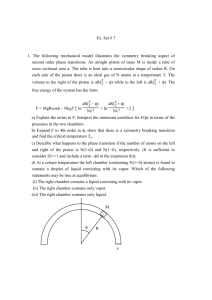
Machine Study of Lubrication Oil Ignition in a Rapid... Under Sporadic Pre-Ignition Conditions
advertisement

Study of Lubrication Oil Ignition in a Rapid Compression Machine Under Sporadic Pre-Ignition Conditions ARCHNES by TITLIT M ASS AC QF V2<'OLOLG'Y Morgen Paul Sullivan JUL 3 0 2015 Bachelor of Science in Mechanical Engineering The University of Texas at Austin, 2013 LIBRARIES Submitted to the Department of Mechanical Engineering in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE IN MECHANICAL ENGINEERING at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2015 Massachusetts Institute of Technology 2015. All Rights Reserved Signature redacted Author Department of Mechanical Engineering April 17, 2015 Signature redacted . Certified by Wai K. Cheng, Professor of Mechanical Engineering Director, Sloan Automotive Laboratory Thesis Supervisor Signature redacted Accepted byDavid E. Hardt, Professor of Mechanical Engineering Chairman, Department Committee on Graduate Theses 2 To my family 3 4 Study of Lubrication Oil Ignition in a Rapid Compression Machine Under Sporadic Pre-Ignition Conditions by Morgen Paul Sullivan Submitted to the Department of Mechanical Engineering on April 17, 2015 in partial fulfillment of the requirements for the degree of Master of Science in Mechanical Engineering Abstract In recent years, the industry has shifted toward down-sizing and turbo-charging spark ignition (SI) engines in an effort to increase fuel conversion efficiency. However, this has given rise to a destructive phenomenon known as sporadic pre-ignition (SPI). At low cranking speeds and high loads, engines have been observed to knock violently for brief and infrequent intervals. If allowed to continue, these periods of knock will result in a destroyed engine. This study looks at the propensity of lube oil vapor appearing in the cylinder as a cause for this phenomenon. The theory is that a local oil vapor/air mixture pocket may auto-ignite and start a flame in the charge. The pre-ignition would produce extreme knock. A rapid compression machine (RCM) was used to simulate this scenario and determine if oil vapor can cause SPI, and if so, to relate the auto-ignition tendency to the oil properties. The RCM was used to measure the ignition delay of a cloud of oil vapor in a stoichiometric gasoline/air mixture. The ignition delays were then correlated to chemical and physical properties of the oils. Finally, the effect of diluting the mixture was assessed. The results suggest that lube oil is a plausible source of SPI. The oil ignition delay times are sufficiently short to produce extreme pre-ignition consistent with SPI. Further supporting evidence lies in the fact that oil ignition delay times concur with SPI behavior in engines. It was found that the base stock, degradation, and chemical additives all play a role in oil ignition delay times. The results also demonstrate. that dilution significantly slows auto-ignition of the oil. Thesis Supervisor: Wai K. Cheng Title: Professor of Mechanical Engineering 5 6 Acknowledgements A number of people deserve my deepest gratitude for their immense support. The first of which is my advisor, Professor Wai Cheng. If it were not for his guidance, devotion, and constant involvement at every step, this work would not have been possible. I am truly grateful for his invaluable support. Secondly, I would like to extend my thanks to all of the members of the Engine and Fuels Consortium for their financial and technical support. A special thanks to Richard Davis and Justin Ketterer from General Motors, Thomas Leone from Ford, David Roth from BorgWarner, and Professor John Heywood from MIT, for their vital contributions to research planning and analysis. I would also like to thank the staff of the Sloan Automotive Lab. I want to especially thank Thane DeWitt, the Lab Supervisor, and Raymond Phan, the Lab Technician. Thane constantly provided helpful advice and assistance throughout the entire project while Raymond offered his expertise with technical issues. Also, I could not have completed this work without the valued collaboration with my lab mates. I would like to especially show my gratitude for Felipe Rodriguez who not only provided valuable advice throughout my time at MIT but also voluntarily helped prepare my experimental setup. I also want to thank Jacob McKenzie for offering essential help during the setup phase. Finally, I would like to thank my entire family for their unwavering support of my goals. Without my parents and brothers, I would not be where I am today. I cannot express how grateful I am to them for everything they have done for me. I owe special thanks to my aunt and uncle, Maureen and Frank Keton, for their incredible support. Without their help, moving to Boston would have been impossible. Morgen Sullivan, Cambridge, MA, April 2015 7 8 Table of Contents Abstract............................................................................................................................... 5 Acknowledgem ents....................................................................................................... 7 Table of Contents................................................................................................................ 9 List of Figures ................................................................................................................... 11 List o f Tables .................................................................................................................... 13 Nom enclature .................................................................................................................... 15 Chapter 1: Introduction ................................................................................................... 17 1.1 M otivation ............................................................................................................... 17 1.2 Background ............................................................................................................. 18 1.3 Objective ................................................................................................................. 22 Chapter 2: Experim ental Setup ..................................................................................... 23 2.1 Rapid Com pression M achine .............................................................................. 23 2.2 Oil Vaporization Electrode and Power Source .................................................... 26 2.3 M ixture Preparation Setup (M ixing Tank)........................................................... 31 Chapter 3: Procedure.........................................................................................................33 3.1 Oil Sam ple Preparation ....................................................................................... 33 3.2 Charge Preparation.............................................................................................. 34 3.3 Firing and D ata Acquisition ................................................................................. 35 Chapter 4: Results ............................................................................................................. 37 4.1 Oil Vapor and Ignition Delay............................................................................... 37 4.2 Am ount of Oil..................................................................................................... 40 4.3 Oil Vapor Ignition Stages and Isolation of Valuable Data................................... 41 4.4 Synthetic Versus Non-Synthetic O il ................................................................... 44 9 4.5 New Versus U sed Oil.......................................................................................... 47 4.6 Effect of Oil Additives........................................................................................ 48 4.7 Effect of Oil Physical Properties........................................................................ 53 4.8 Effect of Charge Dilution................................................................................... 57 Chapter 5: Sum m ary and Conclusions........................................................................... 61 5.1 Oil Vapor and Ignition Delay............................................................................... 61 5.2 Oil Characteristics ................................................................................................ 62 5.3 Charge Dilution................................................................................................... 62 5.4 Applicability to Engines...................................................................................... 62 5.5 Closure .................................................................................................................... 63 Appendix A : Rapid Com pression M achine .................................................................. 65 A .1 RCM Schem atics.................................................................................................... 65 A .2 RCM M echanical Drawings [14] ........................................................................... 69 Appendix B : RCM Operating M anual............................................................................. 81 B. 1 The Control Panel.............................................................................................. 82 B.2 Initial Preparations............................................................................................... 83 B.3 Firing Sequence................................................................................................... 87 B.4 Shut-D own Sequence .......................................................................................... 89 B.5 Troubleshooting................................................................................................... 90 Appendix C : Lubrication Oil Classification ............................................................... 93 References ......................................................................................................................... 95 10 List of Figures Figure 1.1: Pressure traces for a single SPI event with multiple SPI cycles [1]............ 17 Figure 1.2: Potential SPI mechanism proposed by Toyota [7].................................... 20 Figure 1.3: Ignition delay times as a function of oil concentration .............................. 21 Figure 2.1: Schematic of the RCM built by Kitsopanidis [14]................... 25 Figure 2.2: Schematic of electrode used to rapidly vaporize oil within the cylinder ....... 26 Figure 2.3: Results of testing the power source performance....................................... 29 Figure 2.4: Filament properties as a function of the transformer dial setting................ 30 Figure 4.1: Determination of the influence of wire energy on ignition delay (no oil) . 37 Figure 4.2: Effects of liquid oil versus oil vapor and timing of oil vaporization trigger.. 39 Figure 4.3: Varying the amount of oil applied to the wire........................................... 40 Figure 4.4: Illustration of oil-induced-knock stages (NOT ACTUAL DATA)............ 42 Figure 4.5: Wire-initiated flame propagation to isolate oil-specific ignition delay.......... 43 Figure 4.6: Dependence of flame propagation measurement on wire energy (no oil) ..... 44 Figure 4.7: Preliminary test of synthetic versus non-synthetic oil................................. 45 Figure 4.8: Ignition delay times for all 8 oils in milliseconds ....................................... 46 Figure 4.9: Ignition delay times for new versus used oils in milliseconds................... 48 Figure 4.10: Ignition delay versus calcium concentration for all 8 new motor oils ......... 50 Figure 4.11: Ignition delay versus molybdenum concentration for all 8 new motor oils. 50 Figure 4.12: Ignition delay versus phosphorus concentration for all 8 new motor oils.... 51 Figure 4.13: Isolation of the effects of additives in lube oils....................................... 52 Figure 4.14: Published physical data versus oil ignition delay times ........................... 56 Figure 4.15: Dilution with nitrogen (fixed fuel amount and k=l)................................ 58 Figure 4.16: Oil ignition delay times versus nitrogen dilution ..................................... 59 Figure A .1: R CM setup [14]............................................................................................. 65 Figure A.2: Pneumatic (driving) system [14] ............................................................... 66 Figure A.3: Hydraulic (locking-releasing) system [14]............................................... 67 Figure A.4: M ixture preparation setup [14]................................................................. 68 Figure B. 1: The rapid compression machine ................................................................. 81 11 Figure B.2: RCM control panel ........................................................................................ 82 Figure B.3: RCM heater panel.......................................................................................... 83 Figure B.4: RCM oil reservoir ...................................................................................... 