnu H 92 medctinalis gierin H. robWW cytplamtc intermediate faMwnW 3 H. robusta cytopasmtc Intrmediate iamont 9 68 H. tnstinanllsprotein 297340 C.ninsstina s protein 29 448C C. C. H. sapiensdesmin -p--s 9meti 173 H.saien vme~inH. sapiens nwuomt Cn2281 00 F414.C 4 4.9tsinlspoei r91812ane H. s. logans #ta-1 H 10383 73--. 295 $a ~~484 601 C. t";et 372 12H. 160 518 504 ~ ~ 9 390 57 351 584 1F -- M92H. so to 925 2E85 295 1000 H. Saplons keratin sapiens keratin 5 intestinallsprotein 206773 H. 1000 sOwd-keain1 - H. sapiens keratin 9 C. C. slegans ifc-2 ifc-1 tlet protein 220856 oftgens C. C elaainslfd-2 elgans ifd-1 C. C netnlspoen264 oteans tpIII 0 C) In 0 V 0 0 0 070 Supplemental Figure S2. Phylogenetic tree for cytoplasmic intermediate filaments Maximum likelihood tree with 1,000 bootstrap replicates of cytoplasmic intermediate filament genes from all three superphyla of the bilateria, including S. mediterranea if-1. intermediate filament genes were used as an outgroup to root the tree. 197 Nuclear Supplemental Figure S3 B A Head rim ... . . . . F eripheral par enchyma if-I DCdh-19 if-1 Dcdh-19 ii Between cephalic ganglia iv Nerve cord Between ne rve cords C Control RNAi Control RNAi ptc RNAi I' 198 ptc RNAi Supplemental Figure S3. if-1]/pcdh-19+ cells are found in multiple regions (A) Schematic of the body and central nervous system of the planarian. Red dotted boxes indicate regions of interest focused on in (B) and (C). (B) Double FISH for if-1 and pcdh-19 in wild type untreated animls. Images show detail of single cells near the periphery of the head (i), in the parenchyma at the ventral midline between the lobes of the cephalic ganglia (ii), within the ventral lateral parenchyma of the trunk (iii), and embedded in the ventral nerve cord (iv). (C) Double FISH for if-1 and pcdh-19 in animals following inhibition of control gene orptc. Images show detail of the head rim region (i) and the tail region between the ventral nerve cords (v). Anterior up, ventral surface shown for all. Scale bars: 10um for A; 50um for B. 199 Supplemental Figure S4 A gli-2 RNAi gli-I RNAi Control RNAi gli-3 RNAi <L b1 20 D 0 200) Supplemental Figure S4. Further characterization of if-1+/pcdh-19r cells (A) FISH for if-1/pcdh-J9 in animals following inhibition of control gene, gli-1, gli-2, or gli-3. DAPI, nuclei (blue). Anterior up, ventral surface shown for all. (B) FISH for if-1/pcdh-19 in d6 anterior blastemas and trunk region ventral to the pharynges of trunk fragments following inhibition of control gene, hh, or ptc. Images of anterior blastemas show accumulation of if1*/pcdh-J9r cells during regeneration. Images of pharyngeal region show presentation of hh or ptc phenotype. Scale bars: I00um for all. 201 Supplemental Figure S5 A if-I Dcdh-19 Dc2 DAPI B if-I Dcdh-19 svn DAP C if-1 pcdh-19 202 Supplemental Figure S5. if-1 and pcdh-19 expression does not overlap with neuronal marker expression (A-B) Double FISH for if-1/pcdh-19 and (A) pc2 or (B) syn. Detail of four cell clusters shown for each. Images show merged image in top left quadrant and single channels for remaining quadrants. DAPI, nuclei (blue). (C) Double FISH for if-J/pcdh-19 and other described neuronal markers in wild type untreated animals. Anterior up, ventral surface shown for all. Scale bars: I0um for A, B; I00um for C. 203 Supplemental Figure S6 gs C t-1 E . tat 2 G eamt3 0 c\< z J K 2 z OZ NC CQ~ CO Z qCOl 204 Supplemental Figure S6. Further characterization of markers for if-1]Ipcdh-19+ cells (A-H) WISH for (A) gs, (C) gat-], (E) eaat2, and (G) eaat3 with FISH for single cell detail for (B) gs, (D) gat-i, (F) eaat2, and (H) eaat3. DAPI, nuclei (blue). (I-K) Double FISH for ptc and (I) gat-] (J) eaat2, and (K) eaa3 in wild type untreated animals. (L-N) Double FISH for pc2 and (L) gat-], (M) eaat2, and (N) eaat3 in wild type untreated animals. (0) RNA probe specificity controls for gs, gat-], eaat2, and eaat3. 4/4 gs(RNAi) animals display reduced gs expression in the CNS compared to unc-22(RNAi) controls animals. 4/5 gat-J(RNAi) animals display slightly reduced gat-1 expression in the CNS compared to unc-22(RNAi) controls animals. 9/9 eaat2(RNAi) animals display reduced eaat2 expression in the CNS compared to unc-22(RNAi) controls animals. 6/6 eaat3(RNAi) animals display reduced eaat3 expression in the CNS compared to unc-22(RNAi) controls animals. Anterior up, ventral surface shown for A, C, E, G. Anterior up, ventral surface of right cephalic ganglia lobe and lateral head parenchyma shown for I-N. Anterior left, ventral surface shown for 0. Scale bars: 500um for A, C, E, G, 0; 10um for B, D, F, H; 50um for I-N. 205 Supplemental Figure S7 A Control RNAi if-I RNAi B Pcdh-19 RNAi 0 C C Li- D C Control RNAi ptc RNAi IE 0 (U C Co U- (U Co U- 206 Control RNAi ptc RNAi Supplemental Figure S7. IF-i protein accumulates in specific tissues (A) Immunofluorescence of IF-I and Synapsin in animals following inhibition of control gene, if-1, and pcdh-19. (B) Immunofluorescence of IF-I in dorsal (top) domain and the ventral (bottom) domain of the ventral nerve cord. Arrowheads depict lacunae in the IF-1 process bundle. (C) FISH of if-1/pcdh-19 and immunofluorescence of IF-i in the cephalic ganglia of wild type untreated animals. (D) Detail of immunofluorescence of IF-I and Synapsin in lateral ventral parenchyma of the trunk following inhibition of control gene or ptc. (E) Detail of immunofluorescence of IF-I and Synapsin in the head rim of animals following inhibition of control gene or ptc. Dotted box in top row refers to corresponding image in bottom row. Anterior up, ventral surface shown for A, C, D, E. Anterior right, ventral surface shown for B. Scale bars: I00um for A, C, top row of E; IOum for B, D, bottom row of E. 207 Supplemental Figure S8 unc-22 RNAi olig2 RNAi jog nkx2 RNAi gcml-1 RNAi S S gcml-2 RNAi b 9, ee 208 Supplemental Figure S8. Inhibition of if-1 or pcdh-19 does not produce any observable phenotype WISH of if-1/pcdh-19 in whole animals or d6 regenerating head, trunk, and tail fragments following inhibition of control gene or putative glial transcription factor. For each RNAi condition, left image shows intact animal, top right image shows head fragment, middle right image shows trunk fragment, bottom right image shows tail fragment. surface shown. 209 Anterior up, ventral Table S1. Enrichment of neuronal markers and depletion of non-neuronal markers in cephalic ganglia tissue libraries CNS control (RHAI) vs. Head control (RNAI) Conto ID ddSmed_v4_78_0_1 ddSmed_v4_41965_0_1 dd._Smed_v4_7063_0_2 ddSmed_.v4_9474.0.1 ddSmed_v4_10354_0_1 dd_Smed-V4j7470_0_1 dd Smed v4 46372_0_1 ddSmed_v4_17566_.0.1 ddSmed_v4_3135_0_1 ddSmedyv4_6208_0_1 ddSmnedv4_1566_0_1 ddSmed-v4_5133_0_2 dd_Smedv4_9795_0_1 dd_Smed-V4_14852_0_1 dd_Smedv4_8392_0_2 dd_Smedv4_16581_0_1 dd_Smedv4_2688_0_1O ddSmed_v4_28398_0_1 ddSmedv4_7326_0_1 ddSmedv4_14865_0_1 ddSmedyv4_659_0_1 ddSmed-v4_756_0_1 dd_.Smedv4_899_0_1 ddSmedv4_4575_0_1 ddSmedv4_9774_0_1 ddSmedv4_8234_0_1 dd_Srned_v4_4841_0_1 ddSmed v4_34399_0_1 ddSmedv4_19040_0_1 dd_Smedyv4_17385_0_1 ddSmedv4_48430_0_1 ddSmedv4769_0_1 dd_Smed_v4_702_0_1 dd_Smed_v4_3131_0_1 ddSmedv4_7223_0_1 dd_.Smedv4_907_0_1 ddSmed v4 249 ,,0 1 , , Annotation gapdh hedgehog patched smoothened sufu gil-1 gli-2 gli-3 synapsin choline acetyltransferase prohonnone convertase 2 1020HH netrin I nefrin 2 tryptophan hydroxylase tyrosine hydroxylase beta-catenin wnbP-1 wntP-2 wntP-3 smedwi-I smedwi-2 agat-1 cubulin sine oculis pou2/3 carbonic anhydrase tyrosinase, distal-less sp6-9 ovo marginaladhesive gland collagen mboat2 mboat7 met mhc6 Expression References Global CNS Rink 2009 CNS, PHX, PCYM CNS, PHX, PCYM PHX, PCYM GUT, MRG Rink 2009 Rink 2009 Rink 2009 Rink 2009 Rink 2009 Rink 2009 CNS, PNS CNS, PNS CNS, PNS CNS CNS NP CNS, PR CNS, PHX Global Tail Tail