Atmosphere and ocean differences Lesson 2 Atmosphere & ocean properties:
advertisement

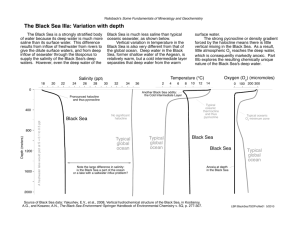
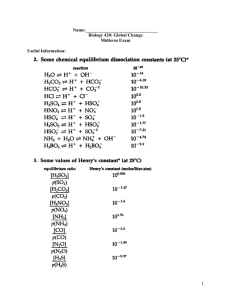
Atmosphere and ocean differences Lesson 2 Atmosphere & ocean properties: equations of state and density Fluids are physically different Review: differences b/t ocean & atmosphere, equations of state for atmosphere & ocean Density Equation of state Density (ρ) is defined as the mass per unit volume: mass ρ= Volume With units: . g or cm 3 General form: Density is related to the specific volume, α, which is the volume per unit mass 3 With units: cm or g The equation of state - The functional relationship between the absolute pressure, specific volume and temperature for a homogenous substance. kg m3 α= Water is incompressible (atm is assumed incompressible for many applications) Ocean has no analogy for “latent heat” source that atm has Oceans are laterally bounded (by land) Atm circulation largely forced by convection – heating from below Ocean circulation forced by convection – heat loss from above – and by wind stress (no analogy in atm) . f ( p, α , T ) = 0 1 ρ 3 m kg 1 Ideal Gas law – Moist atmosphere Ideal Gas law The equation of state – For relatively small pressure values (on the order of (1 atm)), there exists an analytic relationship for the equation of state: Due to Dalton’s law of partial pressures, we can express an ideal gas law for the molecular constituents of the atmosphere p = ρRT R= p = ρR d T * R* m Where And m is the molecular mass with units grams/mole . * 3 And R = 8.3143 × 10 o Joules Kelvin ⋅ kg ⋅ mole 2 Where Rd = 2.8705 × 10 . is the ideal gas constant. This equation is only valid under the conditions that: Molecules exhibit no potential forces between each other. They only interact through elastics collisions like billiard balls. 2. Molecules occupy no volume. They are all treated as “point” masses T* is the virtual temperature and is defined as the temperature a parcel of dry air would have if its pressure and density were those of the parcel of moist air. If W is the mixing ratio in units of gm/kg then 1. Equation of state for the ocean Clearly, the requirements of the ideal gas law break down in the ocean and therefore we are stuck with the general form of the equation of state: f ( p, α , T ) = 0 . Joules is the ideal gas constant for dry air kg ⋅o Kelvin In practice, we are interested in examining how the density (specific volume) of the ocean varies with Pressure, Temperature and Salinity. Density increases with a(n): - increase in Salinity and Pressure - decrease in Temperature T* ≈T + W 6 General properties of ocean Seawater is nearly incompressible (more than fresh water or air), thus seawater density depends (mostly) on salinity & temperature More dense Less dense Cold water Warm water Salty water Fresh(er) water Density units in kg m-3 2 3. (from Pinet, 1998) What are factors that control the growth and structure of the Thermocline? 4 (NORTHERN HEMISPHERE) (from Pinet, 1998) SALINITY General definition - The mass of salt per unit mass of seawater - measured in parts per thousand– It is very difficult to actually find the amount of salt or the amount of water at a given Temperature and pressure – Evaporation alone is not sufficient. Practical Salinity: Salinity measured in accordance with the Practical Salinity Scale (conductivity scale as compared to a standard KCL solution) and has Practical Salinity units (PSUs). 4 (NORTHERN HEMISPHERE) (from Pinet, 1998) 3 Major Constituents high temperate subtropical The concentrations of these major constituents are conservative. They are unaffected by most biological and chemical processes. 7. (from Pinet, 1998) What is the “halocline”? What factors control the growth and structure of the halocline? 11. (from Pinet, 1998) (from the Navy Coastal Ocean Model) Why is the Atlantic saltier (at the surface) than the other global oceans? 