83 Figure B.5: RCM sight tube.............................................................................................. 84 Figure B.6: RCM pneum atic tank.................................................................................. 84 Figure B.7: RCM m ixture preparation setup .................................................................... 85 Figure B.8: RCM pneum atic chamber valve ................................................................ 88 .......................................... 89 Figure B.10: Disassembled RCM head......................................................................... o Figure B.9: RCM firing valve 12 List of Tables Table 4.1: Eight preliminary lube oils tested in the RCM ........................................... 45 Table 4.2: Additive elements in ten new and used oils tested in the RCM .................. 49 Table 4.3: Concentrations of additive elements in the four "doped" oils ..................... 52 Table 4.4: Published and measured physical properties of the test oils........................ 54 Table B. 1: Approximate RCM operating pressures.......................................................... 85 Table C. 1: American Petroleum Institute base stock classification [18]...................... 93 13 14 Nomenclature Recurring Acronyms BMEP Brake Mean Effective Pressure CAD Crank Angle Degree DAQ Data Acquisition System EGR Exhaust Gas Recirculation GM General Motors Company KV40 Kinematic Viscosity at 40*C KV100 Kinematic Viscosity at 1 000 C LSPI Low-Speed Pre-Ignition PAO Polyalphaolefin MoDTC Molybdenum Dithiocarbamate RCM Rapid Compression Machine RON Research Octane Number RTD Resistance Temperature Detector SI Spark Ignition SPI Sporadic Pre-Ignition (or Stochastic Pre-Ignition) SwRI Southwest Research Institute ZDDP Zinc Dialkyldithiophosphate 15 Electric Symbols C Capacitance R Resistance Pr Resistivity iT Time constant U Voltage Geometric Symbols A Area d Diameter L Length V Volume Material Symbols P Density h Heat transfer coefficient V Kinematic viscosity T Temperature k Thermal conductivity a Thermal diffusivity Thermal expansion coefficient Cw Wire heat capacity cw Wire specific heat capacity Dimensionless Symbols Nu Nusselt number Pr Prandtl number Ra Rayleigh number Miscellaneous Symbols g Acceleration due to gravity Air-fuel equivalence ratio 16 Chapter 1: Introduction 1.1 Motivation For many decades, spark ignition (SI) engines have been plagued by the existence of abnormal combustion. In recent years, manufactures have shifted toward down-sizing and turbo-charging their SI engines in an effort to increase fuel conversion efficiency and reduce the relative losses associated with pumping work and friction. Unfortunately, this trend has given rise to a distinct new form of abnormal combustion known as sporadic preignition (SPI), also known as low-speed pre-ignition (LSPI), superknock, or megaknock. SPI typically occurs in highly boosted engines operating at low speeds (1,000 to 3,000 rpm) and high loads (above 18 bar BMEP). At these conditions, an SPI event will typically occur once every 10,000 to 15,000 cycles; however, each event will usually consist of more than one pre-ignition cycle. Figure 1.1, obtained from the Southwest Research Institute (SwRI) [1], shows what a typical SPI event looks like. 150- 140- 120- i 100- 25 0 0 0 *3 s0- 0 0 0 53 25 0~ 0 V C 53M 0 80- 5 Cyd e No, 0 40- 20 TDC" n 330 345 30 Spa*k Timing -5,0deg bTDC 375 390 CAD 405 420 435 450 Figure 1.1: Pressure tracesfor a single SPI event with multiple SPI cycles [1] 17 Figure 1.1 shows several pressure traces for normal cycles and pre-ignition cycles. Five of the six pre-ignition cycles begin early enough to develop into heavy knock, which is characterized by extreme peak pressures and pressure oscillations. These five cycles are considered SPI cycles. One of the six pre-ignition cycles begins slightly later, resulting in light knock. A key difference between SPI and typical knock is that a much larger portion of the end gas is auto-ignited, and in extreme cases more than half of the entire charge is auto-ignited [2]. The cycle peak pressures plotted in the upper right corner of Figure 1.1 demonstrate two points: SPI cycles reach very dangerous peak pressures, and an SPI cycle is almost always followed by a regular cycle. SPI is characterized by a very loud and violent "knocking" noise that is usually obvious to the driver. If allowed to continue, the extreme pressures will result in serious damage, most commonly to the piston crown and top land. Due to the extremely destructive and increasingly ubiquitous nature of SPI, it has become the focus of many studies throughout the world. 1.2 Background Several theories have been raised regarding the cause of SPI. Most hypotheses include one of the following fundamental mechanisms, which have been compiled by the French Institute of Petroleum (IFP) [3]: 1. Hot surface sites 2. Hot soot particles 3. Fuel pooling sites 4. Interaction with lube oil Hot surface sites or "hot spots" (e.g. exhaust valves, spark plug, etc...) have been proposed as possible sources for SPI; however, there is an overwhelming amount of evidence to suggest that hot spots are not to blame [3]. As shown in Figure 1.1, an SPI event is finite, and each SPI cycle is typically followed by a regular cycle. If hot spots in the engine were the source of ignition, then one would expect to see pre-ignition over extended consecutive 18 cycles. In extreme cases, this could even develop into "run-away" pre-ignition, which is a positive feedback loop whereby heat from the hot spots causes pre-ignition, thus increasing the temperature of the hot spot. Hot soot particles or deposits in the cylinder are thought to be another potential source of ignition. This has been ruled out because SPI has been observed immediately following a thorough cleaning of the cylinder [4] [5]. Fuel pooling sites are yet another proposed ignition source. The theory is based on the idea that poor fuel injection techniques are causing liquid fuel to pool in the cylinder, which creates a very rich vapor diffusion zone that ultimately leads to more rapid auto-ignition [3]. This theory can be ruled out since SPI has been detected even with port injection using a gaseous fuel [3]. Interaction with lube oil is the most promising theory and is the focus of this study. The hypothesis is that lube oil is entering the cylinder where it vaporizes and mixes with the charge. Having a characteristically short ignition delay, the oil vapor ignites long before the spark. The resulting heat release starts a flame in the surrounding mixture. As the flame propagates, the ensuing increase in temperature and pressure forces a significant amount of fresh charge to auto-ignite. This theory has been supported by an overwhelming number of studies [4] [5] [6] [7] [8] [9] [10]. Two important questions need to be asked when considering the validity of this theory: 1. How is oil entering the cylinder in the concentrations needed to produce SPI? 2. Can oil vapor in the charge actually produce SPI? One of the many objectives of this project is to help answer the latter question. The first question has been extensively studied by several companies including General Motors [4] [6] and Toyota [5] [7] [8] [10]. Toyota has developed a hypothetical mechanism in hopes of answering question 1. This mechanism is shown in Figure 1.2. 19 [Step 11 : Dilution ISten 21 : Accumulation Oil Film Accumulation of Fuel / Oil Mixture in Piston Top Land Crevice 1 2 irect Injection il Dilution by Fuel Injection Piston Top Land M IStep 31 : Release-Out IStep 41 : Auto-Ignition Oil Droplet released out from Piston Top Land Crevice Oil Droplet Vaporization lame 2 n Lyer Propagatio Oxidation Figure 1.2: PotentialSPI mechanism proposed by Toyota [7] Several studies have indicated that SPI is strongly correlated to liner wetting and fuel dilution [4] [8] [11], which supports Step 1 shown in Figure 1.2. Fuel dilution facilitates oil infiltration into the cylinder by lowering viscosity [12] and compromising film strength [13]. Once the oil is exposed to the charge, fuel dilution advances the process further by increasing volatility and promoting vaporization of the oil droplet [13]. While Steps 1 through 3 in Figure 1.2 have received strong support, some authors have expressed skepticism about Step 4 [10] [11]. One study by Toyota itself even questioned the mechanism by claiming that the time required to auto-ignite a liquid droplet of oil was 20 too long to be causing SPI at normal temperatures. Therefore, they claim that the droplet must be exposed to combustion in cycle 'n' before it causes SPI in cycle 'n+l' [10]. This theory could potentially explain why each SPI cycle is almost always preceded by a regular cycle. A second study found similar results regarding the time required to auto-ignite a liquid droplet of oil; however, while believing that oil was indeed contributing to preignition, they were unable to identify a specific ignition source [11]. Regardless of the mechanism by which oil vapor is appearing in the cylinder, it is yet to be determined whether oil vapor can even ignite fast enough to produce SPI. Measuring the time required to auto-ignite a liquid droplet of oil only tests the specific theory shown in Figure 1.2; however, measuring the ignition delay of oil vapor temporarily neglects the physical mechanism by which the oil vapor appears in the cylinder. As a result, the precursor to this study sought to measure oil vapor ignition delay times. The experiment used a rapid compression machine (RCM) to measure the ignition delay of homogeneous mixtures containing lube oil, fuel, and air. The objective of the experiment was to determine if oil vapor in the mixture could in fact produce ignition delay times that were short enough to cause SPI. The results of this experiment are shown in Figure 1.3. 18 16 14 E 12 10 Vm C 0 8 -4 2 0 I 0% 40% 20% 60% Oil volume in fuel mixture Figure 1.3: Ignition delay times as a function of oil concentration 21 The ignition environment for the experiment was about 660 K and 30 bar, which is comparable to SPI conditions in an engine. As expected, the ignition delay time decreased as oil concentration increased; however, at an oil concentration above 50% by volume in the oil-fuel mixture, the oil began to condense onto the walls of the cylinder. This is an issue because at a concentration of 50% the ignition delay time was still 6 ms, which is too long to produce SPI. Therefore, a new method had to be devised so that higher concentrations could be achieved. 1.3 Objective The main objective of the study is to assess, via ignition delay measurements in an RCM, the plausibility of the oil mechanism for initiating sporadic pre-ignition in modem engines. If oil appears to be a viable cause of SPI, then the next step is to determine how oil characteristics might impact ignition delay times in the RCM and then compare these results to findings in engine studies. The oil properties that are to be analyzed are base stocks, deterioration, chemical additives, and physical properties. Finally, charge dilution will be assessed as a tool for mitigating SPI. The goal is to gain a more fundamental understanding of how charge dilution is reducing pre-ignition frequency in engines, especially as it pertains to SPI. 22 Chapter 2: Experimental Setup 2.1 Rapid Compression Machine As the name implies, an RCM is a device used to compress a mixture of fuel and oxidizer in a combustion chamber by means of a rapidly moving piston, so that there is negligible chemical reaction during the compression process. However, the piston compresses the gases enough to produce an ignition environment resulting in auto-ignition of the charge. The piston in an RCM is held fixed after compression, yielding a constant-volume environment. The RCM used in this study was built at MIT in 2000 by loannis Kitsopanidis [14] based on a design by Affleck and Thomas at Shell's Thornton Research Centre [15]. A schematic of the RCM acquired from Kitsopanidis' thesis [14] is shown in Figure 2.1. Compression occurs in roughly 15 ins. See Appendix A for a detailed schematic of the RCM. The RCM consists of three fundamental chambers: the combustion chamber, the hydraulic chamber, and the pneumatic chamber. Each chamber contains a piston attached to the piston assembly. The combustion chamber piston is analogous to an engine piston and is used to compress the experimental charge. The hydraulic chamber piston is used to lock the piston assembly in the retracted (ready-to-fire) state by means of a large pressure differential being placed across it. The pneumatic chamber piston is responsible for driving the piston across the RCM during compression. The combustion chamber is covered with several resistance heaters and resistance temperature detectors (RTDs) used to control the initial temperature of the charge and to more closely represent actual engine conditions. The cylinder pressure is measured by a Kistler 6125A pressure transducer inserted into the wall of the combustion chamber. The bore is 5.08 cm (2 in) and the stroke is 20.32 cm (8 in). Silicone oil (Dow Coming 200 series) is used in the hydraulic chamber due to its low vapor pressure and low toxicity. The hydraulic piston and hydraulic chamber are designed with a pin and groove (see Figure 2.1), which are used to prevent damage during rapid 23 deceleration of the piston. As the piston nears