PHX NB NB PCYM NPH NPH NPH NPH PR PR, PCYM PR PR RIM MUS GUT GUT GUT PHX 210 Nishimura 2010 Collins 2010 Collins 2010 Cebuda 2005 Cebria 2005 Curie 2013 Fraguas 2011 Petersen 2008 Petersen 2008 Petersen 2008 Petersen 2008 Reddien 2005 Reddien 2005 Eisenhoffer 2008 Scmone 2011 Scimone 2011 Scimone 2011 Scimone 2011 Lapan 2011 Lapan 2011 Lapan 2011 Lapan 2012 Zayas 2010 Witchley 2013 Wenemoser 2010 Lop Fold Change pul -0.11 5.4E-01 5.85 -0.42 0.87 0.41 -2.10 nla 5.7E-57 3.9E-02 3.8E-07 1.OE-01 3.3E-29 2.3E-01 0.40 1.41 2.82 2.65 6.35 1.50 2.51 0.33 2.92 -1.67 1.16 0.36 -3.06 -1.58 -0.75 -2.09 -3.49 0.03 -1.71 -2.48 -2.43 1.77 1.36 -2.77 -4.48 -4.40 0.53 1.24 -2.05 -2.73 3.8E-01 1.7E-25 6.1E-95 5.1E-88 3.OE-304 3.6E-16 1.2E-21 2.7E-01 3.OE-49 9.8E-33 3.2E-01 3.5E-01 1.3E-02 2.OE-31 1.5E-05 2.5E-51 4.8E-104 1.OE+00 7.5E-12 2.1E-34 2.OE-07 1.6E-10 1.4E-04 4.2E-01 4.3E-196 7.4E-197 8.7E-04 3.4E-15 3.1E-44 3.2E-93 Supplemental Table S1. Enrichment of neuronal markers and depletion of non-neuronal markers in cephalic ganglia tissue libraries For each gene, general expression pattern and log2 fold enrichment of CNS tissue expression over head fragment expression is listed. CNS, central nervous system; GUT, intestinal tract; MUS, muscle layer; NB, neoblasts; NP, neuropil; NPH, nephridia; PCYM, parenchyma; PHX, pharynx; PR, photoreceptors; RIM, body peripheral edge. References for previously published genes are listed (Cebria and Newmark, 2005; Collins et al., 2010; Currie and Pearson, 2013; Eisenhoffer et al., 2008; Fraguas et al., 2011; Lapan and Reddien, 2011; 2012; Nishimura et al., 2010; Petersen and Reddien, 2009; Reddien et al., 2005b; Rink et al., 2009; Scimone et al., 2011; Witchley et al., 2013; Zayas et al., 2010). 211 ........ ........ k) Contig ID ddSmed v4_7131 0 1 ddSmedv4_3451_0_1 ddSmed v4_99610_1 ddSmedv4_6605_0_1 ddSmed_v4_433_0_1 ddSmed_v4_12254_0_1 ddSmedv4_648_0_1 ddSmedv4_69_0_2 dd Smed v4_821 0 1 ddSmedv4_332_0_1 ddSmedv4_1206_0_1 ddSmedv4_16532_0_1 ddSmedv4 122 1 1 dd_Smed_v4_13510_0 1 ddSmedv4 284 0 1 ddSmedv4_493_0_1 ddSmedv4_2627 0 1 dd_Smed_v4_331_0_1 dd Smed v4 2649 0 1 dd_Smed_v4_57_0_1 ddSmedv4_750_0_1 ddSmedv4_16360_1 ddSmedv4_2169_0_1 ddSmed v4_1530 0 1 ddSmed_v4_348_0_1 ddSmedv4_1242 0 1 ddSmed v4_628_0_1 ddSmedv4_9223 0 1 ddSmedv4_13594_0_ 1 dd Smed v4 447 0 1- Annotation GLIPRi-like protein 1-like (Schmidtea mediterranea) FAM115A (Homo sapiens) protocadherin-19 (Mus musculus) No Similarity GLIPR1-like protein 1-like (Schmidtea mediterranea) Protein IFA-1 (Caenorhabdiis elegans) tubulin alpha 1A chain (Homo sapiens) No Similarity protein disulfide-isomerase AS (Mus musculus) prog-1 (Schmidtea mediterranea) hematopoetic prostaglandin D synthase (Mus musculus) No Similarity lysosomal acid lipase/cholesteryl ester hydrolase precursor No Similarity Saposin-related (Drosophila melanogaster) reticulocalbin-1 (Schmidtea mediterranea) E3 ubiquitin-protein ligase MYLIP (Homo sapiens) No Similarity fibrillin-2 (Homo sapien) WAP four-disulfide core domain protein 6B (Mus musculus) No Similarity zonadhesin (Homo sapiens) No Similarity GLIPR1-like protein 1-like (Schmidtea mediterranea) peptidyl-prolyl cis-trans isomerase C (Mus musculus) peptidyl-prolyl cis-trans isomerase B (Mus musculus) SCO-spondin-like (Mus musculus) No Similarity No Similarity No Similaritv 2.00E-44 5.00E-46 7.00E-46 3.00E-25 4.00E-33 8.00E-142 7.00E-12 2.00E-64 0.00E+00 3.00E-05 9.00E-104 -1.09 0.51 -0.38 0.21 -0.65 0.33 -0.82 0.05 -1.15 -0.43 -1.84 -0.50 -1.12 -0.50 -0.35 -0.19 -3.24 -3.56 0.26 -1.15 1.OOE-24 2.00E-32 0.OOE+00 2.00E-44 1.00E-82 0.00E+00 -3.00 Change Log2 Fold -2.90 -2.80 -2.40 -2.16 -2.10 -1.19 -1.19 -1.18 -1.16 E Value 2.00E-44 6.00E-38 1.OOE-03 padj 4.58E-21 4.87E-24 1.61 E-24 8.60E-12 1.29E-1 3 0.044 8.02E-03 8.02E-03 0.043 0.023 0.044 0.015 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 1.000 0.851 1.000 1.000 CNS hh (RNA) vs. CNS control (RNAI) Change 1.47 1.15 0.26 1.63 1.40 0.18 1.19 -0.93 1.28 -0.83 -0.13 -2.53 -4.26 -0.91 -0.73 0.90 1.00 1.26 1.29 1.38 1.49 1.55 1.65 1.62 2.00 2.29 2.65 2.75 2.85 2.90 Log2 Fold pad 3.13E-07 0.105 1.000 2.69E-10 2.13E-05 1.000 0.012 0.175 0.850 1.000 1.000 6.96E-14 4.85E-06 8.61 E-05 0.010 0.033 2.97E-05 8.04E-04 2.73E-09 3.88E-04 3.81 E-09 0.005 6.10E-15 1.50E-03 3.99E-06 3.81E-08 4.65E-40 1.IOE-04 2.42E-06 1.71E-07 CNS ptc (RNAI) vs. CNS control (RNAI) Change 0.25 -0.35 3.75 2.25 1.23 4.68 -1.61 -1.15 -0.51 0.46 2.86 7.22 -0.08 6.99 0.72 -1.89 -0.31 -0.45 -1.94 -0.20 0.83 -0.55 0.78 0.84 -0.97 -0.85 -3.34 2.21 2.76 -1.01 Pad 0.737 0.528 9.34E-06 0.034 0.132 7.84E-09 4.86E-12 1.12E-07 0.250 0.242 7.34E-1 2 1.01 E-09 0.941 3.82E-33 0.015 1.24E-40 0.147 0.321 4.75E-46 0.733 0.145 0.434 0.180 0.253 0.016 0.078 6.77E-95 0.143 0.094 0.119 CNS control (RNAI) vs. Head control (RNAI) Log2 Fold Table S2. Genes with significant differential expression levels following inhibition of hh or ptc. Supplemental Table S2. Genes with significant differential expression levels following inhibition of hh or plc Criteria for selecting genes was (1) adjusted p value (padj) of less than 0.05, (2) greater than 1000 RPKM, and (3) greater than 2 fold change in expression level either between control and hh(RNAi) or between control and ptc(RNAi). Annotations by best BLAST hit listed for each gene; No Similarity listed if no significant BLAST hit was found. Two genes, prog-J and reticulocalbin-1, were described in planaria previously (Eisenhoffer et al., 2008; Zayas et al., 2010). Blue text indicates greater than 2 fold change in expression level. Green text indicates enrichment in CNS tissue versus whole head fragment. 213 v7iz I&I- &I &I &I &I I&& I&f ~ 1I I II If X0 -&~- II & l I& I & If If01 00 I I II I [II00 I f41 40j-h8hn ~ 4 eel ~A 0 I" to.. lbi*I .4 NJ -n -n 8 tg g -W -S "vM tog -A "M w ; -& .,0 -4 N g "-qw88 " h. ! 0boo 5* VP9PP I. M! I PP~b~bb~bPPb.bbbPbbb -h 74 1" I w i4ioIs C13 Mg;! N Zbe i 06 Supplemental Table S3. Neuronal markers used in co-expression studies For each gene, log2 fold change between control and hh(RNAi) and between control and ptc(RNAi) cephalic ganglia samples are listed. References for previously published genes are listed (Cebrih and Newmark, 2005; Collins et al., 2010; Cowles et al., 2013; Currie and Pearson, 2013; Fraguas et al., 2011; MArz et al., 2013; Nishimura et al., 2010). 215 Materials and Methods PlanarianCulture Animals were maintained in Ix Montjuic planarian water at 200 C. S2F1L3F2 sexual animals were used in dissection experiments and CIW4 asexual animals were used in all other experiments. RNA Interference 300ml of bacterial culture expressing dsRNA was pelleted and mixed with lml of 70% liver in planarian water as previously described (Reddien et al., 2005a). Asexual animals were fed 6 times at four-day intervals unless otherwise noted. Sexual animals were fed 12 times at four-day intervals. A gene not present in the planarian genome, unc-22, was used as a control in each RNAi experiment. Dissection After four days of starvation, the animals were immersed in a 0.33N HCl solution for 30 seconds, washed once in PBS, washed once in PBS + 1% BSA, and immobilized dorsal-side up on a silicon elastomer pad with insect pins. One longitudinal incision and one lateral incision were made through the dorsal epidermis near the base of the pharynx. The epidermis was peeled away to expose the pharynx and a layer of gut tissue overlying the central nervous system. Collected tissue was placed immediately in Trizol Reagent (Invitrogen) and stored at -80C until all samples were processed. 