RESULTS Pinus Fungi Recovered from Roots, Soil, and Insects Leptographium
advertisement
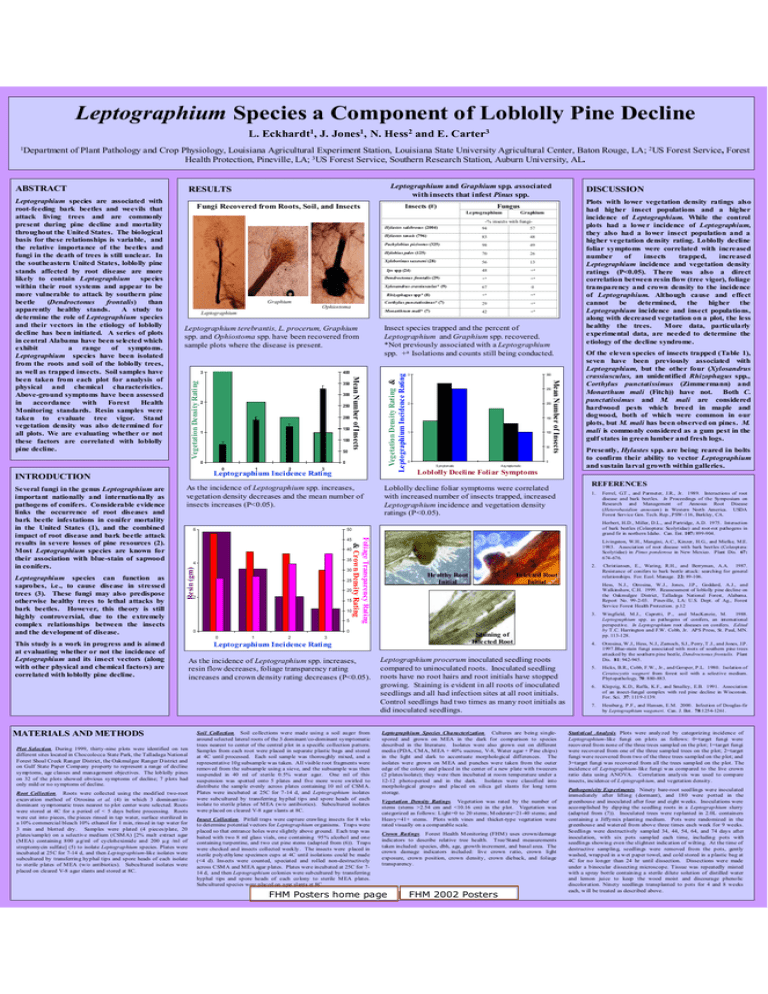
Leptographium Species a Component of Loblolly Pine Decline L. Eckhardt1, J. Jones1, N. Hess2 and E. Carter3 of Plant Pathology and Crop Physiology, Louisiana Agricultural Experiment Station, Louisiana State University Agricultural Center, Baton Rouge, LA; 2US Forest Service, Forest Health Protection, Pineville, LA; 3US Forest Service, Southern Research Station, Auburn University, AL. ABSTRACT Fungi Recovered from Roots, Soil, and Insects Fungus Graphium Hylastes salebrosus (2004) 94 57 Hylastes tenuis (796) 83 48 Pachylobius picivorus (325) 98 49 Hylobius pales (125) 70 26 Xyleborinus saxeseni (28) 56 13 48 +a Dendroctonus frontalis (29) +a +a Xylosandrus crassiusculus* (9) 67 0 +a +a Corthylus punctatissimus* (7) 29 +a Monarthrum mali* (7) 42 +a Ips spp (24) Rhizophagus spp* (8) Graphium Ophiostoma Leptographium Leptographium terebrantis, L. procerum, Graphium spp. and Ophiostoma spp. have been recovered from sample plots where the disease is present. 400 350 300 2 250 200 150 1 100 50 0 1 2 3 Leptographium Incidence Rating INTRODUCTION As the incidence of Leptographium spp. increases, vegetation density decreases and the mean number of insects increases (P<0.05). 6 35 Resin (gm) 4 Plot Selection. During 1999, thirty-nine plots were identified on ten different sites located in Choccolocca State Park, the Talladaga National Forest Shoal Creek Ranger District, the Oakmulgee Ranger D istrict and on Gulf State Paper Company property to represent a range of decline symptoms, age classes and management objectives. The loblolly pines on 32 of the plots showed obvious symptoms of decline; 7 plots had only mild or no symptoms of decline. Root Collection. Roots were collected using the modified two-root excavation method of Otrosina et al. (4) in which 3 dominant/codominant symptomatic trees nearest to plot center were selected. Roots were stored at 4C for a period of < 5 days before processing. Roots were cut into pieces, the pieces rinsed in tap water, surface sterilized in a 10% commercial bleach 10% ethanol for 1 min, rins ed in tap water for 3 min and blotted dry. Samples were plated (4 pieces/plate, 20 plates/sample) on a selective medium (CSM A) [2% malt extract agar (M EA) containing 800 µg/ml of cycloheximide and 200 µg /ml of streptomycin sulfate] (5) to isolate Leptographium species. Plates were incubated at 25C for 7-14 d, and then Leptographium-like isolates were subcultured by transferring hyphal tips and spore heads of each isolate to sterile plates of M EA (w/o antibiotics). Subcultured isolates were placed on cleared V-8 agar slants and stored at 8C. 300 250 2 200 150 1 100 50 0 0 Symptomatic Asy mptomatic Loblolly Decline Foliar Symptoms Loblolly decline foliar symptoms were correlated with increased number of insects trapped, increased Leptographium incidence and vegetation density ratings (P<0.05). Of the eleven species of insects trapped (Table 1), seven have been previously associated with Leptographium, but the other four (Xylosandrus crassiusculus, an unidentified Rhizophagus spp., Corthylus punctatissimus (Zimmermann) and Monarthum mali (Fitch)) have not. Both C. punctatissimus and M. mali are considered hardwood pests which breed in maple and dogwood, both of which were common in our plots, but M. mali has been observed on pines. M. mali is commonly considered as a gum pest in the gulf states in green lumber and fresh logs. Presently, Hylastes spp. are being reared in bolts to confirm their ability to vector Leptographium and sustain larval growth within galleries. REFERENCES 1. 30 25 20 2 15 10 5 0 Livingston, W.H., Mangini, A.C., Kinzer, H.G., and Mielke, M.E. 1983. Association of root disease with bark beetles (Coleoptera: Scolytidae) in Pinus ponderosa in New Mexico. Plant Dis. 67: 674-676. 