the end of the stroke, the pin must enter the groove in the chamber where trapped oil is forced through a 0.7 mm clearance between the pin and groove. The viscous friction creates a significant deceleration force on the piston, preventing the assembly from violently slamming into the end of the chamber at full speed. The opposite side of the hydraulic piston contains an 0-ring to seal off the rear portion of the hydraulic chamber. When the piston assembly is fully retracted, the hydraulic piston 0-ring contacts the rear surface, sealing the two portions of the chamber from each other. This allows a pressure differential to be placed across the piston. The pneumatic chamber is driven by compressed nitrogen stored in a large reservoir, which is about 50 times the volume of the pneumatic chamber. The reservoir and pneumatic chamber are connected by a pipe with a very large diameter. The large diameter and large reservoir volume both promote a relatively constant driving pressure, despite the volume in the pneumatic chamber rapidly increasing with piston motion. Overall, the piston assembly is about 6 kg (13.2 lbs). The pneumatic piston has a diameter of 12.7 cm (5 in), making it 6.25 times the area of the combustion chamber piston and about 5 times the area of the hydraulic piston. This means that high pressures can be achieved in the combustion chamber with relatively low driving pressure; however, the locking pressure applied to the hydraulic chamber must be at least 5 times the driving pressure in order to create a force balance and lock the piston in the retracted state before firing. See Appendix B for detailed steps on how to operate the RCM. 24 DETAIL combustion combustion hydraulic hydraulic chamber piston chamber piston pneumatic chamber pneumatic piston 0.22 m (AM j DRAWN IN SCALE 1.17 m Figure2.1: Schematic of the RCM built by Kitsopanidis [14] - pin and groove mechanism 2.2 Oil Vaporization Electrode and Power Source As mentioned before, the study needs to produce highly concentrated pockets of oil vapor within the cylinder to simulate and observe one possible mechanism by which sporadic pre-ignition is thought to occur. In order to create high concentrations at the ideal moment (end of compression), a small nichrome filament inside the combustion chamber is used to rapidly vaporize a coating of liquid oil just when the piston is reaching the end of the stroke. The filament is very quickly heated by driving a large but brief pulse of current across the wire. A schematic of the electrode is shown in Figure 2.2. ground termina - positive terminal electric insulator nichrom e filament -70 mm Figure 2.2: Schematic of electrode used to rapidly vaporize oil within the cylinder The electrode shown in Figure 2.2 is fixed in the head of the RCM with the electrical terminals external to the machine. When installed, it yields a compression ratio of just under 9.1. The nichrome wire is about 2 cm (0.8 in) in length and 255 pm (0.01 in) in 26 diameter (#30 AWG) with an electrical resistance of roughly 0.43 Q, which was calculated with the following equation: PrL AX where pr is the resistivity of nichrome (about 1.1 x 10-6 Q-m), L is the length of the wire, and AX is the cross-sectional area of the wire. When fully coated, the oil film has a mass of approximately 1 mg. In order to accurately measure this small mass with a microgram scale, it was necessary to design the entire part to be under 200 g. The power source used to drive current across the wire consists of four parts: a transformer, a rectifying circuit, a capacitor, and a triggering circuit used to release the energy stored on the capacitor. The four components work together to produce an electric pulse and vaporize the oil film in the following steps: 1. AC voltage is sent into the transformer which reduces it to a specified voltage. 2. The resulting voltage is then rectified to DC and used to charge the capacitor. 3. At the precise moment that the oil is ready to be vaporized, a signal is sent to the triggering circuit which flips a switch, closing the circuit between the charged capacitor and the oil-coated filament. 4. The current from the capacitor is then allowed to flow across the wire which heats it enough to vaporize the oil film and create a locally concentrated cloud of oil vapor around the wire. The capacitor has a capacitance of about 2040 pF and requires at least 20 seconds to be sufficiently charged. After the power source has been triggered, the capacitor and wire can be modeled as a discharging RC circuit. Assuming for simplicity that the wire is adiabatic, then the electric power in the circuit is used entirely to heat the wire and is modeled by the following equation: 27 dT C Wdt U) - 2 ( e-t 12 )] R ;r=R RC where C, is the heat capacity of the wire (about 0.0039 J/K), T is the wire temperature, U0 is the initial voltage on the capacitor, T is the electrical time constant of the circuit (about 0.88 ms), and C is the capacitance. After rearranging and integrating, the following equation can be used to determine the adiabatic wire temperature change as a function of capacitor voltage and time: [ (T - TO) = -[e2t/] The time constant for the above two equations is half that of the voltage time constant (Tthermal= 0.44 ms), therefore 90% of the energy is deposited on the wire after 1 ms. For the above calculations to be accurate, convection losses from the wire must be negligible for this time scale. Assuming the wire is a horizontal cylinder under free convection, the Nusselt number can be used to determine the ratio of convective to conductive heat transfer. This is given by the following equation: i 0.387R aL/L Nu = 10.6 + [1 + (0.559/Pr)9/16]8/27 [16] where Pr is the Prandtl number (taken as 0.7), and Ra is the Rayleigh number governed by the following equation: Ra = fATgd av [16] where fl is the thermal expansion coefficient of the gas (1/T for ideal gases), AT is the difference in temperature between the wire and gas (about 300 K), g is the acceleration due to gravity, a is the thermal diffusivity of the gas (about 4x10 5 m 2 /s), and v is the kinematic viscosity of the gas (about 5x 10-5 m2 /s). With these values, the Rayleigh number 28 is about 0.08, resulting in a Nusselt number of 0.66. This suggests that heat transfer is almost purely via conduction. The Nusselt number can be used to determine the thermal time constant due to convective cooling of the wire with the following equations: Tconvection = hAs [17] k h = Nud where h is the heat transfer coefficient, As is the surface area of the wire, and k is the thermal conductivity of the surrounding gas (about 0.05 W/m-K). This equation gives a time constant of 1.86 sec, which is orders of magnitude larger than the 0.44 ms associated with heating the wire. As a result, convective losses from the wire can be neglected. The calculations above agree very well with real-world measurements obtained from the power source which can be seen in Figure 2.3. Tek TRiGGER M Pos: -1.000ns J ea Jr Type Trigger Signal .E 1Capacitor Voltage Mode itch,.ma RC. (MOSFET Drain) CH1 CH3 20NJ9 H ,0 V M1.00ms 28-Mar-1414:25 * Coupling T is about 0.9 ms CH /4, H Figure2.3: Results of testing the power sourceperformance 29 The results in Figure 2.3 show a discharge time constant of about 0.9 ms, which agrees very well with the calculated 0.88 ms time constant described earlier. Again, the time constant in Figure 2.3 corresponds to the voltage on the capacitor; the time constant for the energy deposited on the wire and the corresponding temperature rise are expected to be half as long. Since it was demonstrated that convective heat loss is negligible over this time scale, the wire is assumed to be adiabatic. Under this assumption, the maximum wire temperature rise can easily be calculated for the range of transformer voltage settings. The results are tabulated in Figure 2.4. 4500 - 16 - 14 - - 12 3000 . .. ... ........ ~ (j) -6 - - - .. - --.... -4 ..... - -... - - . 1000 .. ~ - 1500 - 10 Energy of Electric Pulse - 8 - Adiabad ic 2500 Wire Temp Rise 2000 (0 C) - . ...-.. 3500 -2 500 - ..... ... - 4000 0 10 20 I I 1 1 30 40 50 60 0 70 80 Transformer Dial Setting (V) Figure 2.4: Filamentpropertiesas a function of the transformerdial setting Again, the values in Figure 2.4 correspond to the adiabatic temperature increase, so the actual temperature increase is expected to be lower. 30 The power source is triggered by a digital output signal from the data acquisition system (DAQ) controlled by LabVIEW. The software uses the pressure measurements from the combustion chamber to identify the end of compression and then triggers the power source to heat the wire after a specified delay. 2.3 Mixture Preparation Setup (Mixing Tank) Each firing of the RCM in this study required the combustion chamber to be filled with a mixture of gasoline (Haltermann 437; 97 RON) and air. To promote consistency, a separate heated tank, referred to as the "mixing tank," is used to prepare a relatively large amount of stoichiometric mixture. Gasoline is injected into a small port in the tank using a syringe. Compressed air is then metered into the tank until stoichiometry is achieved. This mixing tank has a much larger volume than the combustion chamber, and the gases are held at a high pressure to allow for several firings with the same mixture. Other gases can also be metered into this tank to produce different types of mixtures. The mixing tank contains a rotating fan to ensure that the various gases are well mixed and homogeneous. See Appendix B for a more detailed description on how the mixtures are prepared. 31 32 Chapter 3: Procedure 3.1 Oil Sample Preparation For most of the runs in this study, a sample of oil was being tested in the RCM. There were six steps in preparing these samples: Step 1. Detach the power source and remove the vaporization electrode from the RCM Step 2. Connect a fresh nichrome filament to the electrode Step 3. Record the electrode weight before oil has been applied Step 4. Apply the oil sample to the filament using a cotton swab Step 5. Record the electrode weight after oil has been applied Step 6. Replace the electrode into the RCM and reattach the power source Typically, Steps 3 and 5 were omitted, except in cases where it was imperative to know the mass of the oil on the wire (more on this in Section 4.2). Step 1 required the head of the RCM to be removed, which is held in by four bolts. Once removed, it was deemed necessary to use a new wire for each run due to slight oxidation of the wire after each firing, which leads to a change in electrical and thermal properties. It also was found that used wires can contain trace amounts of oil residue, which can contaminate the results. The mass of the oil was determined by measuring the difference in overall mass before and after oil had been applied. The oil specimen was applied with a cotton swab, and typically the entire surface area was coated (-1 mg). Finally, the electrode was placed back into the head of the RCM with its new wire and coating of oil. The power source was reattached to the terminals making the sample ready to be tested. In order to promote consistency, while the head was removed, the cylinder and electrode were very thoroughly cleaned with acetone and then flushed with air to prevent deposits or contamination from previous oil samples from impacting the results. It should be noted that while the oil visibly created a film across the entire coated surface of the wire, surface tension did cause small portions of accumulation to form. 33 3.2 Charge Preparation Every experiment presented in this study required some sort of gaseous mixture to be compressed in the RCM. There were six steps to preparing that mixture: Step 1. Evacuate the mixing tank Step 2. Inject gasoline into the evacuated tank Step 3. Meter in air to achieve stoichiometry using partial pressures Step 4. Evacuate the combustion chamber Step 5. Fill the combustion chamber with stoichiometric charge from the mixing tank Step 6. Add any additional gases specific to each experiment (e.g. dilution) Steps 1 through 3 needed to be performed roughly once every 5 to 10 runs (until the mixture was used up) while Steps 4 through 6 needed to be repeated for each run. In Step 1, the mixing tank was evacuated to a pressure reading between 1.0 and 1.5 torr (-0.13 to 0.20 kPa), which depended on temperature since the pressure gauge drifts when heated. Then roughly 30 to 40 torr (-4.0 to 5.3 kPa) of gasoline were injected into the evacuated mixing tank. Finally, air was metered into the mixing chamber, accounting for the initial 1.0 to 1.5 torr pressures, until a stoichiometric mixture was produced. At this stage, the mixing tank contained around 2.5 bar (250 kPa) of fresh air-fuel charge, which is the pressure limit of the mixing tank. At this pressure, the mixing tank supplied charge for 5 to 10 runs. Next, the charge had to be transferred to the combustion chamber before each run. This was done by evacuating the cylinder down to 2.0 torr (0.27 kPa) and then metering fresh charge from the mixing tank to the evacuated chamber. For nearly every run in this study, 1.490 bar (149.0 kPa) of fresh stoichiometric charge was supplied to the combustion chamber. Finally, for a select few runs, diluent gases were metered into the chamber. For nearly every run, the mixing tank and combustion chamber were held at 315 K (42C) before compression, and all the pressures during Steps 1 through 6 above were monitored with extreme care to promote consistency. See Appendix B for a more detailed procedure on mixture preparation. 