216 mRNA-Seq Analysis Illumina libraries were generated with 1.Oug total RNA from head fragments and 0.2ug total RNA from dissected CNS samples using Illumina TruSeq kits. Libraries were prepared in duplicate and sequenced with Illumina HiSeq. Reads were mapped to the Dresden Assembly with Bowtie and read counts were analyzed with DESeq R package (10% false discovery rate). MolecularBiology cDNA libraries from planarian multi-stage total RNA were synthesized using SuperScriptIll (Invitrogen). DNA fragments were amplified from cDNA with primers designed from Dresden Assembly sequences and cloned into pGEM (Promega). For RNAi constructs, inserts were amplified from pGEM constructs and introduced using BP clonase (Invitrogen) into a Gateway vector containing flanking LacZ inducible promoters. Full-length sequences for if-1 and pcdh-19 were obtained with 5' and 3' RACE (Ambion). Phylogenetics Amino acid sequences for full-length if-1 were aligned to previously published intermediate filament protein sequences using MUSCLE (EMBL-EBI) with default parameters (Kuo and Weisblat, 2011). Poorly aligned segments were eliminated using GBlocks (Castresana Lab). Phylogenetic trees were constructed using maximum likelihood with 1000 bootstraps and adjusted with FigTree. 217 RNA in situ Hybridizationand Immunofluorescence RNA probes were synthesized with digoxygenin, fluorescein, or dinitrolphenyl nucleotides (Roche). For whole-mount in situ hybridization (WISH) and fluorescent in situ hybridization (FISH), animals were fixed in 4% formaldehyde according to published protocols (Pearson et al., 2009). FISH protocols were followed as previously described using RNA probe dilutions at 1:1000, anti-digoxygenin peroxidase at 1:500, anti-fluorescein peroxidase at 1:300, and antidinitrophenyl at 1:100 (Pearson et al., 2009). Probes for if- 1 and pcdh-19 typically combined into single channel to improve coverage and signal intensity. Rabbit polyclonal antibodies for IF-1 protein were raised against peptides with amino acid sequence "TENNQIENSKEKTVC" (GenScript). For immunofluorescence, animals were fixed in Carnoy's fixative and stained as previously described (Newmark and Sanchez Alvarado, 2000; Wenemoser and Reddien, 2010). Anti-IF-1 antibody was used at 0.4ug/ml, anti-Synapsin antibody (Anti-SYNORF, Developmental Studies Hybridoma Bank) at 1:1000, and anti-a-tubulin (DM1 A, NeoMarkers) at 1:1000, and were developed with tyramide signal amplification (Invitrogen). To detect nuclei, animals were stained in DAPI overnight prior to mounting in VectaShield (Vector Labs). Samples were imaged by confocal microscopy (Zeiss LSM 700) and processed with Fiji/ImageJ. Cell counts for neuropil regions were normalized to cross-sectional area of the cephalic ganglia lobes. Cell counts for heads excluding the neuropil region were normalized to the cross- sectional area of the head. 218 RNA Probe Specificity RNA probe specificity for a target gene was determined by performing whole-mount in situ hybridization on animals following inhibition of the gene. Animals were fed one to four times with bacteria expressing dsRNA for a control gene or the target gene. After the last feeding, the animals were given five days to clear the intestine of lingering RNAi food. Irradiation Animals were exposed to 6,000 rads of ionizing radiation (GammaCell) to ablate all dividing cells. Treated animals were subsequently fed dsRNA-expressing bacteria three times at dO, d4, and d8. Animals displayed signs of anterior regression at dl I and were fixed immediately. 219 References Adell, T., Sal6, E., Boutros, M., and Bartscherer, K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development (Cambridge, England) 136, 905-910. Anderson, C.M., and Swanson, R.A. (2000). Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32, 1-14. Bhat, K.M. (1999). Segment polarity genes in neuroblast formation and identity specification during Drosophilaneurogenesis. Bioessays 21, 472-485. Boeckh, J., and Tolbert, L.P. (1993). Synaptic organization and development of the antennal lobe in insects. Microsc. Res. Tech. 24, 260-280. Cahoy, J.D., Emery, B., Kaushal, A., Foo, L.C., Zamanian, J.L., Christopherson, K.S., Xing, Y., Lubischer, J.L., Krieg, P.A., Krupenko, S.A., et al. (2008). A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. Journal of Neuroscience 28,264-278. Cebrii, F., and Newmark, P.A. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development (Cambridge, England) 132, 3691-3703. Colamarino, S.A., and Tessier-Lavigne, M. (1995). The role of the floor plate in axon guidance. Annu. Rev. Neurosci. 18, 497-529. Collins, J.J., III, Hou, X., Romanova, E.V., Lambrus, B.G., Miller, C.M., Saberi, A., Sweedler, J.V., and Newmark, P.A. (2010). Genome-Wide Analyses Reveal a Role for Peptide Hormones in Planarian Germline Development. PLoS Biol 8, e1000509. Cornell, R.A., and Ohlen, T.V. (2000). Vnd/nkx, ind/gsh, and msh/msx: conserved regulators of dorsoventral neural patterning? Current Opinion in Neurobiology 10, 63-71. Cowles, M.W., Brown, D.D.R., Nisperos, S.V., Stanley, B.N., Pearson, B.J., and Zayas, R.M. (2013). Genome-wide analysis of the bHLH gene family in planarians identifies factors required for adult neurogenesis and neuronal regeneration. Development (Cambridge, England) 140, 4691-4702. Currie, K.W., and Pearson, B.J. (2013). Transcription factors lhxl/5-1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development (Cambridge, England) 140, 3577-3588. Deitmer, J.W., Rose, C.R., Munsch, T., Schmidt, J., Nett, W., Schneider, H.P., and Lohr, C. (1999). Leech giant glial cell: functional role in a simple nervous system. Glia 28, 175-182. 220 Dessaud, E., McMahon, A.P., and Briscoe, J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development (Cambridge, England) 135, 2489-2503. Eisenhoffer, G.T., Kang, H., and SAnchez Alvarado, A. (2008). Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea.Cell Stem Cell 3, 327-339. Evans, T.A., and Bashaw, G.J. (2010). Axon guidance at the midline: of mice and flies. Current Opinion in Neurobiology 20, 79-85. Featherstone, D.E. (2011). Glial solute carrier transporters in Drosophilaand mice. Glia 59, 1351-1363. Fraguas, S., Barberin, S., and CebriA, F. (2011). EGFR signaling regulates cell proliferation, differentiation and morphogenesis during planarian regeneration and homeostasis. Developmental Biology 354, 87-101. Frank, M., and Kemler, R. (2002). Protocadherins. Current Opinion in Cell Biology 14, 557-562. Freeman, M.R., Delrow, J., Kim, J., Johnson, E., and Doe, C.Q. (2003). Unwrapping Glial Biology: Gem Target Genes Regulating Glial Development, Diversification, and Function. Neuron. Freeman, M.R., and Rowitch, D.H. (2013). Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613-623. Garcia, A.D.R., Petrova, R., Eng, L., and Joyner, A.L. (2010). Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. Journal of Neuroscience 30, 1359713608. Golubev, A.I. (1988). Glia and neuroglia relationships in the central nervous system of the Turbellaria (Electron microscopic data). Fortschr Zool 36, 31-37. Hartenstein, V. (2011). Morphological diversity and development of glia in Drosophila.Glia 59, 1237-1252. Hartline, D.K. (2011). The evolutionary origins of glia. Glia 59, 1215-1236. Herrmann, H., Bar, H., Kreplak, L., Strelkov, S.V., and Aebi, U. (2007). Intermediate filaments: from cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 8, 562-573. Hosoya, T., Takizawa, K., Nitta, K., and Hotta, Y. (1995). glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82, 1025-1036. Ingham, P.W., Nakano, Y., and Seger, C. (2011). Mechanisms and functions of Hedgehog 221 signalling across the metazoa. Nat Rev Genet 12, 393-406. Kuo, D.-H., and Weisblat, D.A. (2011). Intermediate filament genes as differentiation markers in the leech Helobdella. Dev. Genes Evol. 221, 225-240. Lapan, S.W., and Reddien, P.W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genet. 7, e1002226. Lapan, S.W., and Reddien, P.W. (2012). Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep 2, 294-307. Leise, E.M. (1990). Modular construction of nervous systems: a basic principle of design for invertebrates and vertebrates. Brain Res. Brain Res. Rev. 15, 1-23. Manev, H., Favaron, M., Guidotti, A., and Costa, E. (1989). Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 36, 106-112. MArz, M., Seebeck, F., and Bartscherer, K. (2013). A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development (Cambridge, England) 140, 4499-4509. Morgan, T.H. (1898). Experimental studies of the regeneration of Planariamaculata. Dev. Genes Evol. 7,364-397. Morita, M., and Best, J.B. (1965). Electron microscopic studies on Planaria. II. Fine structure of the neurosecretory system in the planarian Dugesiadorotocephala.J. Ultrastruct. Res. 13, 396408. Morita, M., and Best, J.B. (1966). Electron microscopic studies of planaria. III. Some observations on the fine structure of planarian nervous tissue. J. Exp. Zool. 161, 391-411. Nakazawa, M., CebriA, F., Mineta, K., Ikeo, K., Agata, K., and Gojobori, T. (2003). Search for the evolutionary origin of a brain: planarian brain characterized by microarray. Mol. Biol. Evol. 20, 784-791. Newmark, P.A., and Sanchez Alvarado, A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental Biology 220, 142-153. Nishimura, K., Kitamura, Y., Taniguchi, T., and Agata, K. (2010). Analysis of motor function modulated by cholinergic neurons in planarian Dugesiajaponica.Neuroscience 168, 18-30. Oikonomou, G., and Shaham, S. (2011). The glia of Caenorhabditiselegans. Glia 59, 12531263. Parra, L.M., and Zou, Y. (2010). Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat. Neurosci. 13, 29-35. 222 Pearson, B.J., Eisenhoffer, G.T., Gurley, K.A., Rink, J.C., Miller, D.E., and SAnchez Alvarado, A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev. Dyn. 238,443-450. Perea, G., and Araque, A. (2010). GLIA modulates synaptic transmission. Brain Res Rev 63, 93102. Petersen, C.P., and Reddien, P.W. (2009). A wound-induced Wnt expression program controls planarian regeneration polarity. Proceedings of the National Academy of Sciences 106, 1706 117066. Pfeiffer, S.E., Warrington, A.E., and Bansal, R. (1993). The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3, 191-197. Qi, Y., Cai, J., Wu, Y., Wu, R., Lee, J., Fu, H., Rao, M., Sussel, L., Rubenstein, J., and Qiu, M. (2001). Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development (Cambridge, England) 128, 2723-2733. Reddien, P.W., and SAnchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725-757. Reddien, P.W., Bermange, A.L., Murfitt, K.J., Jennings, J.R., and SAnchez Alvarado, A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635-649. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and SAnchez Alvarado, A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. Reinecke, M. (1976). The glial cells of the cerebral ganglia of Helix pomatia L. (Gastropoda, Pulmonata). Cell Tissue Res 169. Reuter, M., Mhntyli, K., and Gustafsson, M.K.S. (1998). Organization of the orthogon - main and minor nerve cords - Springer. Hydrobiologia 383, 175-182. Rink, J.C., Gurley, K.A., Elliott, S.A., and Sanchez Alvarado, A. (2009). Planarian Hh Signaling Regulates Regeneration Polarity and Links Hh Pathway Evolution to Cilia. Science 326, 14061410. Robel, S., Berninger, B., and G6tz, M. (2011). The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 12, 88-104. Rowitch, D.H. (2004). Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 5, 409-419. Ruiz i Altaba, A., Nguyen, V., and Palma, V. (2003).The emergent design of the neural tube: prepattern, SHH morphogen and GLI code. Curr. Opin. Genet. Dev. 13, 513-521. 223 Sanchez Alvarado, A., and Newmark, P.A. (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. U.S.a. 96, 5049-5054. Scimone, M.L., Srivastava, M., Bell, G.W., and Reddien, P.W. (2011). A regulatory program for excretory system regeneration in planarians. Development (Cambridge, England) 138, 43874398. Silver, J., and Miller, J.H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146-156. Sirko, S., Behrendt, G., Johansson, P.A., Tripathi, P., Costa, M., Bek, S., Heinrich, C., Tiedt, S., Colak, D., Dichgans, M., et al. (2013). Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell 12, 426-439. Sofroniew, M.V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638-647. Sofroniew, M.V., and Vinters, H.V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119, 7-35. Treherne, J.E., Harrison, J.B., Treherne, J.M., and Lane, N.J. (1984). Glial repair in an insect central nervous system: effects of surgical lesioning. J. Neurosci. 4, 2689-2697. Varjosalo, M., and Taipale, J. (2008). Hedgehog: functions and mechanisms. Genes Dev. 22, 2454-2472. Wagner, D.E., Wang, I.E., and Reddien, P.W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816. Watson, J.D., Wheeler, S.R., Stagg, S.B., and Crews, S.T. (2011). Drosophilahedgehog signaling and engrailed-runt mutual repression direct midline glia to alternative ensheathing and non-ensheathing fates. Development (Cambridge, England) 138, 1285-1295. Wenemoser, D., and Reddien, P.W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental Biology 344, 979-991. Wilhelmsson, U., Li, L., Pekna, M., Berthold, C.-H., Blom, S., Eliasson, C., Renner, 0., Bushong, E., Ellisman, M., Morgan, T.E., et al. (2004). Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. Journal of Neuroscience 24, 5016-5021. Witchley, J.N., Mayer, M., Wagner, D.E., Owen, J.H., and Reddien, P.W. (2013). Muscle cells provide instructions for planarian regeneration. Cell Rep 4, 633-641. Wolff, E., and Dubois, F. (1948). Sur la migration des cellules de regdndration chez les planaires. Rev Suisse Zool 55, 218-227. 224 Yazawa, S., Umesono, Y., Hayashi, T., Tarui, H., and Agata, K. (2009). Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proceedings of the National Academy of Sciences 106,22329-22334. Yu, K., McGlynn, S., and Matise, M.P. (2013). Floor plate-derived sonic hedgehog regulates glial and ependymal cell fates in the developing spinal cord. Development (Cambridge, England) 140, 1594-1604. Zayas, R.M., Cebria, F., Guo, T., Feng, J., and Newmark, P.A. (2010). The use of lectins as markers for differentiated secretory cells in planarians. Dev. Dyn. 239, 2888-2897. Zhou, Q., and Anderson, D.J. (2002). The bHLH transcription factors OLIG2 and OLIGI couple neuronal and glial subtype specification. Cell 109, 61-73. 225 226 Chapter 4 Discussion 227 I. Pluripotent adult stem cells The existence of a pluripotent stem cell type in planarians had been speculated upon but not conclusively demonstrated until the use of sub-lethal irradiation and single cell transplantation. Unfortunately the types of experiments that can be performed using clonogenic neoblasts are currently limited, as there is no way to determine a priori whether any particular neoblast is pluripotent. A neoblast is clonogenic if it is capable of forming a colony, which currently cannot be assayed in vivo. Also, there is increasing evidence that the neoblast population is highly heterogeneous, containing clonogenic neoblasts as well as lineage restricted stem cells that express both neoblast markers and differentiated cell markers. These properties limit certain types of experiments that require pure populations of cells, such as transcriptomic, epigenetic, and proteomic analysis. Even so, the development of transplantation has opened the field to a number of experiments that can further both our understanding of stem cell biology and a number of technologies that can improve planarians as a model system. Many questions of the nature of clonogenic neoblasts remain unanswered. Are neoblasts able to sense their environment and decide to divide symmetrically or asymmetrically? We estimate based on our data that the cells undergo symmetric