4 Effect of pressure on density In-situ versus laboratory θ = T − ∫ Γdp Theoretical analysis of the ocean is important but is of little use without the experimental results to accompany it. There are many ways of obtaining the thermodynamics properties of the ocean. These can broken into two main categories: . o Γ= Data off Antarctica Cp ≈ 4 × 104 J /(kg oC ) Γ is the adiabatic lapse rate In-situ – measuring the quantities of interest at depth in the ocean. Often, the use of a CTD can be used for these type measurements. Laboratory – Measuring the properties of interest by taking a sample at depth and bringing it to the surface. One must then account for variations in pressure and, to a lesser degree, temperature, in transporting the fluid parcel. Many chemical properties of sea-water require this type of measurement. – potential in-situ ~0.1º change at 1000 m ~0.3º change at 3000 m One must be careful though since neglect of pressure may mislead the observer into indications of an instability when actually does not exist. North Atlantic Deep Water (with higher potential density) over Antarctic Bottom Water (with lesser potential density) Γ can also be obtained from Unesco Technical Papers in Marine Science # 44 by Fofonoff and Millard, Unesco 1983 Ocean density representation Ocean density representation Given that we know density depends on salinity, temperature and pressure, we can approximate density as a linear function of its state variables using a Taylor series. If we expand about reference values, To, So and po we obtain Density is actually a non-linear function of T,S and p so we must use different techniques to get an accurate representation of density over a wide range of state variable values ρ (T , S , p ) ≈ ρ o (1 − α T (T − To ) + β S (S − S o ) + K ( p − p o )) The international equation of state for seawater is a widely accepted empirical technique to obtain accurate values of density to order 10-5. Where αT = − . βS = K= C ∂T g = ≈ 1 − 2 × 10− 4 ∂p C p db 1 ∂ρ - Thermal expansion coefficient ρ ∂T 1 ∂ρ - Haline contraction coefficient ρ ∂S 1 ∂ρ = - Isothermal compressibility coefficient ρ ∂p The above representation is only useful locally (with little change in the domain or parameters) . The international equation of state for seawater is not static (fixed), and note that the Intergovernmental Oceanographic Commission has approved a modification, TEOS-10 http://www.teos-10.org/ An amazingly detailed (179 pg) brief is hosted by the Australian government (Commonwealth Scientific and Industrial Research Organisation) at: http://www.marine.csiro.au/~jackett/TEOS-10/TEOS-10_Manual_08Jan2010.pdf 5 International equation of state for seawater International equation of state for seawater The international equation of state for seawater calculates density by finding the secant bulk modulus, K, which is the slope of a segment on a graph of density versus fractional rate of change of volume. Its value is K = α o p = ρ p αo − α Solving for density in the previous equation we obtain ρ − ρo ρ (S , T , p ) = ρ o (S , T , po ) 1− p K (S , T , p ) p . . K= p o = 0, αo ρ p= p αo − α ρ − ρo αo −α =0 αo αo − α αo International equation of state for seawater K (S , T , p ) = There are other more recent techniques for finding the density of seawater. −2 + 148.4206T − 2.327105T 2 999.842594 + 6.793952 × 10 T + 1.360477 × 10 − 2 T 3 − 5.155288× 10 −5 T 4 − 9.095290 × 10 −3 T 2 + 1.001685 × 10 −4 T 3 + 3.239908 p + 1.43713× 10 −3 Tp −4 −7 + 1.16092 × 10 T p − 5.77905 × 10 T p 2 3 + 8.50935 × 10 −5 p 2 − 6.12293 × 10 −6 Tp 2 . Representing the density of seawater ρ (S , T ,0 ) = 19652.21 + 8.24493 × 10 −1 S − 4.0899 × 10 −3 TS + 7.6438 × 10 −5 T 2 S − 8.2467 × 10 −7 T 3 S + 54.6746S − 0.603459TS + 5.3875 × 10 −9 T 4 S − 5.72466 × 10 −3 S 3 / 2 + 1.09987 × 10 − 2 T 2 S − 6.1670 × 10 −5 T 3 S + 7.944 × 10 − 2 S 3 / 2 + 1.6483 × 10 − 2 TS 3 / 2 − 5.3009 × 10 T S 2 3/ 2 −3 + 2.2838 × 10 pS Some recent approaches are by McDougall et al (2002) or Feistel (2003) − 1.120083 × 10 −6 T 4 + 6.536332 × 10 −9 T 5 + 5.2787 × 10 −8 T 2 p 2 −4 K and ρο=0 are then empirically fit to different values of salinity, temperature and pressure. The accepted expansion of K and ρο are . . + 1.0227 × 10 − 4 TS 3 / 2 − 1.6546 × 10 − 6 T 2 S 3 / 2 + 4.8314 × 10 − 4 S 2 . McDougall T.J., Jackett D. R., Wright D. G., Feistel R., 2002, Accurate and computationally efficient formulae for potential temperature and density. J. Atmos. Ocean. Tech., 20, 730-741 Feistel R., 2003. A new extended thermodynamic potential of seawater. Prog. Oceanography, 36, 249-347. − 1.0981× 10 −5 TpS − 1.6078 × 10 −6 T 2 pS + 1.91075 × 10 −4 pS 3 / 2 − 9.9348 × 10 −7 p 2 S + 2.0816 × 10 −8 Tp 2 S + 9.1697 × 10 −10 T 2 p 2 S 6 Tables A good java program that allows use to find the thermodynamic properties of the ocean is found . . . here . 7