34 3.3 Firing and Data Acquisition Once the oil specimen was prepared for testing and the cylinder had been filled with a fresh charge, the RCM was almost ready to be fired. Before the gases could be compressed, there were four steps that needed to occur: Step 1. Apply pressure to the hydraulic chamber to lock the piston in place Step 2. Add driving pressure to the pneumatic chamber Step 3. Wait 10 minutes for the gas pressure and temperature to reach equilibrium Step 4. Release the pressure from the hydraulic chamber to fire the RCM The locking pressure in Step 1 was about 1750 psi (-12,000 kPa), and the driving pressure in Step 2 was roughly 275 psi (-1,900 kPa). It was determined that the 10 minute equilibration period was essential for repeatability. For a more detailed step-by-step process of operating the RCM see Appendix B. After the four steps had been completed, the gases in the combustion chamber began to compress. Vaporization of the oil needed to occur at a specified point after compression had begun. This was handled by the DAQ. There were four steps performed by the DAQ: Step 0. The RCM has been fired and compression has begun Step 1. The DAQ continuously monitors the cylinder pressure Step 2. Once a specified pressure threshold has been crossed, the DAQ reacts Step 3. The DAQ triggers the vaporization process after an optional delay Step 4. Pressure data is recorded and stored over a specified period of time For Step 1, the DAQ sampled the cylinder pressure at a rate of 10 kHz, and for most runs in this study the pressure threshold in Step 2 was set to 23 bar, to indicate the approximate end of compression. In a few experiments, the use of a vaporization delay was required (Step 3); however, in most runs this delay was not used (i.e. vaporization occurred immediately after detecting the pressure threshold). The pressure was then recorded at the 10 kHz sampling rate and stored for analysis. 35 36 Chapter 4: Results 4.1 Oil Vapor and Ignition Delay The first step was to confirm that the setup performed as expected. In other words, if everything went to plan the wire should successfully vaporize a small film of oil, and there should be a significant decrease in ignition delay as a result of rapid auto-ignition of the oil vapor. Before this could be observed, it was necessary to confirm that the individual effects of the liquid oil (un-vaporized film) and the wire heat had insignificant impacts on ignition delay. First, an appropriate wire energy had to be selected such that the oil was vaporized, but the wire heat did not impact ignition delay. This was done by repeatedly exposing a dry wire (no oil-coating) to a stoichiometric environment while sweeping a range of voltage settings on the transformer. The objective was to observe at what minimum setting the ignition delay of the charge was decreased (i.e. at which point the heat was no longer negligible). The results of this experiment are shown in Figure 4.1. 160 Wire Ignites Mixture V ire Does NOT Ignite Mixture 32 Volt Setting 3 0 Volt Setting (4 trials) Wire AT: ~660*C V ire AT: ~58 0 1C 140 - 120 100 80 J 40 S40 20 0 0 40 20 J 80 60 100 120 140 Time (ms) Figure4.1: Determination of the influence of wire energy on ignition delay (no oil) 37 The range of swept voltage settings between 10 and 30 volts had negligible effects on the stoichiometric ignition delay; however, Figure 4.1 shows that the 32 volt setting on the transformer actually started a flame in the mixture. The flame caused ignition to occur significantly earlier than when lower settings were used. This implies that a transformer setting of 30 volts was the maximum that could be used. Although the 2 volt difference seems negligible, it actually corresponds to a change in the adiabatic temperature rise of 800C, which is not negligible. The 30 volt setting on the transformer corresponds to about 2.2 J of energy being deposited onto the wire which translates into an adiabatic temperature increase of 5801C in the wire. This experiment made the maximum limit clear, but it was still unclear if this setting was above the minimum energy required to vaporize a film of oil. Testing this was much less quantitative and was determined by simply observing what happened when the wire was coated and subsequently heated. The 30 volt setting was observed to successfully vaporize a full coating of oil outside the cylinder of the RCM. As a result, the 30 volt setting was used from then on to vaporize the oil film. Next was to test the difference in ignition delays between liquid oil and vaporized oil when exposed to the stoichiometric ignition environment. This was done by first testing a control case with no oil and no current sent across the wire. Then, the liquid oil case was tested in which the wire was coated in a film of oil, but the wire was never heated. Finally, the wire was coated in oil and heated at three different times during and after compression. The results of the experiment are shown in Figure 4.2. 38 200Oil 180 End of Compression: ~26 bar ~65O K Oil Vaporized at Late Trigger (3 Trials) Vaporized at Normal Trigger (3 Trials) 160 Oil Vaporized at Early Trigger (3 Trials) 140 160 Trials) (33 C L) Normal S80 60Noi/otwr NorTmals 40 60 att TrralTrggrgONRO Early Trigger 20 0 20 40 80 60 100 120 140 Time (ins) Figure4.2: Effects of liquid oil versus oil vapor and timing of oil vaporization trigger The results shown in Figure 4.2 suggest that the heat from the wire and the liquid oil did not independently influence the ignition delay; however, when combined and the oil was vaporized, a flame was started in the cylinder due to rapid auto-ignition of the oil vapor pocket. The flame propagated throughout the stoichiometric charge and resulted in violent end-gas auto-ignition. This produced knock, which is characterized by the extreme peak pressures and oscillatory pressure waves. The onset of knock is not clear in Figure 4.2, however, it will become much more evident in later plots. Finally, the trigger timing had little impact on ignition delay of the oil. This suggests that the physical processes associated with vaporization and mixing were not significantly influenced by the final stages of compression. On the same token, this was evidence that the delay associated with physical processes was not insignificant. 39 The results followed expectations very well. The wire successfully vaporized the film of oil without significantly effecting the charge. The oil vapor auto-ignited very early, resulting in end-gas auto-ignition and knock. 4.2 Amount of Oil Figure 4.2 shows that when the proper wire setting is used, wire heat and vaporization timing have negligible effects on the results, but up to this point it was not clear how the mass of the oil was impacting the timing. To find out, a range of oil masses were applied to the wire and tested. A full coating of the wire corresponds to approximately 1 mg, so it was only feasible to decrease the amount from there. Three different masses were tested: 1.0 mg, 0.5 mg, and 0.2 mg. The digital scale that was used could only accurately measure down to 0.2 mg. Nevertheless, the three quantities were tested, and the results are shown in Figure 4.3. 180 160 11.0 mg of Oil (-90 pM film) 140 10.5 mg of Oil (~50 pm film) 120 a- 10.2 mg of Oil (~20 p.m film) 100 80 Trigger -o 40 20 0 20 60 40 80 100 120 Time (ms) Figure 4.3: Varying the amount of oil appliedto the wire Figure 4.3 shows that the amount of oil over the quantities tested had little effect on ignition time. This would suggest that the 0.2 mg film was above the minimum amount of oil 40 required to produce a fully concentrated pocket of oil vapor. A mass of 0.2 mg is characteristic of typical oil droplets observed in a test engine at MIT (about 0.1 to 0.2 mg). 4.3 Oil Vapor Ignition Stages and Isolation of Valuable Data Before looking at the effects of the different oils, it was important to understand what was actually happening after the oil was vaporized and to understand which specific portions of the data were influenced by oil properties. Ultimately, the goal was to isolate the impacts that were unique to each oil, so that a better understanding of ideal oil properties could be achieved. There are four fundamental stages associated with oil-induced-knock: Stage 1. Pre-Vaporization Compression Stage 2. Oil-Specific Ignition Delay Stage 3. Oil-Independent Flame Propagation Stage 4. Knock Stage 2 began at the end of compression when the wire was heated, vaporizing the oil film in approximately 0.5 ms. The oil vapor had to then diffuse away from the wire and mix with the oxygen in the charge. Once exposed to the ignition environment, there was a short chemical delay that was specific to each oil. Finally, the oil vapor auto-ignited, starting a small flame in the stoichiometric mixture, which initiated Stage 3. Stage 3 was independent of which oils were used, but instead depended on the properties of the stoichiometric mixture, which was kept constant for these experiments. During this stage, the flame spread throughout the stoichiometric mixture. This increased the temperature and pressure of the end gases, ultimately resulting in detonation (occurring around 70 bar). Stage 4 was characterized by the onset of knock, which was visibly apparent as a sudden change in slope. Knock is characterized by very large peak pressures and pressure 41 oscillations. Substantial heat loss through the cylinder walls occurred during this stage. The four stages are illustrated in Figure 4.4. 200 GRAPHIC: NOT ACTUALDATA 180 Oil-Specific Ignition Delay 160 Oil-independent Flame Propagation Period 140 120 Vaporization 100 Mixing, & 80 Chemcial Delay - CL) 60 I Oil Vaporization Trigger 40 Highly Exaggerated Oil Auto-Ignition Bump Starts a I Ii. Onset of Knock Flame 20 0 0 5 10 10 15 Time (ms) 20 25 30 Figure4.4: Illustrationof oil-induced-knock stages (NOT ACTUAL DATA) In actual data, the oil auto-ignition bump was not discemable outside of the noise of the transducer, which made it difficult to determine the oil-specific ignition delay time. Since this study is focused on how oil might influence sporadic pre-ignition, we were really only concerned with the duration of Stage 2 for each individual oil. The total time from the oil vaporization trigger to the onset of knock can be measured, since both times are known. Therefore, the combined duration for Stages 2 and 3 can be obtained from the data. This means that if the duration of Stage 3 can be measured then the duration of Stage 2 can be calculated, which is exactly the purpose of the next experiment. In the next experiment, the heat from the wire was used to simulate auto-ignition of the oil and initiation of flame propagation (Stage 3). By significantly increasing the heat of the 42 wire, a flame could be started at the time of the trigger allowing us to isolate and measure Stage 3. Subtracting the measured duration of flame propagation (Stage 3) from the total time required for the onset of knock (Stages 2 & 3), we were left with the oil-specific ignition delay time (Stage 2). The results of this experiment are shown in Figure 4.5. 200 Oil-Specific Oil-independent Flame Propagation Ignition (7.7 ms) Delay Time 180 160 140 A Black Curve is WITH Oil (Vapor-Ignited) 120 S120 (A00Tige 100 C ~0 80 Hot Wire Oil Vaporization Trigger (30V Setting) 80(70V Flame Initiation Trigge Setting) 60 Red Curve is NO Oil (Wire-Ignited) 40 20 00 5 15 10 20 25 30 Time (ms) Figure4.5: Wire-initiatedflame propagationto isolate oil-specific ignition delay Using the wire to initiate the flame resulted in a flame propagation duration measurement of 7.7 ms. To ensure that the heat was actually igniting the mixture at the trigger point, the transformer dial setting was increased to 70 volts. At lower voltages, there was a slight delay between the trigger and when flame propagation actually began. To make sure that the 70 volt setting was immediately starting a flame, a range of voltage settings were tested between 40 and 70 volts. The corresponding flame propagation measurement for each setting was plotted versus wire energy, and the results are shown in Figure 4.6. 43 11 E 40V 10 Time to knock is no longer dependent upon 45V transformer voltage setting 0 0 E 55V 0 2 4 6 8 -65V 10 '70V 12 14 Wire Energy (J) Figure4.6: Dependence offlame propagationmeasurement on wire energy (no oil) Since the time between the trigger and onset of knock reached an asymptotic minimum around 8 J, this minimum was attributed to the time required for the flame to propagate. This would mean the time to initiate the flame had approached zero. Therefore, it can be inferred from the results that flame propagation took about 7.7 ms, under the current conditions. 4.4 Synthetic Versus Non-Synthetic Oil It has been proposed by some automotive companies that different base oils lead to different SPI behavior in engine tests. The American Petroleum Institute (API) has divided all base stocks into five categories, which are detailed in Table C. 1 in Appendix C. Groups I, II, and III are refined from petroleum crude oil and are considered mineral oils while Group IV is made up of polyalphaolefins (PAO) which are 100% synthetic. Group V is a catch-all for any oil that does not fall into the first four groups [18]. General Motors (GM) has observed significantly lower SPI frequency when using a PAO base stock (synthetic) instead of a standard hydrocrack base stock (non-synthetic) [6]. A similar claim has been made by Toyota in that the use of Group IV (synthetic) base oils resulted in lower frequency of SPI than Groups I or II (non-synthetic) [5]. In theory, these results should translate into synthetic PAO oils having longer ignition delays. 