division on average once every two days. However, the number of cells per colony can vary widely at specific time points after single cell transplant, with some colonies examined consisting of only one cell. This may result from varying rates of cell division, where colonies with fewer cells stem from a clonogenic neoblast that was cycling more slowly. Another possibility that is not necessarily mutually exclusive is that the cells of the early colony underwent one or more rounds of asymmetric 228 division, producing a clonogenic neoblast and a differentiated cell. One line of evidence that argues against the ability of a clonogenic neoblast to sense population depletion is from experiments done with shielded irradiation. When a portion of the animal is protected against ionizing radiation, the neoblasts within that region survive while the remainder of the animal is left devoid of proliferative cells. In this situation, few smedwi-J+ cells are observed migrating into the areas lacking neoblasts unless the animal is wounded (Guedelhoefer and SAnchez Alvarado, 2012). Therefore, depletion of the neoblast population does not appear to stimulate a response in these cells. It would also be interesting to see what types of cells are produced by the clonogenic neoblasts early after transplantation into irradiated hosts and whether the transplantation location has an effect on the identity of the cells. Two possibilities are evident. The first is that the progeny of clonogenic neoblasts are completely naYve of their environment and differentiate randomly. If this were the case, then we would expect either the progeny cell to migrate long distances to its destination tissue or undergo apoptosis if unable to find a niche. The second possibility is that the progeny cell is capable of sensing its surroundings and engages a differentiation pathway befitting its location. Recent improvements in the reliability of BrdU labeling will enhance clonal analysis by single cell injection, as it will allow the tracing of the differentiated progeny of the transplanted cell (van Wolfswinkel et al., 2014). What proportion of the smedwi-1] cell population consists of clonogenic neoblasts? We can determine the success rate of neoblast transplantation by performing an in situ hybridization for smedwi-J on transplant hosts immediately following the procedure. We can also determine the Pate of successful clonogenic neoblast grafting by examining colony formation several days 229 later. However, attempting to infer the proportion of clonogenic neoblasts in the neoblast population by calculating the ratio between these two rates will not likely to be accurate. One possibility for an underestimate is that the transplanted cell is damaged during the procedure and, although it may survive for a short time it may fail to engraft and divide. Evidence for this hypothesis is the presence of dynamic processes on X1(FS) cells which have been observed to break off upon loading or ejection from the injection needle. Another source of error in this estimate is the use of visual identification of cells to be transplanted, which biases the transplanted cells towards colony forming cells. Still another possibility is whether the state of the cell, such as cell cycle or region of the animal it was collected from, has any bearing on its success in colony formation upon transplantation. This illustrates the need for specific markers for clonogenic neoblasts, as the transplant protocol is limited to certain types of experiments. One major benefit offered by the development of single cell transplantation is its utility for the generation of transgenic animals. If the cell can be manipulated prior to transplant into an irradiated host, one could imagine creating entire transgenic lines using this technique. Unfortunately, the current success rate of creating lines from single cells is prohibitively low (about 1 in 45 transplant hosts recovered from lethal irradiation in the most successful experiments). A reason for this low success rate is the inability of any given expanding colony to restore regeneration before the entire animal succumbs to lethal irradiation. One possible solution to this issue is to alter the state of the host to be more amenable to colony expansion. Upon wounding, planarians undergo two waves of synchronized mitoses (Wenemoser and Reddien, 2010). Transplant hosts may be fed or lightly wounded to promote cell division during early colony expansion phases. However, wounding an animal also increases the rate of 230 programmed cell death, so attempting to stimulate proliferation this way may prove more detrimental than beneficial (Pellettieri et al., 2010). More protocol development, especially for methods to isolate a purer population of clonogenic neoblasts and to prevent hosts from succumbing to lethal irradiation before the graft can restore regeneration, will be required to allow transplantation to be a viable approach to transgenics. Asexual planarians are incapable of sexual reproduction. Instead, asexual planarians of sufficient size will undergo fission, where the tail will detach from the body and both pieces will regenerate into two complete individuals. In this fissiparous form of reproduction, pre-existing neoblasts are partitioned between the two individuals. It is conceivable that, over time, neoblasts accrue mutations, thus generating a mixed population of cells with varying genomes. A unique situation arises where each variant neoblast genotype can be thought of as an individual subpopulation with its own fitness as well as a contribution to the overall fitness of the animal. Heterogeneity in the clonogenic neoblast population may confer selective advantage to the host animal by allowing adapted subpopulations to thrive under stress conditions. Selection against deleterious mutations may be carried out by creating an environment where clonogenic neoblasts must compete for limited resources. Currently these theories do not have any supporting evidence, but can be explored by examining the level of DNA sequence polymorphism within an animal and seeing if strains generated from single cells have any differences from wild type animals. 231 I. Functions of Planarian Glia The function of planarian glia is currently unknown. This is unsurprising because, despite finding a signaling pathway that regulates the identity of the cell, we have no way of completely ablating the cell. Previous studies have inhibited transcription factor expression by RNAi to prevent the formation of a specific cell type during regeneration, but we currently do not know of any transcription factors to be expressed in glial cells (Lapan and Reddien, 2012; Scimone et al., 2011). Toxic small molecules that specifically act on Glutamine Synthetase have been used to ablate glial cells in vertebrate systems, but two issues may confound analysis. First, a second gene encoding a glutamine synthetase enzyme has been identified in the planarian genome and initial in situ hybridization studies have indicated that it is expressed in cells outside the nervous system. Second, the gs gene that has been identified in planarian glia is also expressed in other tissues, most notably the intestine. Thus, a pharmacological approach will likely result in offtarget effects that cannot be divorced from the glia ablation phenotype. Inhibition of the expression of transcription factors necessary for specification is commonly used to ablate a cell type in the study of planarian regeneration, but none have yet been identified as necessary for glia formation. A candidate approach using homologs of transcription factors known to play a role in glial cell specification in mouse and fly, such as Gcm and Olig2, has been attempted to no avail (Hosoya et al., 1995; Zhou and Anderson, 