2. Healthy Root Initial 0 0 1 2 Ferrel, G.T ., and Parmeter, J.R., Jr. 1989. Interactions of root disease and bark beetles. In Proceedings of the Symposium on Research and Management of Annosus Root Disease (Heterobasidion annosum) in Western North America. USDA Forest Service Gen. Tech. Rep., PSW-116, Berkley, CA. Herbert, H.D., Miller, D.L., and Partridge, A.D. 1975. Interaction of bark beetles (Coleoptera: Scolytidae) and root-rot pathogens in grand fir in northern Idaho. Can. Ent. 107: 899-904. Foliage Transparency Rating & Crown Density Rating 40 MATERIALS AND METHODS 3 Plots with lower vegetation density ratings also had higher insect populations and a higher incidence of Leptographium. While the control plots had a lowe r incidence of Leptographium, they also had a lower insect population and a higher vegetation density rating. Loblolly decline foliar symptoms were correlated with increased number of insects trapped, increased Leptographium incidence and vegetation density ratings (P<0.05). There was also a direct correlation between resin flow (tree vigor), foliage transparency and crown density to the incidence of Leptographium. Although cause and effect cannot be determined, the higher the Leptographium incidence and insect populations, along with decreased vegetation on a plot, the less healthy the trees. More data, particularly experimental data, are needed to determine the etiology of the decline syndrome. 50 45 This study is a work in progress and is aimed at evaluating whether or not the incidence of Leptographium and its insect vectors (along with other physical and chemical factors) are correlated with loblolly pine decline. Insect species trapped and the percent of Leptographium and Graphium spp. recovered. *Not previously associated with a Leptographium spp. +a Isolations and counts still being conducted. Mean Number of Insects Vegetation Density Rating 3 0 Leptographium species can function as saprobes, i.e., to cause disease in stressed trees (3). These fungi may also predispose otherwise healthy trees to lethal attacks by bark beetles. However, this theory is still highly controversial, due to the extremely complex relationships between the insects and the development of disease. DISCUSSION -% insects with fungi- 0 Several fungi in the genus Leptographium are important nationally and internationally as pathogens of conifers. Considerable evidence links the occurrence of root diseases and bark beetle infestations in conifer mortality in the United States (1), and the combined impact of root disease and bark beetle attack results in severe losses of pine resources (2). Most Leptographium species are known for their association with blue-stain of sapwood in conifers. Insects (#) Le ptographium Mean Number of Insects Leptographium species are associated with root-feeding bark beetles and weevils that attack living trees and are commonly present during pine decline and mortality throughout the United States. The biological basis for these relationships is variable, and the relative importance of the beetles and fungi in the death of trees is still unclear. In the southeastern United States, loblolly pine stands affected by root disease are more likely to contain Leptographium species within their root systems and appear to be more vulnerable to attack by southern pine beetle (Dendroctonus frontalis) than apparently healthy stands. A study to determine the role of Leptographium species and their vectors in the etiology of loblolly decline has been initiated. A series of plots in central Alabama have been selected which exhibit a range of symptoms. Leptographium species have been isolated from the roots and soil of the loblolly