44 Eight different oils were tested in the RCM: 2 non-synthetics, 3 synthetic blends, and 3 full synthetics (one of which was a gear lube). Two of the oils were recommended by GM after producing notably dissimilar SPI behavior in engine tests. The oils are listed in Table 4.1. Table 4.1: Eight preliminary lube oils tested in the RCM | Oil B 15W-40 oil C Oil D Oil E Gear Lube 5W-20 5W-30 15W-50 75W-90 Blend Blend Full synthetic Full synthetic GM A GM B 5W-30 5W-30 Blend Full synthetic Non-synthetic Each of the eight oils were vaporized in the RCM at the end of compression, and the total time from the vaporization trigger to the onset of knock was compared between them. Figure 4.7 shows representative curves for Oil A and Oil E. 200 180 Oil A Non-Synthetic 15W-40 160 140 (2 trials) 120 MJ L (2 trials) 100 80 C Oil E Full Synthetic 15W-50 Trigger LI- 60 40 20 0 0 5 15 10 20 25 Time (ms) Figure4.7: Preliminarytest of synthetic versus non-synthetic oil 45 30 After testing for all eight oils had been thoroughly repeated in the RCM, an average time to the onset of knock was calculated for each. Using the method explained in Section 4.3, the auto-ignition time for each oil was calculated by removing the time associated with flame propagation. The resulting oil-specific auto-ignition times for all eight oils are tabulated in Figure 4.8. These times include vaporization, mixing, and chemical ignition delay. 5.9 6.1 5.4 5.1 4.4 6.0 4.6 4.0 Oil C Oil A (5W-2 D) (15W-40) GM B Oil B GMA (5W-30) (15W-40) (5W-30) Oil D Gear Lube Oil E (5W-30) (75W-90) 15W-50) Figure 4.8: Ignition delay times for all 8 oils in milliseconds Figure 4.8 shows that in general the oils with full synthetic base stocks had longer ignition delays than those with non-synthetic base stocks. There was no definitive difference in the ignition delays between non-synthetics and blends. The difference between the two GM recommended oil ignition delay times was about 0.8 ms, which corresponds to just under 10 CAD in an engine operating at 2000 rpm. This was small compared to the 2.1 ms difference between Oil C and Oil E, which is approximately 25 CAD at 2000 rpm. This suggests that Oil C is significantly more likely to initiate pre- 46 ignition than Oil E when exposed as vapor to the ignition environment. It was not clear why the GM recommended oils produced such similar ignition delays in the RCM after behaving very differently in GM's engine tests. One speculation is that since the oils used at GM and the two used here at MIT were purchased at different times and locations, there may have been some influential difference between the batches tested. Overall, there was a correlation between the base stocks and ignition delay times measured in the RCM. In an engine, we would expect the synthetic oils (longer delays) to have a lower probability of producing SPI while the use of non-synthetic oils (shorter delays) should be more likely to produce SPI. These findings agree with those published by GM [6] and Toyota [5]. 4.5 New Versus Used Oil The results in Section 4.4suggested that the base oil had some effect on ignition delay in the RCM. It was likely then that degradation of the base oil should have some sort of impact as well. Over time, repeated exposure to heat and pressure causes lube oils to become thermally unstable and oxidize, especially after the depletion of oxidation inhibitors (such as zinc dithiophosphate) [19]. Toyota has suggested that this oxidation instability is a key element in SPI [5]. A new and used version of two different oils were tested in the RCM. The two oils tested were Oil D and GM A. The used Oil D sample came from a GM LNF experimental engine in the Sloan Automotive Lab at MIT, and the used sample of GM A was provided by GM. The two used samples had very similar fuel dilution of about 8% by weight, and both had a KV100 (kinematic viscosity at 100 0C) below the allowable limit for 5W-30 grade oils. This amount of fuel dilution is considered very high [13]. The results from the RCM are shown in Figure 4.9. 47 5.9 4.6 4.1 3.8 GM A Oil D Figure4.9: Ignition delay times for new versus used oils in milliseconds Although the used version of both oils produced shorter ignition delays, Figure 4.9 shows a much more significant change for Oil D (-2.1 ms) than for GM A (-0.5 ms). One possible explanation for the discrepancy is that both Oil D samples (new and used) originated from the same 5 gallon bucket, while the new and used versions of GM A came from different batches. Therefore, variations in the sample batches might have been influencing the results for GM A. Nevertheless, degradation of the oil decreased ignition delay, which should result in a higher probability of SPI occurring in an engine. This would suggest that the likelihood of SPI happening should increase with extended use, until the oil is replaced. Due to variations in base stocks and additives amongst different oils, we would expect to have a range of sensitivity to degradation. This would imply that some oils can maintain a level of SPI resistance for longer than others. 4.6 Effect of Oil Additives As mentioned in Section 4.5, lubrication oils contain a variety of chemical additives which promote maintenance of the engine such as detergents, corrosion inhibitors, anti-wear additives, and many more. It was proposed by Toyota that several of these additive 48 compounds have conflicting catalytic effects on oxidation, and they have found a strong correlation between oxidation catalysis and SPI frequency [8]. General Motors provided their resources to perform an elemental analysis on all ten oils tested in the RCM so that the additives could be compared to ignition delay times. The results of that analysis are tabulated in Table 4.2. Table 4.2: Additive elements in ten new and used oils tested in the RCM Oil 6 98 <10 28 531'" 1953 10 603 8 3.8 219 11885 <2 <5 <10 32 649 2554 1 828 0 4.0 6 52 1552 <2 59 2 64 580 2065 17 62 Oil A <3 49 2616 <2 <5 301 <5 1013 5209 3 1085 0 4.4 GMA <3 192 1860 <2 <5 <10 64 630 1912 3 733 0 4.6 Oil B <3 488 1415 <2 <5 372 88 1069 2686 5 1184 0 5.1 GM B <3 95 1165 <2 <5 918 74 663 1563 4 821 0 5.4 Oil D <3 144 1645 <2 <5 11 31 623 2071 3 792 0 5.9 Gear Lube <3 252 16 <2 <5 <10 <5 1839 12170 <1 10 0 6.0 USED D Oil C USED GMV A 6" 60 <3 1L270 7. 4. There are four additives that have been linked to SPI promotion: calcium, iron, molybdenum, and phosphorus. Toyota has found that the first two increased SPI through oxidation catalysis, while the second two decreased it through peroxide decomposition [8]. In order to minimize competing effects, only the new motor oils were plotted (i.e. excluding the gear lube and used oils). Since the base stock had an influence on ignition delay, it was not reasonable to compare oils with dramatically different bases. The effect of calcium is plotted in Figure 4.10. 49 6.5 Used oils and gear lube are excluded from this plot 6 , 55 * 0 Sb 45 ** 0 3.5 4- ---------0.15% 0.10% - 5% 0.20% 0.25% 0.30% Calcium Concentration (mass %) Figure4.10: Ignition delay versus calcium concentrationfor all 8 new motor oils Although the correlation was weak, delay times decreased as calcium concentrations increased. It was not surprising that the correlation was weak since there were so many competing effects involved (e.g. other additives and variations in base stocks). Nevertheless, the trend agreed with claims made by Toyota [8]. The same plot was made for molybdenum concentrations and is shown in Figure 4.11. 6.5 6 0 5... 55t 0 o Used oils and gear lube are excluded from this plot 4 0.000% 0.002% 0.004% 0.006% 0.008% 0.010% Molybdenum Concentration (mass %) Figure 4.11: Ignition delay versus molybdenum concentrationfor all 8 new motor oils 50 Once again, there was a weak correlation; however, the ignition delay times increased with increasing molybdenum. Again, it was likely a weak correlation due to the multitude of competing effects. The direction of the trend agreed with Toyota's findings [8]. A third plot was made for the phosphorus concentrations and is shown in Figure 4.12. 6.5 6 5.5 0 5 0 0 Used oils and gear lube are excluded from this plot 4 3.5 , 0.00% ------0,10% 0.05% ---0.15% 0.20% Phosphorus Concentration (mass %) Figure 4.12: Ignition delay versus phosphorus concentrationfor all 8 new motor oils Phosphorus concentrations produced no correlation with the ignition delay times of the oils. At this point, it was not clear whether the competing effects were simply negating any potential correlation; however, from Toyota's results [8] we would expect to see ignition delay times increase with increasing phosphorus concentration. Iron concentrations were not plotted since only the used oils contained measurable traces of the element. Toyota found iron to have similar effects to calcium [8], so we would expect to see ignition delay times decrease with increasing iron concentrations. It was clear that the effects of each of these additives needed to be isolated before any conclusions could be made. This would be done by artificially "doping" each of the oils with additives to see how the ignition delay times would be effected. The range of additive element concentrations and base oils are shown in Table 4.3. 51 Table 4.3: Concentrationsof additive elements in thefour "doped" oils Calcium GM B Oil B GMIA Oil D Calcium Sulfonate I Phosphorus Iron ZDDIP Iron Naphthenate 1165 1415 630 <5 5000 5000 40GO 500 Each oil was selected for having a low initial concentration of the element to be added. Compounds were added so that a range of concentrations could be tested up to the maximum concentrations tabulated in Table 4.3. The results are plotted in Figure 4.13. 6.5 6.5 1~ i 6 2.2 ms drop 5.5 6 0.9 ms drop 5.5 5 5 0 0 -E4.5 :E 4.5 Calcium GMB 4 3.5 -- -r-- 0 Calcium 04 - - r------ .... -..... ...... 3.5 0 1000 2000 3000 4000 5000 Calcium Concentration (ppm) 6.5 6.5 E 0.2 ms range S6 4 0.3 ms range 5.5 5.5 0 5 5 4.5 4.5 Phosphorus 4 3.5 - 0 -r-- 1000 Iron Oil D 34 GM A - 0 1000 2000 3000 4000 5000 Calcium Concentration (ppm) - 2000 --- 3000 ...-. r --. r --- r - - 3.5 , 4000 0 100 200 300 400 Iron Concentration (ppm) Phosphorous Concentration (ppm) Figure 4.13: Isolation of the effects of additives in lube oils 52 500 The results show that calcium did have an influence on ignition delay times measured in the RCM. The effect was especially significant in GM B, which saw a decrease in delay time that was nearly equal to the entire range of oils tested. The 2.2 ms drop for GM A would correspond to about 26 CAD in an engine operating at 2000 rpm. The same trend was observed for Oil B. While the 0.9 ms drop was less significant, it corresponds to about 11 CAD at 2000 rpm, which is not trivial. It should be noted that GM B was a fully synthetic oil while Oil B was completely non-synthetic. Furthermore, the two oils had different initial concentrations and delays. The results show that the oils had dissimilar sensitivities to the effects of the additives. As for phosphorus and iron, the effects claimed by Toyota [8] were undetectable in the RCM measurements. In both cases, relatively high concentrations were added with negligible changes in ignition delay times (<0.5 ms spreads). The effects of varied molybdenum concentration were never tested in the RCM since molybdenum dithiocarbamate (MoDTC) was never sourced. Overall, the RCM results for calcium and potentially molybdenum helped explain why these elements have been observed to influence SPI frequency in engine tests. Calcium decreased ignition delay, which would increase the probability of SPI, while the correlation for molybdenum indicated that higher concentrations may be increasing delay times, which should decrease SPI frequency. These findings agree with claims from a study done by Toyota [8]; however, concentrations of phosphorus and iron had little effect on ignition delay in the RCM, which does not support corresponding claims made by the same Toyota study [8]. 