2002). Compiling a list of transcription factors expressed in planarian glia would require RNA-Seq on either a pure population of cells or a single cell, both of which we currently have no method of obtaining. Until a means of ablating 232 the cell type is discovered, any functional data will have to be inferred based on morphology and gene expression. We have identified four planarian glial genes encoding orthologs of vertebrate and invertebrate proteins with known functions. Interestingly, each of the four genes are predicted to be involved in either neurotransmitter reuptake or neurotransmitter metabolism, and the orthologs of two of the four genes, eaat2 and gs, are commonly used markers for astrocytes in vertebrates and astrocyte-like glia in Drosophila. Three of these genes, eaat2, eaat3, and gat-], encode members of the solute carrier family (Hediger et al., 2004). The excitatory amino acid transporters are often co-expressed with glutamine synthetase enzymes, which catalyze the processing of glutamate into glutamine (Anderson and Swanson, 2000). lonotropic glutamate receptors and the glutamate biosynthesis gene glutaminase have been identified in the planarian genome, thus providing evidence towards both the presence of glutamatergic neurons and a need for a system to maintain extracellular levels of excitotoxic glutamate. The GABA transporter gat-1 encodes a member of the SLC6 solute carrier family, of which gat-3 is expressed in vertebrate glia and gat-] is expressed in insect glia (Kinjo et al., 2013; Oland et al., 2010). Although we can predict that neurotransmitter uptake is performed by these cells, we have no direct evidence. RNAi of these genes may result in a behavioral phenotype, but that alone is insufficient to determine the solute specificity of these transporters. Ideally, radiolabelled neurotransmitters would be applied to live cells to track transport into the cell. The planarian glial cell morphology is an indication of the putative extracellular environment maintenance function. The processes of planarian glia are embedded in the axon bundles of the neuropil and the peripheral nervous system, and also form a continuous layer 233 around synaptic glomeruli in the ventral nerve cords. We posit that planarian glia extend processes along axons to reuptake neurotransmitter molecules released at synapses. Although we have described the highly branched nature of these cells as well as their close association with neurons, axons and synapses, the actual shape of the cell is still unknown. Immunofluorescence of GFAP labels cytoskeletal elements within vertebrate astrocytes and does not provide any information of the topology of the cell membrane. Actin and Ezrin antibodies label thousands of smaller peripheral astrocyte processes that emerge from the major GFAP+ processes to interact with synapses (Derouiche and Frotscher, 2001). We currently have no means of observing the true structure of the cell, as we have no antibodies for other structures in these cells, but we know that the neurotransmitter transporters are transmembrane domain proteins and are likely to accumulate near synapses. Producing antibodies against these targets will aid in the determining the morphology of the cell. RNAi of if-1 and pcdh-19 in unamputated and regenerating fragments has not produced any detectable phenotype. It is possible that these two genes are involved in a specific neuropil glia function that would not have been tested in our assays. Intermediate filaments play two well-known functions in cells of other organisms: providing mechanical support and increasing cell size. These functions are well conserved among cytoplasmic intermediate filaments such as GFAP (Herrmann et al., 2007). Given the density of synapses within the neuropil compared to the peripheral nervous system, more regulation of the extracellular environment may be required. Increasing the size of the cell may allow wider coverage of synapses by neuropil glia. Another possibility is that the central nervous system is more sensitive to damage, given the greater number of axons in a confined region that are more important to protect. The formation of IF- 1' 234 glial processes primarily in the neuropil may be a means of directing resources towards the central nervous system at the expense of the more expendable peripheral nervous system. Assays that specifically probe the sensitivity of animals to neuropil damage or neurotransmitter reuptake may reveal a previously undescribed aspect of the phenotype. 235 III. Roles of Glia Cells in Regeneration Although it is known that clonogenic neoblasts are the source of new tissue in planarian regeneration, the mechanism by which the progeny of clonogenic neoblasts differentiate into planarian glia has yet to be established. Given that glia are capable of proliferating in adulthood in both vertebrates and invertebrates, it would be interesting to determine whether any of the mechanisms regulating adult gliogenesis are present in planarians, or if they specifically follow developmental paths of determination. Heterogeneity observed in the neoblast compartment suggests that there are intermediate stages between naYve stem cell and terminally differentiated cell, and the characterization of other cell types within the neoblast population is currently the focus of many projects in the planarian community. One question is whether planarian glia arise from the same progenitor lineage as neurons. This would entail an intermediate cell committed to an ectodermal lineage but retains the capacity to form either neurons or glia, as well as another system for determining the fate of the cell. The Notch signaling pathway is a principle mediator of neuron versus glia decisions both during development and adult gliogenesis (Gaiano and Fishell, 2002; Wheeler et al., 2008), and is an attractive target for continued study of planarian glia. It is also possible that glia progenitors, are specified independently of neural progenitors as well as all other progenitors, which is an interesting possibility as it would suggest the presence of a master glial regulatory factor. The existence of such a factor would have implications in both comparisons of differentiation mechanisms between species and the understanding of the evolution of the glial cell type. 236 Glial involvement in axon guidance has been well established. Glia in C. elegans, the midline glia in Drosophila, and the floor plate glia in vertebrates all provide cues that control midline crossing of axons (Mitchell et al., 1996; Serafini et al., 1994; Wadsworth et al., 1996). In adulthood, however, the glia are involved repression of outgrowth, as seen in neurite collapse by myelinating oligodendrocytes and astroglial scars (McKeon et al., 1991; Wang et al., 2002). Currently, there is no evidence that planarian glia provide instructive cues to neurons during regeneration. Neural networks form prior to IF-W processes in the regeneration blastema. Also, IF-1 processes also always contact with Synapsin+ or a-Tubulin+ axon bundles, but not all axon bundles are associated with IF-1* processes. However, these experiments use the IF-I antibody to trace the location of glial processes. It is possible that glial processes exist prior to the expression of if-1, as evidenced by the persistence of glial cells following inhibition of hh, the possible existence of if-J glia in the peripheral nervous system, and the branched morphology of gs+1'fF cells. One could imagine that planarian glia extend processes first and then stabilize them with intermediate filaments upon induction by Hedgehog signaling. The study of homeostatic effects of glia on neural architecture may also reveal any roles in synapse pruning, maintenance of connectivity, and overall stability of the