trees, as well as trapped insects. Soil samples have been taken from each plot for analysis of physical and chemical characteristics. Above-ground symptoms have been assessed in accordance with Forest Health Monitoring standards. Resin samples were taken to evaluate tree vigor. Stand vegetation density was also determined for all plots. We are evaluating whether or not these factors are correlated with loblolly pine decline. Leptographium and Graphium spp. associated with insects that infest Pinus spp. RESULTS Vegetation Density Rating & Leptographium Incidence Rating 1Department Staining of Infected Root 3 Leptographium Incidence Rating As the incidence of Leptographium spp. increases, resin flow decreases, foliage transparency rating increases and crown density rating decreases (P<0.05). Soil Collection. Soil collections were made using a soil auger from around selected lateral roots of the 3 dominant/co-dominant symptomatic trees nearest to center of the central plot in a specific collection pattern. Samples from each root were placed in separate plastic bags and stored at 4C until processed. Each soil sample was thoroughly mixed, and a representative 10g subsample w as taken. All visible root fragments were removed from the subsample using a sieve, and the subsample was then suspended in 40 ml of sterile 0.5% water agar. One ml of this suspension was spotted onto 5 plates and five more were swirled to distribute the sample evenly across plates containing 10 ml of CSM A. Plates were incubated at 25C for 7-14 d, and Leptographium isolates were subcultured by transferring hyphal tips and spore heads of each isolate to sterile plates of M EA (w/o antibiotics). Subcultured isolates were placed on cleared V-8 agar s lants at 8C. Insect Collection. Pitfall traps were capture crawling insects for 8 wks to determine potential vectors for Leptographium organisms. Traps were placed so that entrance holes were slightly above ground. Each trap was baited with two 8 ml glass vials, one containing 95% alcohol and one containing turpentine, and two cut pine stems (adapted from (6)). Traps were checked and insects collected weekly. The insects were placed in sterile polyethylene specimen cups at 4C until isolations could be made (<4 d). Ins ects were counted, speciated and rolled non-destructively across CSM A and M EA agar plates. Plates were incubated at 25C for 714 d, and then Leptographium colonies were subcultured by transferring hyphal tips and spore heads of each colony to sterile M EA plates. Subcultured species were placed on agar slants at 8C. Infected Root Initial Leptographium procerum inoculated seedling roots compared to uninoculated roots. Inoculated seedling roots have no root hairs and root initials have stopped growing. Staining is evident in all roots of inoculated seedlings and all had infection sites at all root initials. Control seedlings had two times as many root initials as did inoculated seedlings. Leptographium Species Characterization. Cultures are being singlespored and grown on M EA in the dark for comparison to species described in the literature. Isolates were also grown out on different media (PDA, CM A, M EA + 40% sucrose, V-8, Water agar + P ine chips) in the light and dark to accentuate morphological differences. The isolates were grown on M EA and punches were taken from the outer edge of the colony and placed in the center of a new plate with tweezers (2 plates/isolate); they were then incubated at room temperature under a 12-12 photo-period and in the dark. Isolates were classified into morphological groups and placed on silica gel slants for long term storage. Vegetation Density Ratings. Vegetation was rated by the number of stems (stems >2.54 cm and <10.16 cm) in the plot. Vegetation was categorized as follows: Light=0 to 20 stems; M oderate=21-40 stems; and Heavy=41+ stems. Plots with vines and thicket-type vegetation were rated visually on a comparable scale. Crown Ratings. Forest Health M onitoring (FHM ) uses crown/damage indicators to describe relative tree health. Tree/Stand measurements Crown taken included: species, dbh, age, growth increment, and basal area. The Outline crown damage indicators included: live crown ratio, crown light exposure, crown position, crown density, crown dieback, and foliage transparency. FHM Posters home page | FHM 2002 Posters Christiansen, E., Waring, R.H., and Berryman, A.A. 1987. Resistance of conifers to bark beetle attack: searching for general relationships. For. Ecol. Manage. 22: 89-106. Hess, N.J., Otrosina, W.J., Jones, J.P., Goddard, A.J., and Walkinshaw, C.H. 1999. Reassessment of loblolly pine decline on the Oakmulgee District, Talladega National Forest, Alabama. Report No. 99-2-03. Pineville, LA: U.S. Dept. of Ag., Forest Service Forest Health Protection. p.12 3. Wingfield, M.J., Capretti, P., and MacKenzie, M. 1988. Leptographium spp. as pathogens of conifers, an international perspective. In Leptographium root diseases on conifers. Edited by T .C. Harrington and F.W. Cobb, Jr. APS Press, St. Paul, MN. pp. 113-128. 4. Otrosina, W.J., Hess, N.J., Zarnoch, S.J., Perry, T .J., and Jones, J.P. 1997.Blue-stain fungi associated with roots of southern pine trees attacked by the southern pine beetle, Dendroctonus frontalis. Plant Dis. 81: 942-945. 5. Hicks, B.R., Cobb, F.W., Jr., and Gersper, P.L. 1980. Isolation of Ceratocystis wagneri from forest soil with a selective medium. Phytopathology, 70: 880-883. 6. Klepzig, K.D., Raffa, K.F., and Smalley, E.B. 1991. Association of an insect-fungal complex with red pine decline in Wisconsin. For. Sci. 37: 1119-1139. 7. Hessbur g, P.F., and Hansen, E.M. 2000. Infection of Douglas-fir by Leptographium wageneri. Can. J. Bot. 78:1254-1261. Statistical Analysis. Plots were analyzed by categorizing incidence of Leptographium-like fungi on plots as follows: 0=target fungi were recovered from none of the three trees sampled on the plot; 1=target fungi were recovered from one of the three sampled trees on the plot; 2=target fungi were recovered from two of the three trees sampled on the plot; and 3=target fungi was recovered from all the trees sampled on the plot. The incidence of Leptographium-like fungi w as compared to the live crown ratio data using ANOVA. Correlation analysis was used to compare insects, incidence of L eptographium, and vegetation density. Pathogenicity Experiments. Ninety bare-root seedlings were inoculated immediately after lifting (dormant), and 180 were potted in the greenhous e and inoculated after four and eight w eeks. Inoculations were accomplished by dipping the seedling roots in a Leptographium slurry (adapted from (7)). Inoculated trees were replanted in 2.0L containers containing a Jiffymix planting medium. Pots were randomized in the greenhous e and watered from above three times each week for 9 weeks. Seedlings were destructively sampled 34, 44, 54, 64, and 74 days after inoculation, with six pots sampled each time, including pots with seedlings showing even the slightest indication of wilting. At the time of destructive sampling, seedlings were removed from the pots, gently washed, wrapped in a w et paper towel, and cold stored in a plastic bag at 4C for no longer than 24 hr until dissection. Dissections were made under a binocular dissecting microscope. Tissue was repeatedly misted with a spray bottle containing a sterile dilute solution of distilled water and lemon juice to keep the wood moist and discourage phenolic discoloration. Ninety seedlings transplanted to pots for 4 and 8 weeks each, w ill be treated as described above.