4.7 Effect of Oil Physical Properties The previous three sections focused on the chemical characteristics of the oils. It was logical then to look into their various physical properties. Since all of the oils tested are commercially available, physical data is published and readily accessible. Five physical parameters are commonly published for each oil: 53 * NOACK Volatility (ASTM D5800) -measured wt% loss due to evaporation [20] * Pour Point (ASTM D97) - min. temperature associatedwith oilflow [21] * Flash Point (ASTM D92) - min. temperature associatedvaporflammability [22] * KV40 (ASTM D445) - kinematic viscosity at 40'C measured in cSt * KV100 (ASTM D445) - kinematic viscosity at 1000 C measured in cSt The material safety data sheets (MSDS) for each oil were studied to collect information on the five parameters. Any information that was not readily available was obtained through correspondence with the manufacturers for each oil. The published data along with KV100 values measured by GM are tabulated in Table 4.4. NOACK USED Oil D PourPoint FlashPoint KV40 KV100 ----- XV100 , Table 4.4: Publishedand measuredphysical properties of the test oils 6.868 3.8 oil C 14.3 -39 185 67.0 11.1 8.94 4.0 Oil A 12.00 -30 199 118.0 15.2 15.59 4.4 GM A 7.90 -39 225 63.3 10.9 11.17 4.6 oil BB 18.34 -30 204 1625.0 153 15372 5.1 Oil D 14.70 -42 135 63.5 10.5 10:24 5.9 Gear Lube N/A 40 165 125.0 14.6 14.55 6.0 oil E 6.40 -39 232 125.0 18.0 1.64 6.1 It should be made clear that the published values are expected to differ somewhat from the actual physical properties for the specific oils tested in the RCM. Since there are variations between batches, it is highly unlikely that the test oils will match published data exactly. This was evident when comparing the measured and published KV100 values. Oil C was 54 measured to have approximately a 20% lower KV100 value than what is published while Oil E had a roughly 10% higher KV100 value than what is published. Nevertheless, the ignition delay times were plotted against the five published physical properties, and the results are shown in Figure 4.14. The used oils were excluded since their physical properties were expected to be drastically different from their new counterparts. Figure 4.14 shows that there was no correlation between published physical data and ignition delay times measured in the RCM. It is not clear if the discrepancy is due to disparities between published and actual values or whether the experimental procedure is possibly making the physical characteristics non-limiting, but there is no discernable trend for any of the physical properties. 55 NOACK 6.5 6 5.5 0 Gear lube does not have a published NOACK value 5 4.5 4 0 3.5 4.0% 19.0% 14.0% 9.0% NOACK (wt% loss) Pour Point Flash Point 6.5 6.5 6 6 55 5.5 5 5 4.5 4.5 0 4 - - 3 .5 -45 -40 - - - - --35 Pour -30 - .. -. - - - --.- 3.5 ---25 100 150 200 250 KV0sh Pint (C) Pnint (oC) KV40 KV100 6.5 6.5 6 60 55 5.5 0 50 5 4.5 4.5 40 0 4 3.5 3.5 0.0 50.0 100.0 150.0 9.0 KV40 (cSt) 11.0 13.0 15.0 17.0 KV100 (cSt) Figure 4.14: Publishedphysical data versus oil ignition delay times 56 19.0 4.8 Effect of Charge Dilution It has been observed that charge dilution could reduce SPI. Dilution is typically in the form of exhaust gas recirculation (EGR). The Southwest Research Institute has claimed that reasonable levels of EGR produce a substantial reduction in SPI frequency [1]. Since the RCM ignition delay results correlate well with SPI observations in engines, observing dilution effects on ignition delay should help to fundamentally understand how EGR is mitigating engine knock. There are three potential explanations for how dilution could prevent pre-ignition, assuming oil vapor is the ignition source: Scenario 1. Auto-ignition of the oil is fully suppressed by the inert gases Scenario 2. Auto-ignition occurs but a flame does not start in the diluted mixture Scenario 3. Auto-ignition occurs and starts a flame but dilution slows the flame propagation, hence mitigating the compression ignition of the end gas To find out if any of the above are occurring, dilution in the form of nitrogen gas was added to the mixture in the RCM. Nitrogen was added in increments of 10% by volume up to 50% in the air-nitrogen mixture. In other words, at 50% dilution, the mixture would have approximately 10.5% oxygen by volume and about 89.5% nitrogen by volume, assuming air is composed entirely of oxygen and nitrogen. The fuel mass was kept constant, and k was fixed at I for every case. Since ignition delay times are highly sensitive to temperature, it was imperative to keep the end of compression temperatures the same, despite reaching higher pressures. This was done by lowering the pre-compression temperatures such that the gases reached a temperature of about 650 K after compression, assuming adiabatic conditions. Oil A (reference oil) was the oil used in this experiment, which again had a non-synthetic base oil and produced a relatively short ignition delay time in the RCM (4.4 ms). The results for dilution with nitrogen up to 50% are shown in Figure 4.15. 57 - 180 Piston retr action and push-back due t o overpressure 20% - 160 1 0% 30% - 140 40% 50% 0% Dilution - 120 100 80 Trigger 60 20 - 40 0 0 20 40 80 60 100 120 140 Time (mns) Figure 4.15: Dilution with nitrogen (fixedfuel amount and2=1) As expected, the total times to end-gas auto-ignition increased with increasing dilution. It can also be seen from the slopes of each curve that flame propagation occurred in every case, which would imply that the oil vapor auto-ignited in each case. This would suggest that we are observing Scenario 3: oil auto-ignition occurred and started a flame but the entire process was significantly delayed by dilution. In an engine, it is expected that at high levels of dilution the time required for the end-gases to auto-ignite would be so long that the piston would already be travelling down the expansion stroke, thus preventing auto-ignition from occurring. Although it was very clear from the results that dilution delayed knock, it was not obvious whether that delay was mostly due to slower flame propagation or if the oil auto-ignition was also substantially delayed. Flame propagation is not comparable between the RCM and an engine because the RCM utilizes constant-volume combustion, simple geometry, and research grade fuel, 58 while in an engine, volume is always changing, turbulence is promoted, and fuels vary. As a result, the effect of dilution on oil auto-ignition time is much more valuable when comparing RCM results to engine data. As described in Section 4.3, the oil-specific ignition delay times for dilution up to 30% were calculated by subtracting measured flame propagation times from the total times to knock for each dilution case. The results from this experiment are shown in Figure 4.16. - 30 - 32.1 35 Oil A (Reference Oil) 23.1 25 am Time to Knock Onset e agation Prop Dura tion 16.4 12.7 15 12.1 010 t 91. 00 W E I at 6.3 - 10 M * , 0 . - do - - - O 20 -""a 4.41 , Time to Oil Auto-Ignition 00'- 0 0% 5% 15% 10% 20% 25% 30% Nitrogen Dilution Figure4.16: Oil ignition delay times versus nitrogen dilution Looking at the range of oil ignition delays, there was a very significant effect from the dilution. Even at 10% dilution, which is typically considered a modest amount, the ignition delay for Oil A was already slowed from 4.4 to 6.3 ins, which was a longer delay than any of the oils tested. At 30% dilution, the ignition delay time was almost tripled. In this experiment, flame propagation and oil auto-ignition were proportionately delayed since the 59 oil-specific delay accounts for roughly 40% of the total time to knock for all four cases. It should be noted that there was more opportunity for error in the dilution cases, mostly due to mixture preparation uncertainties associated with adding nitrogen; however, the trends are still very clear. Overall, dilution had a much more significant impact on ignition delay times than chemical or physical properties of the oils. This finding agrees with a claim made by SwRI that 10% EGR totally eliminated SPI from the 0% case, which produced approximately 4 SPI events per 30,000 cycles, with each event averaging about 4 pre-ignition cycles [1] (i.e. relatively severe SPI). 60 Chapter 5: Summary and Conclusions 5.1 Oil Vapor and Ignition Delay It has been observed that oil vapor and air mixture in the cylinder can initiate combustion and lead to compression ignition of the end gas. Thus, lube oil is confirmed as the ignition source. The amount of oil required to initiate a flame in the mixture is consistent with masses seen in typical oil droplets found in the engine cylinder. The oil ignition delay measurements in the RCM use oil vapor evaporated from a heated wire. Therefore, the delay includes both physical and chemical processes. The wire must be heated, the oil vaporized, the vapor mixed, and finally chemical reactions must occur before the oil vapor can auto-ignite. As shown in Section 2.2, wire heating is short, on the order of 0.5 ms; however, the mixing time is not trivial. The chemical ignition delay of the oil is of the order of milliseconds since the overall ignition delay is around 5 ms. Evidence that mixing time constitutes a substantial part of the delay lies in Figure 4.2. Since the overall time from the trigger to the onset of knock remained relatively unchanged between the "Normal Trigger" and "Early Trigger," the physical processes are on the order of roughly 3 or 4 ms. The chemical processes are heavily dependent upon temperature, therefore, if the physical processes were negligible the "Early Trigger" would produce a longer delay since the vapor-air mixture is prepared at a low temperature and pressure (beginning of compression). For example, if we assume for a moment that vaporization and mixing occur instantaneously and chemical delay accounts for 100% of the oil ignition delay, then in the "Early Trigger" case the ignition delay would be much longer since for about 4 ms the oil vapor is exposed to the lower temperatures and pressures seen during compression, which would increase the delay. However, this is not the case, which means that for the majority of that 4 ms the ignition delay is relatively independent of temperature, suggesting that mixing is taking place, rather than substantial chemical reactions. The reason this is important is that if indeed the physical processes are taking about 3 to 4 ms then the chemical ignition delay would be on the order of 1 to 2 ms, which would undoubtedly cause SPI in an engine. 61 5.2 Oil Characteristics In general, ignition delay times correlated fairly well with engine results. The findings suggest that oil base stock and deterioration effect the ignition delay, with synthetic oils being less likely to produce SPI than non-synthetics, and new oils being less likely to produce SPI than their used counterparts. Additives in the oils also play a role. Calcium significantly increases the likelihood of SPI. Any effect from phosphorus and iron was undetectable in the RCM results. Molybdenum could potentially have a positive effect in mitigating SPI; however, its effects were not isolated in the RCM experiments, and no meaningful conclusion can be made. The physical properties of the oils play little role as no correlation has been seen between published physical data and ignition delay times. It should be noted that the published data may differ from the actual values of the particular samples of oil tested. The heated wire vaporization process may also render the physical properties to be non-limiting. 5.3 Charge Dilution Charge dilution significantly delays flame propagation and oil auto-ignition. Even at modest levels of dilution, oil ignition was significantly delayed. At 10% dilution with nitrogen the oil uto-igrnition was delayed by nearly 45% from the 0% dilution case. The effect of dilution far outweighed any effects due to variations in oil properties. A maximum difference in delays of 2.3 ms was seen across the wide variations in oil base stock, deterioration, chemical additives, and physical properties, while more than 8 ms was achieved just through dilution. 5.4 Applicability to Engines The end of compression temperature and pressure in the RCM were chosen to match SPI conditions seen in engines. After compression, the gases are roughly 650 K and 26 bar, which compares well with SPI conditions seen by GM in which the ignition environment is also about 650 K and between 20 and 30 bar. 62 The range in delay times of 2.3 ms between the best and worst of the ten oils corresponds to 27.6 CAD at a speed of 2000 rpm, which is fairly substantial. This wide range should impact SPI behavior. In addition, several of the ignition delay correlations presented in this study agree reasonably well with actual engine tests, suggesting that the ignition delay measurements in the RCM are a decent indicator of SPI probability. 5.5 Closure The results of this study indicate that oil vapor is an ignition source for initiating flame propagation, which leads to end gas knock. The observed ignition delay of approximately 5 ms results from both physical and chemical processes. The delay, which corresponds to 60 CAD at 2000 rpm, is short enough so that oil vapor that is introduced during the intake or early compression can ignite the charge. This delay is sensitive to oil composition. Calcium additives have been found to decrease the delay. Charge dilution is found to be a much more effective tool for SPI mitigation than alteration of oil characteristics. On the other hand, the range of delay times measured is large enough that the different oils should exhibit different SPI behavior in an engine. Base stock, degradation, and additives all impact ignition delay. Fully synthetic oils generally produced longer ignition delays, hence they are less likely to produce SPI. Degraded oils produced shorter ignition delays making them more likely to produce SPI. Calcium in the oil acts as a catalyst for oil auto-ignition and would increase the likelihood of SPI. New fully synthetic oil with low concentrations of calcium combined with the use of moderate levels of EGR are expected to significantly help reduce the likelihood of SPI. 63 64 Appendix A: Rapid Compression Machine A.1 RCM Schematics All of the following images have been adapted from the thesis of Ioannis Kitsopanidis [14]. ............. ....... ............... 