neural network, but without a means of determining the minutiae of glial morphology, this will be difficult to do. Whether glia play a role in the AP polarity defect observed in hh(RNAi) and ptc(RNAi) animals has not been fully explored. The defective tail regeneration resulting from inhibition of hh or the regeneration of anterior tails from inhibition of ptc is a striking but rare phenotype, requiring long-term inhibition by RNAi as well as multiple rounds of regeneration to clear existing, pre-patterned tissues (Rink et al., 2009; Yazawa et al., 2009). Even then, a range of 237 expressivity of the respective phenotypes is observed. hh(RNAi) animals can regenerate bifurcated tails, half tails, or no tails. ptc(RNAi) animals can regenerate heads with cyclopic photoreceptors or anterior tails (Rink et al., 2009; Yazawa et al., 2009). Additionally, it is possible that hh is expressed at low levels in other cells throughout the animal, but the major site of hh production is the medial cortex of the cephalic ganglia and the anterior-most portions of the ventral nerve cords. The distance between the primary source of ligand, the head, and the apparent target of signaling, the tail, raises questions of how Hedgehog signaling is controlling polarity decisions so remotely. These data raise the possibility that Hedgehog signaling may be indirectly involved in the establishment of AP polarity. In contrast to the late-appearing AP polarity defect, changes in if-1 and pcdh-19 expression occur as early as 12 days after the onset of RNAi treatment. Also, planarian glia express ptc, providing evidence that these cells are direct targets of Hedgehog signaling. One possible role of glia in polarity regeneration is to provide a route for hh ligand to travel to its site of action. The Hedgehog protein has a cholesterol modification that allows it to associate with cell membranes and limits its diffusion (Varjosalo and Taipale, 2008). One group has shown, using fluorescently-tagged Sonic hedgehog, that the ligand binds to and is transported along filopodia in order to cover longer distances (Sanders et al., 2013). A similar mechanism may exist in planarians, where hh not only is transported by a glia track, but also promotes the formation of that track. If that is the case, then it would suggest that if-1 or pcdh-19 are directly involved in this mechanism, since only expression of these two genes and not the entire cell is ablated by hh inhibition. Long-term inhibition of if-1 or pcdh-19 does not recapitulate the hh 238 RNAi morphological defect. It is possible that inhibition of multiple genes regulated by Hedgehog signaling is required to produce the AP defect. 239 IV. Glial Cell Evolution Continued study of planarian glia will offer insight into whether the glia of protostomes and deuterostomes originated from a single primordial glial cell type, or whether glia arose independently in those two branches of the bilateria. Although we have found three planarian genes, gs, gat-], and eaat2, that encode orthologs expressed in both vertebrate and Drosophila glia, they are not glia-specific and cannot be used as evidence of common origin. Glutamine Synthetase is found in the vertebrate liver (Gebhardt and Mecke, 1983), and related solute carrier family members of gat-1 and eaat2 are also expressed in neurons (Jin et al., 2011; Zhou and Danbolt, 2013). Comparisons of morphology are limited because of the lack of ultrastructural resolution, but there are similarities, such as encapsulation of synaptic glomeruli (Boeckh and Tolbert, 1993; Leise, 1990). Interestingly, the glial processes observed in the peripheral nervous system do not appear to wrap orthogonal commissures. Complete encasement of neurons or axons is thought to be a trait of "well-developed" glia, suggesting that the glial form we see in the peripheral nervous system may have a morphology similar to the hypothetical ancestral glial cell type and offer an opportunity to study the origins of glia (Hartline, 2011). Whereas invertebrate and vertebrate glia share a number of commonalities, such as morphology and function, the developmental pathways of the two are dissimilar. We do not yet know the key factors responsible for specifying planarian glia, but we have evidence that orthologs for Drosophila Gcm and mammalian Olig2 are not involved. If planarian glia are specified in a way unrelated to vertebrates and other invertebrates, it would be consistent with the theory that the cell type arose multiple times during the course of animal evolution. 240 Identification of a role for Hedgehog signaling in glial cell identity in planarians draws interesting parallels. In vertebrates, Sonic hedgehog released from neurons has been implicated in generating heterogeneity in the astrocyte population (Garcia et al., 2010). In Drosophila, Hedgehog signaling confers posterior midline glia identity during differentiation of midline glia (Watson et al., 2011). Hedgehog signaling therefore may be involved in the specification of a neuropil-localized subtype of planarian glia. The similarity between if-1/pcdh-19 glia and reactive astrocytes should also be considered. Sonic hedgehog is one of the extrinsic factors that activate astrocytes to undergo hypertrophy during astrocyte scar formation (Sirko et al., 2013). Expression of GFAP, an intermediate filament, is upregulated during hypertrophy to increase the size of the cell and allow more efficient sealing of the wound site (Wilhelmsson et al., 2004). In planaria, glia in the vicinity of hh ligand upregulate expression of if-1. The functions may be different, but there is striking resemblance between the effect of Hedgehog signaling and cellular response. Hedgehog signaling does not appear to control central nervous system patterning in planarians, based on both expression patterns and expression levels of neurogenic transcription factors following inhibition of hh or ptc. In vertebrates, Sonic hedgehog is critical for specifying the floor plate and patterning the ventral domain of the neural tube, and in Drosophila, Hedgehog controls cell identity specification in developing neuroblasts (Bhat, 1999; Dessaud et al., 2008). A question arises about whether the role of Hedgehog signaling in nervous system patterning arose in the last common Bilaterian ancestor and was lost in the planarian lineage, or whether Hedgehog signaling has been co-opted into nervous system patterning multiple times throughout evolution, or a combination of the two. Clearly many more organisms from many 241 other phyla will need to be analyzed before any conclusions can be made, but our results begin to support a certain viewpoint. The ventral to dorsal arrangement of neurogenic transcription factor expression domains is similar to that of Drosophila. However, unlike vertebrates, expression and localization of ventral neuroblastsdefective (vnd), intermediate neuroblastsdefective (ind) and eyeless (ey) (which encode Drosophilaorthologs of Nkx2.1, Gsh2, and Pax6, respectively) is independent of Hedgehog signaling (Cornell and Ohlen, 2000). Planaria have a medial to lateral distribution of Nkx2 and Pax6 domains similar to the ventral to dorsal distribution in fly and vertebrate, and those domains too are independent of Hedgehog signaling. Given these data, it is likely that the involvement of Hedgehog signaling in patterning these neurogenic domains is a vertebrate innovation. A secondary topic raised by the discovery of if-1 in planarian glia is the evolution of intermediate filaments in the context of glia. Intermediate filament proteins such as Vimentin, Nestin, and GFAP are used as markers for glia, neural stem cells, and reactive astrocytes, respectively. These subtypes of cytoplasmic intermediate filaments, however, arose when the intermediate filaments radiated into six different families in the vertebrate lineage (Erber et al., 1998). Even so, intermediate filaments have been found in invertebrate glia, the major exception being Drosophila (Goldstein and Gunawardena, 2000; Hartline, 2011). Given the highly specialized morphology of glial cells, the need for additional cytoskeletal structure elements is not surprising. Whether intermediate filaments impart a structural property especially advantageous to glia such that it would be incorporated multiple times throughout evolution, or whether the use of intermediate filaments was inherited from a common glial cell ancestor is unknown. 