0- - LAJ _j >-J" LY > LJ 17 CL C. 17 C, CL II Ofa a_ 65 vENT VACUUM PNEUMATIC CHAMBER BYPASS HIGH RELIEF VALVE PRESSURE N2 PRESSURE GAUGE HIGH PRESSURE TANW DRAIN FigureA.2: Pneumatic (driving) system [14] VENT r7 PRESSURE GAUGE HIGH PRESSURE N2 DAMP OILOIL SIGHT TUBE OIL -N2VA SEPARATION TANK HYDRAULIC LOCK OIL PUMP PRESSURE GAUGE - C-lF IRING E PIN GROOVE MECHANISM OIL DRAIN A Hydrau=: ( OIL RESERVOIR FigureA.3: Hydraulic (locking-r-eleasinig) syvstemn [14] 67 MIXING FAN METERING VALVE AIR L VACUUM PUMP PRESSURE Q GIASE DLiNE INE CTIEN P RI 1IXIN TPANK TEMPERAT URE (THERMIST OR) VENT TEMPERATUR E (THERMISTO VACUUM PUMP PRESSURE (I mbar) TEMPERATURE (THERMISTOR) TEMPERATURE (THERMISTOR) N2 PRESSURE (0,1 Torr) TEMPERATURE (THER ISTOR) COMBUSTION CHAMBER ALL LINES & VESSELS ABOVE ARE HEATED AND INSULATED Figure A.4: Mixture preparation setup [14] 68 A.2 RCM Mechanical Drawings [14] No. 1 2 3 4 5 PART NAME HEAD WINDOW OTY 1 1 1 228 U-RING HEAD PLATE 113 D-RING 4 I"-L14-/2" HOLLOW SOCKET SET SCREW 4 1 6 7 8 5-938 0-RNG COMBUSTION CYLINDER 1/8"-18 NPT CONNECTOR 9 10 3/8"-16-1 1/2" SOCKET HEAD CAP SCREW HYDRAULIC STOP RING 3/8"-16-2 3/4" SOCKET HEAD CAP SCREW 350 0-RING 11 12 13 14 15 16 17 I 1 6 1 8 1 1 HYDRAULIC CYLINDER 1/2"-14 NPT CONNECTOR ACKNG 2 1 RING 1 342 0-RING 1/2"-13 HEX NUT & 1/2" FLAT WASHER 6 1 18 19 STROKE ADJUSTMENT RING 20 21 1/4"-18 NPT CONNECTOR 1/2"-13-5" STUD 1 6 22 PNEUMATIC CYLINDER 23 358 U-RING 1 24 25 26 27 28 FLANGE 1/2"-13 HEX NUT L 1/2" FLAT WASHER 1/4"-20-1 1/4" SOCKET HEAD CAP SCREW L 1/4" LOCK WASHER STROKE ADJUSTMENT SPACER 1/2"-t3-5" SOCKET HEAD CAP SCREV & 1/2" FLAT WASHER 29 30 31 32 33 34 SPACER HEAD) CAP SCREW & 1/2" FLAT WASHER STAND 3" SCH. 80 PIPE SECTION PNEUMATIC PISTON 1/2"-13-4" STUD 1 6 L 6 I I I 6 6 4 2 1 38 PNEUMAT1C SHAFT 1 39 40 41 SLC4300A90BT12501000-250 SEAL 8-32-1 1/4" SOCKET HEAD CAP SCREW SHAFT COUPLER 1 4 1 1 210 STUD 2 4 42 1/4"-20-1" 43 45 HYDRAULIC LOCK RING 141 0-RING 4-40-1/4" FLAT HEAD SCREW 46 210 47 48 49 HYDRAULIC P[STON 1/2~-13-I 1/2" SOCKET HEAD LOCK CAP SCREW S LOCK WASHER HYDRAULIC SHAFT SLC4300A90BT12501000-250 SEAL 52 BRACKET 53 54 1/2" FLAT COPPER L RUBBER WASHER SIDE WINDOW HEAD WINDOW RETAINING RING 1/2"-13-5" SOCKET HEAD CAP SCREW SOCKET HEAD CAP SCREW L LOCK WASHER 1 57 58 0-RING 1 1 1 L 4 1 4 4 &4 4 iMSSACHUSETTS INSTITUTE OF TECHNOLDGY SLOAN AUTOMOTIVE LABORATORY ASSEMBLY SECTION AND VIEWS RAPID COMPRESSION MACHINE 69 1 1& 1 4 4 HEX NUT & FLAT WASHER 3/8"-16-1 1/2" HEAD CAP SCREW DRAWN BY IOANNIS KITSOPANIDIS DATE. 08/21/00 1 2 1/2"-13 HNITSi in &1 1 1 8 50 51 N427418701625 SEAL 56 6 L4 6 N4274A854622E40804500 SEAL 0-RING 1/2"-13-3" 55 L 1 1 35 36 37 44 6 1 1/2"-13-1 1/2" 1/2"-13 NUT L 6 Duv r% I OF 10 11 9 7 5 55 15 13 16 18 22 20 24 23 25 26 3732 53 51 s 49 36 30 4 46 44 42 40 39 36 35 33 34 31 3-10 1/4' -.1 0 VIEW A 57 56 VIEV B 55 19 27 20 OML 18 52 58 Note: Part 1 has been replaced with the electrode for this study 28 At'. 30 29 SECTION A-A' DtA 4751 213 040 MOiVE GEUIVE Wr 11-U41 -u -I f - -- PART No. 3 ISTLEW 4 CA I tILL 33t" IS A% 4,71 K A' MA S624. 4- &M STAP 0.33 , 1.3 so03 103n(3 UafS Re NOT SH@" I--4 3/1-54, L 35165 -46 - L AP - 3A &5 SECTION A-A' A e f PART No. 55 / -NMI \\~~ 304 001SAO1SETTS VISS7T MAT No. 3 U) Aw OFr TEOELSXI 4 (A ON A4.71 K wolUS in PMR mw5S SUART WEf6 T ILT NA1C3I5Sim ITIL L17 Sf6 VISSN3 WETAMM IS SI1 mrmkESl. MlL 111.17 MOW IV I3 WG #I*. MUMI KIJWASMS I I.f W0 IAT URI " \\ PART PART No. 43 PART No. 1 SECTION A PART No. 54 No. 19 PART No. 19 A-A' tRILL 3/ 4-4 ElAt he"C j W6 DRL L -----1---- k) PART No. 41 PART No. 30 SECTION A-A'A ,-0 A D# #4 A M"40K A' .873AnMILL 336 1\ 0DRILL 4 L 33M L i5 IUAM UTMWIVE 047WUTELAUMTMN or1CH& M$$AO4JSCUS DAMWTTY PrMT WME PWRT U. It TMO 3 K6"TA .U NATE&A I IWALIC LINK 0551 43 "TERIM, STEEL SIL1? I A WAD Ow~ I RATEMILM.'riSILXA POEER 19 PATOUK-. Sr. 11t,17 SWT WATEOIM, WIE RA7I3M 41 54 UNT KIT3WPSIDIS DATE, Ora, .IOAM4 I COUPLE* STEEL 1IL17 VIIOV FWES SILIA 4 30of PART No. 7 SECTION A-A' few A I- T I, DRILL 33 MA INA43 Jr I-o Mw 8' DRIL r .7413t3 DRILLV/ sSacCti 9NSUTWt? OF TCOOGY UAN AMXTIVC LAIPAYPYit Ph? low auwtnvY PART ND 7 3S1STUE CVLIMK DAtrlfn Sim Imp I a &Ms -oo 3.99* F **0** 0 OMM -----j - L PART No. 49 - ~~"r' im -- TAP fort'1 1! /N ..... 195 SECTII3N A-A' TUp 3M9-16 PART No. 10 \A' OW v a EA $5004000 GM~3VE A 7 ac MMSOMTS UOA PART IRISER an mail L)b* Not 49 If stw@S --- 97--. WMTV in rw IMUT W TncNI@.m MsaavIVE LAATiPT me, PMT OW NTrOMIC 36II OATERIM.' 3TEEL 1IL17 WIMOKXIC STOP RM VATCWIALj STIM IILII awnR KSTSWOZ lI ATE, S/Utd JRTITY I I S I sVt 19 PART No. 13 SECTION A-A' TAP 3/4-6 NPT AP I-I 410 TAPIgI... -i M 3-- ---- ~-~~ -~~ -----r SM Y MASSACHJSETTS INSTITUTE Tr TEL0GM SLOAN OAUTTtVC LAKIArTMY PART No. 13 UNITS, in PART NAME HYDRAULIC CYLINDER MATERIAL STEL 1IL17 QUANTITY DAWN BY IDANNS KITSOPANII I DATYE NOW&FO OVO N 6 of to I PART No. 32 PART No, 27 SECTION A-A' ~I3s -r z .- A 7 X / Z / I z Z 4 Z;l _ L - MW PART No. 47 a SECTION A-A' CA W 4v 141 * Vi i Wc O-4PN G4/0C f2 a- A SECTD4S MDT IM TIC UWLxv F ~I? P3114. 33/64 IT, LIKS( WIT 3147"I MSSAISEITTS 1"SlIbTUT W T1C14CGY SLOM AUTUNDTiVE LABMIATy PART ft PARTaIARTIv AWA " - DRILL 3V6Ad 38 23 .- 7 PJCAiiMM PTitch MATRIAL- iALON.IMM 6m-T4 I STRIE ADpAJwwTNT SAcEI 6 MATEmM01 STEL lity - I - - / I 1123 ___j 47 MYONA&A.K PITUO MTEMAL: STEEL 9LIt7 LMTS in RJMMIS KITSM'AIDIS DAME P411W I I of l I 1i WA &9% VIEV A-A' MA -- A TAP 1/4-0 T*p A' KOM_ - PART No. 38 A SECTION A-A wY TAPI1/4-I0 I ~~1 -L PART No. 18 00 rACE OwU tI x win5 w a-am GMW FSA40IW1S panfEstvi w MWrO= SLOW AItUlvt LAMTMY WM 3W$* PT la Mt. no?"C K 39 4 CA amA 7 PT WeC PNCUNMIC S9I 9WITY I EW. IG? 18 TEUIdL' It UEAUIlENT -' )MIhM.s ST1UL17~t 449054W MAW Iht"YV I mlb b% WEWM KIYSUPWDII~h of'I I WO aIS/lI PART No. 22 SECTION A-A' --- ( A 00 -- - ------------TAP V/SA r~a SPOT tm xS 6CA OSNA Ix SLOW MITakuIv tlA1TM gftt v PA UK Peal ft I W(IUW CIIUIM AR NATUM SWilL MI.1 "" .&V5mSI W WS= WIU sW am~ or" _.- PART No. 24 PART No. 15 A SECTION A-A' 0~,7 6- r wi~ PILL 33W4 6 EA ONA7 X -. 5 4- -.SQ iasZ wsiSrt MTrUE 15 fidealt 84 M GTtLis $ac=N "a MIUM.. M~iAUI Ut64-4 MTEMM.. licKSL SILlY in own Kitm to of to 80 Appendix B: RCM Operating Manual The following operation manual has been adapted from the original operation protocol outlined by loannis Kitsopanidis [14], who is the creator of the RCM at MIT. These guidelines are specific to the RCM in the Sloan Automotive Laboratory shown in Figure B.1, which was built in the summer of 2000. Figure B.1: The rapid compression machine 81 B.1 The Control Panel Figure H.2: HCM control panel 82 B.2 Initial Preparations Heating the RCM In experiments where the initial temperature is needed to be ambient above temperature, there are eight temperature controllers used to regulate the temperature of the combustion chamber, mixing tank, and supply network. This is the first step in preparation due to the large amount of time required for the RCM to heat up and reach thermal equilibrium. Use the following steps to heat FigureB.3: RCM heaterpanel the RCM: 1. Turn on the main "POWER" for the controllers 2. Turn on the power to the 8 sets of heating elements (6 switches) 3. Use the controller menus to select and initiate the temperature set-point 4. Allow at least an hour for equilibrium to be achieved (usually longer) Filling the RCM with Oil The RCM must be filled to the "suggested level" of oil before it can be operated. The oil prevents damage to the RCM by gradually decelerating the piston at the end of compression. First, the oil reservoir below the RCM must have enough Figure B.4: RCM oil reservoir oil. If oil is not visible within the plastic sight tube on the side of the reservoir then perform the following steps: 1. Remove the 4 bolts (7/16" wrench) holding the top plate of the reservoir 2. Fill the reservoir to the line marked on the plastic sight tube with oil (Dow Corning Series 200 silicone oil is recommended) 3. After filling, reattach the top plate with the 4 bolts 83 When the reservoir has enough oil, the next step is to ensure that there is enough oil in the RCM. Check the glass sight tube on the control panel and if the oil is well below the "suggested level" perform the following steps to supply oil to the RCM: ' 1. Make sure the "Compressed N2 Vent" valve (2) is open 2. Make sure the Firing Valve (11) is set to "VENT' 3. Make sure the "Oil-N2 Separation" valve (1) is open 4. Make sure the "Sight Tube Supply" valve (5) is open 5. Turn on the "Oil Pump" 6. Open the "Oil Supply" valve (4) to begin supplying the oil 7. Use the "Oil Flow Regulation" valve (6) to slow the oil flow 8. Supply to "suggested level" and close the "Oil Supply" valve (4) 9. Allow the oil to settle and repeat steps 6 through 8 as needed 10. Close the following valves in this order: 6, 2, 11, 5 11. Turn off the "Oil Pump" Note that due to variations in pipe cross sections, the oil level in the sight Figure B.5: tube will fall slightly after the "Oil Supply" valve has been closed. RCM sight tube Filling the Pneumatic Driving Tank Before each run, there must be adequate driving pressure in the large pneumatic tank beneath the RCM. The driving pressure generally should not exceed 275 psi. At 250 psi, Figure B.6: RCMpneumatic tank compression time is roughly 15 ms. The pneumatic tank has a pressure relief valve set to 450 psi. Driving pressure should be calculated knowing that the area ratios between the pneumatic piston, hydraulic piston, and 84 combustion chamber piston are roughly 6.25:1.25:1 respectively. Use Table B.1 as an approximate guide for RCM pressures. Table B.1: Approximate RCM operatingpressures 750 50 150 900 60 175 200 225 1050 1200 1350 70 80 90 250 1500 100 275 1650 110 300 1800 120 125 To fill the pneumatic tank to the desired pressure, perform the following steps: 1. Set the desired driving pressure using the pressure regulator on the right N2 bottle 2. Turn the "CompressedAir" valve (7) to "Supply" 3. Allow ample time for the desired pressure to be reached 4. Close the "CompressedAir" valve (7) Preparing the Mixture The mixing tank allows for fuel and oxidizer to be well mixed and homogenous before compressing them in the RCM. This process will likely vary greatly from project to project; however, the fundamentals should be similar. It FigureB. 7: RCM mixture preparationsetup should be noted that it is not ideal to prepare the mixture while the mixing tank is heating up since metering pressures will not 85 be consistent while the temperature is in a transient state. Follow these steps to prepare the mixture: 1. Vent the mixing tank 2. Replace the gasoline injection port septum if it is worn 3. Turn on the "MIXING FAN" 4. Make sure the combustion chamber valve is closed 5. Open the valves between the 1000 torr Baratron pressure gauge and the tank 6. Turn on the mixing tank "VAC PUMP" and open the vacuum pump valve 7. After sufficient vacuum has been made close the valve to the vacuum pump 8. Turn off the mixing tank "VAC PUMP" 9. Use the syringe to inject liquid fuel into the tank using the 1000 torr pressure gauge 10. Close the valves between the 1000 torr gauge and mixing tank to avoid damage 11. Meter in any desired gases using the 4 bar LEO 2 pressure gauge and meter valves 12. Do not exceed 2.5 bar in the mixing tank or the fan seal may fail Retracting the Piston In order to fully retract the piston in the RCM (equivalent to BDC in an engine), a vacuum must be applied to the pneumatic chamber so that the piston is essentially sucked to the back of the RCM. This also allows for contact to be made between the "HydraulicLock 0ring" and the back of the hydraulic chamber to enable good sealing. To fully retract the piston assembly, perform the following steps: 1. Make sure the "Rear Chamber Vent" valve (8) is open 2. Turn on the "Vacuum Pump" 3. Turn the "Pneumatic Chamber" valve (9) to the "Vacuum" position 4. Make sure the blue retraction mark on the piston shaft is visible 5. Close the "Rear Chamber Vent" valve (8) 6. Close the "Pneumatic Chamber" valve (9) 7. Turn off the "Vacuum Pump" 86 Supplying the Charge Once the piston is fully retracted, the chamber is ready to be filled with the mixture from the mixing tank. Follow these steps to fill the combustion chamber with the test gases: 1. Open the valve between the combustion chamber and the 1000 torr pressure gauge 2. Turn on the "Vacuum Pump" from the control panel 3. Open the valve between the chamber and vacuum to evacuate the cylinder 4. After sufficient vacuum has been made close the valve to the vacuum pump 5. Turn off the "Vacuum Pump" 6. Close the valve to the 1000 torr gauge to avoid damage 7. Meter in the mixture from the mixing tank using the 4 bar LEO 2 pressure gauge 8. Meter in any additional gases 9. Close the combustion chamber valve 10. Allow to sit for 10 minutes to reach thermal equilibrium B.3 Firing Sequence Locking the Piston Once the piston assembly is retracted, it is ready to be locked in place with a pressure differential being applied across the hydraulic chamber. This process should be performed with care since high pressures are involved and there is potential for damage to the RCM. After the O-ring on the back of the hydraulic piston makes contact with the back of the hydraulic chamber, the two halves of the chamber are sealed off from one another. Applying pressure to the forward end of the chamber places a differential across the two sides of the piston, thus locking it in place. This hydraulic locking allows driving pressure to be applied to the pneumatic chamber without the piston moving. The locking pressure is applied to the oil using compressed nitrogen gas. Follow these step to lock the retracted piston in place: 87 Set the desired locking pressure using the pressure regulator on the left N2 bottle. 