242 V. Conclusion This work presents a number of opportunities for continued study of planarian stem cells and glia. Of particular interest are the means by which glia are specified at the clonogenic neoblast level and how their differentiation is modulated by the neural network into which they must integrate. Not only would elucidating this mechanism increase out understanding of the specification of cell types in a highly regenerative model organism, but will also permit comparisons between extant species with glia and provide insight into long standing questions of the evolution of the cell type. In addition to understanding how glia are regenerated, continued investigation of whether glia participate in the regeneration of other cell types and structures is also pertinent, as it would aid in our understanding of adult gliogenesis and nervous system repair in mammalian systems. 243 References Anderson, C.M., and Swanson, R.A. (2000). Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32, 1-14. Bhat, K.M. (1999). Segment polarity genes in neuroblast formation and identity specification during Drosophilaneurogenesis. Bioessays 21, 472-485. Boeckh, J., and Tolbert, L.P. (1993). Synaptic organization and development of the antennal lobe in insects. Microsc. Res. Tech. 24,260-280. Cornell, R.A., and Ohlen, T.V. (2000). Vnd/nkx, ind/gsh, and msh/msx: conserved regulators of dorsoventral neural patterning? Current Opinion in Neurobiology 10, 63-71. Derouiche, A., and Frotscher, M. (2001). Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia 36, 330-341. Dessaud, E., McMahon, A.P., and Briscoe, J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development (Cambridge, England) 135, 2489-2503. Erber, A., Riemer, D., Bovenschulte, M., and Weber, K. (1998). Molecular Phylogeny of Metazoan Intermediate Filament Proteins. J Mol Evol 47, 751-762. Gaiano, N., and Fishell, G. (2002). The role of Notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 25, 471-490. Garcia, A.D.R., Petrova, R., Eng, L., and Joyner, A.L. (2010). Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. Journal of Neuroscience 30, 1359713608. Gebhardt, R., and Mecke, D. (1983). Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. Embo J. 2, 567-570. Goldstein, L.S., and Gunawardena, S. (2000). Flying through the Drosophilacytoskeletal genome. J. Cell Biol. 150, F63-F68. Guedelhoefer, O.C., and Sdnchez Alvarado, A. (2012). Amputation induces stem cell mobilization to sites of injury during planarian regeneration. Development (Cambridge, England) 139, 3510-3520. Hartline, D.K. (2011). The evolutionary origins of glia. Glia 59, 1215-1236. Hediger, M.A., Romero, M.F., Peng, J.-B., Rolfs, A., Takanaga, H., and Bruford, E.A. (2004). The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 447, 465-468. 244 Herrmann, H., Bar, H., Kreplak, L., Strelkov, S.V., and Aebi, U. (2007). Intermediate filaments: from cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 8, 562-573. Hosoya, T., Takizawa, K., Nitta, K., and Hotta, Y. (1995). glial cells missing: a binary switch between neuronal and glial determination in Drosophila.Cell 82, 1025-1036. Jin, X.-T., Galvan, A., Wichmann, T., and Smith, Y. (2011). Localization and Function of GABA Transporters GAT-1 and GAT-3 in the Basal Ganglia. Front Syst Neurosci 5, 63. Kinjo, A., Koito, T., Kawaguchi, S., and Inoue, K. (2013). Evolutionary history of the GABA transporter (GAT) group revealed by marine invertebrate GAT- 1. PLoS ONE 8, e8241 0. Lapan, S.W., and Reddien, P.W. (2012). Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep 2, 294-307. Leise, E.M. (1990). Modular construction of nervous systems: a basic principle of design for invertebrates and vertebrates. Brain Res. Brain Res. Rev. 15, 1-23. McKeon, R.J., Schreiber, R.C., Rudge, J.S., and Silver, J. (1991). Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 11, 3398-3411. Mitchell, K.J., Doyle, J.L., Serafini, T., Kennedy, T.E., Tessier-Lavigne, M., Goodman, C.S., and Dickson, B.J. (1996). Genetic analysis of Netrin genes in Drosophila:Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203-215. Oland, L.A., Gibson, N.J., and Tolbert, L.P. (2010). Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. J. Comp. Neurol. 518, 815-838. Pellettieri, J., Fitzgerald, P., Watanabe, S., Mancuso, J., Green, D.R., and SAnchez Alvarado, A. (2010). Cell death and tissue remodeling in planarian regeneration. Developmental Biology 338, 76-85. Rink, J.C., Gurley, K.A., Elliott, S.A., and Sanchez Alvarado, A. (2009). Planarian Hh Signaling Regulates Regeneration Polarity and Links Hh Pathway Evolution to Cilia. Science 326, 14061410. Sanders, T.A., Llagostera, E., and Barna, M. (2013). Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497, 628-632. Scimone, M.L., Srivastava, M., Bell, G.W., and Reddien, P.W. (2011). A regulatory program for excretory system regeneration in planarians. Development (Cambridge, England) 138, 43874398. Serafini, T., Kennedy, T.E., Galko, M.J., Mirzayan, C., Jessell, T.M., and Tessier-Lavigne, M. 245 (1994). The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78,409-424. Sirko, S., Behrendt, G., Johansson, P.A., Tripathi, P., Costa, M., Bek, S., Heinrich, C., Tiedt, S., Colak, D., Dichgans, M., et al. (2013). Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell 12, 426-439. van Wolfswinkel, J.C., Wagner, D.E., and Reddien, P.W. (2014). Single-Cell Analysis Reveals Functionally Distinct Classes within the Planarian Stem Cell Compartment. Cell Stem Cell. Varjosalo, M., and Taipale, J. (2008). Hedgehog: functions and mechanisms. Genes Dev. 22, 2454-2472. Wadsworth, W.G., Bhatt, H., and Hedgecock, E.M. (1996). Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16, 35-46. Wang, K.C., Koprivica, V., Kim, J.A., Sivasankaran, R., Guo, Y., Neve, R.L., and He, Z. (2002). Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941-944. Watson, J.D., Wheeler, S.R., Stagg, S.B., and Crews, S.T. (2011). Drosophilahedgehog signaling and engrailed-runt mutual repression direct midline glia to alternative ensheathing and non-ensheathing fates. Development (Cambridge, England) 138, 1285-1295. Wenemoser, D., and Reddien, P.W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental Biology 344, 979-991. Wheeler, S.R., Stagg, S.B., and Crews, S.T. (2008). Multiple Notch signaling events control DrosophilaCNS midline neurogenesis, gliogenesis and neuronal identity. Development (Cambridge, England) 135, 3071-3079. Wilhelmsson, U., Li, L., Pekna, M., Berthold, C.-H., Blom, S., Eliasson, C., Renner, 0., Bushong, E., Ellisman, M., Morgan, T.E., et al. (2004). Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. Journal of Neuroscience 24, 5016-5021. Yazawa, S., Umesono, Y., Hayashi, T., Tarui, H., and Agata, K. (2009). Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proceedings of the National Academy of Sciences 106, 22329-22334. Zhou, Q., and Anderson, D.J. (2002). The bHLH transcription factors OLIG2 and OLIGI couple neuronal and glial subtype specification. Cell 109, 61-73. Zhou, Y., and Danbolt, N.C. (2013). GABA and Glutamate Transporters in Brain. Front Endocrinol (Lausanne) 4, 165. 246