1. (See Table B. 1 for recommended hydraulic pressures) 2. Make sure the "Sight Tube Supply" valve (5) is closed to avoid damaging it 3. Make sure the "Compressed N2 Vent" valve (2) is closed 4. Make sure the Firing Valve (11) is closed 5. Make sure the "Oil-N2 Separation" valve (1) is open 6. Ensure a good seal is being made by retracting the piston as described before 7. Carefully open the "CompressedN2 Supply" valve (10) to lock the piston 8. Close the "Oil-N2 Separation" valve (1) 9. Close the "Compressed N2 Supply" valve (10) Loading the RCM Once the piston assembly is locked in place, driving pressure can be applied to the pneumatic chamber without moving the piston. Once this pressure is applied, the RCM is analogous to a loaded gun and is essentially ready to fire. The RCM has been known to fire unexpectedly when in this state, so FigureB.8: RCM pneumatic chamber valve use caution (See Appendix B.5 for why this might happen). Follow these steps to bring the RCM to the ready-to-fire state: 1. Slowly turn the "Compressed Air" valve (7) to "Bypass" to remove the pressure differential across the yellow 3 inch pneumatic ball valve 2. Slowly open the yellow 3 inch pneumatic ball valve 3. Close the "Compressed Air" valve (7) 4. OPTIONAL: Turn the "Compressed Air" valve (7) to "Supply" to compensate for pressure loss due to filling the pneumatic chamber 88 Firing the RCM After following the procedures above, the RCM is ready to fire. The piston is rapidly forced across the RCM by removing the hydraulic locking pressure from the hydraulic chamber, allowing the force from the driving pressure to quickly accelerate the piston. Follow these FigureB.9: RCMfiring valve steps to fire the RCM and begin compression: 1. Make sure the combustion chamber valve is closed 2. Make sure the yellow 3 inch ball valve is open 3. Make sure each valve on the control panel is pointing toward the red "F" 4. Set the charge amplifier to "Operate" mode 5. Initiate the data acquisition system ("RCM Pulse Trigger.VI" in LabVIEW) 6. Quickly rotate the Firing Valve (11) counter-clockwise to release the hydraulic pressure and fire the RCM 7. Turn the Firing Valve (11) 180 degrees to the "VENT' position B.4 Shut-Down Sequence Venting the RCM Once the RCM has been fired, the first step is to relieve the pressures from the machine so that the piston can be returned to the retracted position. Follow these steps to vent the RCM: 1. Set the charge amplifier to "Reset" mode 2. Close the yellow 3 inch pneumatic ball valve 3. Slowly open the "Compressed N2 Vent" valve (2) until the pressure is ambient 4. Open the "Oil-N2 Separation" valve (1) after pressure in step 3 is fully relieved 5. Open the "Sight Tube Supply" valve (5) 6. Open the "Rear Chamber Vent" valve (8) 7. Turn the "CompressedAir" valve (7) to "Supply" only to prepare for the next run 89 8. Turn the "Pneumatic Chamber" valve (9) to "Vent" to begin retraction of the piston 9. Once fully vented, turn on the "Vacuum Pump" on the control panel 10. Turn the "Pneumatic Chamber" valve (9) to "Vacuum" to complete retraction 11. After retraction is complete, close the "Pneumatic Chamber" valve (9) 12. Turn off the "Vacuum Pump" 13. Exhaust the combustion chamber with the three-way chamber valve Cleaning the Cylinder are complete, Once the experiments it is essential to clean the combustion chamber to ensure that corrosion and deposits do not form. Follow these steps to clean the cylinder: FigureB.10: DisassembledRCM head 1. Remove the 4 head bolts (3/4" wrench) 2. Using acetone and lint-free paper clean the cylinder walls and piston head 3. Clean the crevices (e.g. window plugs, 0-ring grooves, etc...) 4. Replace the head plate and 4 bolts B.5 Troubleshooting Issue Runs are inexplicably inconsistent (if these fixes do not help look at other issues as many things effect consistency) Combustion chamber is leaking Fixes Possible Causes Mixtures are not consistent Use more care when metering and injecting fuel Wait for 10 minutes after filling the combustion chamber with charge Gases are not at thermal equilibrium Likelihood Hi High Combustion chamber is dirty Clean the combustion chamber and/or replace the piston seals and 0-rings High Head is not fully tightened down Tighten the four head bolts Medium Piston seals have failed Pressure transducer seal failed Replace the piston seals Replace the transducer seal High Medium Plate 0-ring has failed Head 0-ring has failed Window plug 0-rings failed Replace the 0-ring Replace the 0-ring Replace the 0-rings Medium Low Very Low 90 Mixing chamber is leaking Needle getting clogged Temperature sensors are not working Piston will not retract Large pressure spikes during compression The RCM unexpectedly fires The RCM sounds significantly different Low Vent is slightly open Vacuum valve is slightly open Ensure the vent is closed Ensure the valve is closed Mixing fan shaft seal has failed Check for brown liquid around the shaft and if so have the seal repaired Medium Top bolts are loose Tighten the top bolts Very Low Gasoline injection port septum is too worn Replace the septum High Wires are disconnected Ensure that all temperature wires are connected Medium Controllers have become uncalibrated Temperature sensors are damaged Use the controller menus to "self tune" the controllers Medium Replace the temperature sensors High Wires are damaged Piston seals are too worn Replace the wires Replace the piston seals Low High Pneumatic chamber seal has failed Replace the pneumatic chamber seal Low The piston is much hotter than Insulate or even heat the High The hydraulic pressure is too low Safely increase the pressure regulator pressure High The N 2 bottle is empty Replace the N 2 bottle High The hydraulic pressure lines are leaking The piston was not sufficiently Use Snoop to check leaks in the lines Medium flsure atthe piston is Medium retracted before locking locking The hydraulic lock 0-ring failed Replace the O-ring Medium The yellow 3 inch ball valve was not open before firing The N 2 bottle is "empty" The pneumatic chamber is Open the valve before firing Replace the N 2 bottle Replace the pneumatic High leaking piston seal Low the hydraulic chamber hydraulic chamber 91 Low High ... continued Data was not recorded The pneumatic tank was not sufficiently supplied with N 2 Ensure proper regulator pressure and check tank pressure Low The head plate was not sufficiently tightened down There is little oil in the system Tighten the head bolts more Low Check the sight tube and add oil if none is seen Very Low LabVIEW was not initiated Press start in the top left corner of the front panel before firing High The LabVIEW pressure threshold is too high Enter a lower pressure trigger on the front panel High The charge amplifier is on "Reset" mode Set the charge amplifier to "Operate"when firing High The DAQ glitched due to filtering No fix necessary, rare anomaly Very Low The combustion chamber valve was open during firing Make sure the valve is closed Low The piston seals are extremely damaged Replace the piston seals Medium The head was not tightened down Tighten the head down better Medium An O-ring is missing from the Ensure that all cylinder The presur ressure traces look significantly different than normal The pressure gauges are not working in place O-rings are Medium The driving pressure is too low Use Table B. 1 to determine proper pressures High The cylinder is contaminated The pressure transducer wire is damaged The pressure transducer is damaged chamber Medium Replace the transducer wire Medium Replace the pressure transducer Low The charge amplifier is damaged Replace the charge amplifier Very Low The DAQ system is damaged Replace the DAQ Replace the batteries Ensure the proper valves are open Very Low Replace the pressure gauge High The batteries are dead The valves to the gauge are closed The pressure gauge is damaged 92 High Medium Appendix C: Lubrication Oil Classification Table C.]: American Petroleum Institute base stock classification[18] Base Stock Group Typical Process Saturates (wt%) Group I Seivent Refined <90 Group 11 Hydrocracked Group Ill Hydrocracked Group IV Synthesized Group V Various Gr__p VIVarious Sulfur (wt%) Viscosity Index and/or >0.03 80-120 90 and 50.03 80- 120 90 and 50.03 120 Mineral/ Synthetic Mineral* Polyalphaolefins (PAO) Synthetic Any base stock not included in the Groups I through IV Various Various *In the US Group III is typically marketed as synthetic due to significant processing and high performance 93 94 References [1] M. Amann, T. Alger and D. Mehta, "The Effect of EGR on Low-Speed Pre-Ignition in Boosted SI Engines," SAE Int. J. Engines, vol. 4, no. 1, pp. 235-245, 2011, doi: 10.4271/2011-01-0339. [2] J. Zaccardi, L. Duval and A. Pagot, "Development of Specific Tools for Analysis and Quantification of Pre-ignition in a Boosted SI Engine," SAE Int. J. Engines, vol. 2, no. 1, pp. 1587-1600, 2009, doi: 10.4271/2009-01-1795. [3] J. Zaccardi and D. Serrano, "A Comparative Low Speed Pre-Ignition (LSPI) Study in Downsized SI Gasoline and CI Diesel-Methane Dual Fuel Engines," SAE Int. J. Engines, vol. 7, no. 4, pp. 1931-1944, 2014, doi: 10.4271/2014-01-2688. [4] A. Zahdeh, P. Rothenberger, W. Nguyen, M. Anbarasu, S. Schmuck-Soldan, J. Schaefer and T. Goebel, "Fundamental Approach to Investigate Pre-Ignition in Boosted SI Engines," SAE Int. J. Engines, vol. 4, no. 1, pp. 246-273, 2011, doi: 10.4271/2011-01-0340. [5] K. Takeuchi, K. Fujimoto, S. Hirano and M. Yamashita, "Investigation of Engine Oil Effect on Abnormal Combustion in Turbocharged Direct Injection - Spark Ignition Engines," SAE Int. J. Fuels Lubr., vol. 5, no. 3, pp. 1017-1024, 2012, doi: 10.4271/2012-01-1615. [6] P. Rothenberger, A. Zahdeh, M. Anbarasu, T. G6bel, J. Schafer and S. SchmuckSoldan, "Experimental Pre-ignition Investigations of Boosted Spark Ignition Engines in Combination with Lightintensified High Speed Camera and CFD," in 3. Tagung OttoinotorischesKlopfen - irreguldre Verbrennung, Berlin, 2010. 95 [7] S. Hirano, M. Yamashita, K. Fujimoto and K. Kato, "Investigation of Engine Oil Effect on Abnormal Combustion in Turbocharged Direct Injection Spark Ignition Engines (Part 2)," SAE Technical Paper 2013-01-2569, 2013, doi:10.4271/2013-012569. [8] S. Hirano, N. Yokoo, K. Nakata, M. Yamashita, K. Fujimoto and K. Kato, "Investigation of Fuel and Engine Oil Effect on Abnormal Combustion in Turbocharged Direct Injection - Spark Ignition Engines," in Advanced Fuels for SustainableMobility, Nirburg, 2014. [9] W. Attard, E. Toulson, H. Watson and F. Hamori, "Abnormal Combustion including Mega Knock in a 60% Downsized Highly Turbocharged PFI Engine," SAE Technical Paper 2010-01-1456, 2010, doi:10.4271/2010-01-1456. [10] M. Ohtomo, H. Miyagawa, M. Koike, N. Yokoo and K. Nakata, "Pre-Ignition of Gasoline-Air Mixture Triggered by a Lubricant Oil Droplet," SAE Int. J. Fuels Lubr., vol. 7, no. 3, pp. 673-682, 2014, doi:10.4271/2014-01-2627. [11] S. Palaveev, M. Magar, H. Kubach, R. Schiessl, U. Spicher and U. Maas, "Premature FLame "nitiatIon II a Turbocha~rge 1 DI-A Eine - Numerical and Experimental Investigations," SAE Int. J. Engines, vol. 6, no. 1, pp. 54-66, 2013, doi:10.4271/2013- 01-0252. [12] M. J. Plumley, V. Wong, M. Molewyk and S. Y. Park, "Optimizing Base Oil Viscosity Temperature Dependence For Power Cylinder Friction Reduction," SAE Technical Paper 2014-01-1658, 2014, doi: 10.4271/2014-01-1658. [13] Amsoil Technical Service Bulletin, "Fuel Dilution Causes and Effects," 14 July 2004. [Online]. Available: https://www.amsoil.com/. [Accessed 30 March 2015]. 96 [14] I. Kitsopanidis, "Experimental and Computational Study of Soot Formation Under Diesel Engine Conditions," Massachusetts Institute of Technology, Cambridge, 2004. [15] W. S. Affleck and A. Thomas, "An Opposed Piston Rapid Compression Machine For Preflame Reaction Studies," Proc. ofInstn. Mech. Engr., vol. 183, pp. 365-387, 1968, doi: 10.1243/PIMEPROC_1968_183_034_02. [16] F. P. Incropera, D. P. DeWitt, T. L. Bergman and A. S. Lavine, Fundamentals of Heat and Mass Transfer, 6th ed., Hoboken, NJ: John Wiley & Sons, 2007, pp. 580-581. [17] R. W. Lewis, P. Nithiarasu and K. N. Seetharamu, Fundamentals of the Finite Method for Heat and Fluid Flow, Chichester: John Wiley & Sons, 2004, p. 152. [18] American Petroleum Institute, "Engine Oil and Licensing Certification," in API Publication1509, Seventeenth Edition, 2012. [19] K. Inoue and Y. Yamanaka, "Change in Performance of Engine Oils with Degradation," SAE Technical Paper 902122, 1990, doi: 10.4271/902122. [20] ASTM Standard D5800, 2014, "Standard Test Method for Evaporation Loss of Lubricating Oils by the Noack Method," West Conshohocken, PA, 2014, doi: 10.1520/D5800-14E02, www.astm.org. [21] ASTM Standard D97, 2012, "Standard Test Method for Pour Point of Petroleum Products," West Conshohocken, PA, 2012, doi: 10.1520/D0097-12, www.astm.org. [22] ASTM Standard D92, 2012, "Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester," West Conshohocken, PA, 2012, doi: 10.1520/D009212B, www.astm.org. 97