Experiment 15 ANALYSIS OF VINEGAR
advertisement

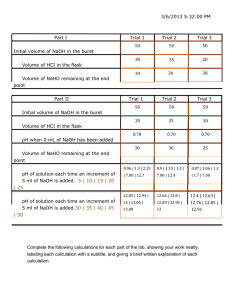
Experiment 15 FV 6/21/13 ANALYSIS OF VINEGAR MATERIALS: Four 250 mL Erlenmeyer flasks, two 50 mL burets, liquid funnel, one 150 mL beaker, one 50 mL beaker, 5.00 mL pipet, weighing dish, approximately 0.1 M NaOH, C6H4(COOH)COOK (potassium hydrogen phthalate), phenolphthalein, grease pencil. PURPOSE: The purpose of this experiment is two-fold: (1) to standardize a solution of sodium hydroxide; (2) to use the standardized sodium hydroxide solution to titrate the acetic acid in vinegar to determine its percent by mass in vinegar. LEARNING OBJECTIVE: 1. 2. 3. 4. 5. 6. By the end of this experiment, the midshipman should be able to demonstrate the following proficiencies: Use pipets and burets correctly. Explain the term hygroscopic. Explain the function and necessary qualities of a primary standard. Perform a titration accurately and precisely. Calculate the molarity and the mass of components in a solution from stoichiometry information. Calculate the percent by mass of a component in a solution. DISCUSSION: Vinegar is a solution of acetic acid in water. Acetic acid, CH3COOH, is a weak monoprotic acid with a molar mass of 60.05 g/mole. The percent by mass of acetic acid in vinegar can be determined by titrating a known amount of vinegar with a standardized solution of sodium hydroxide, i.e., a sodium hydroxide solution of accurately known concentration. Acetic acid and sodium hydroxide react as shown below: CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) 60.05 g/mole Sodium hydroxide is a hygroscopic solid which means it absorbs water from the air. A weighed quantity of sodium hydroxide therefore contains an unknown mass of water. Therefore, a solution of known molarity cannot be made by dissolving a known mass of solid sodium hydroxide in water. The concentration of a sodium hydroxide solution must be determined experimentally. This is done by titrating the sodium hydroxide solution against a primary standard. A primary standard is a substance from which a solution of known concentration can be prepared. The primary standard used in this experiment is potassium hydrogen phthalate, C6H4(COOH)COOK, which is referred to by the shorthand notation of "KHPh". KHPh has several advantages: it does not absorb moisture readily (i.e., is not hygroscopic); it is easily dried; it can be accurately weighed; it can be obtained in very pure form; it has a high molar mass of 204.22 g/mole; and it is very soluble in water. KHPh is an acid, which reacts in aqueous solution to neutralize the base, sodium hydroxide, as shown below: C6H4(COOH)COOK (aq) + NaOH (aq) C6H4(COONa)COOK (aq) + H2O (l) 204.22 g/mole KHPh E15-1 PROCEDURE: Part A. Standardization of the Sodium Hydroxide Solution 1. Use the top-loading balance to pre-weigh about 0.51 grams of KHPh into a weighing dish. 2. Use the analytical balance to obtain the mass of a clean 250 mL Erlenmeyer flask. Transfer the KHPh to the clean 250 mL Erlenmeyer flask. Obtain the mass of the flask and the KHPh sample. 3. Add approximately 100 mL of distilled water to the flask to dissolve the KHPh and then add two to three drops of phenolphthalein indicator. Gently swirl the solution. It is not necessary for all the KHPh to dissolve because the undissolved KHPh will dissolve as the titration proceeds. 4. Add ~50 mL of NaOH solution (approximately 0.1 M) to a 150 mL beaker. Pour the NaOH solution from the beaker, through a funnel, into the buret. Make sure there is no air bubbles in the buret tip. Record your initial buret reading with the proper number of significant figures. 5. Titrate the KHPh solution with the NaOH solution to a very pale pink endpoint. Record the final buret reading to the correct number of significant figures. 6. Repeat steps 1 – 5. After doing your second titration, do a quick calculation to determine whether your precision is acceptable. Divide the volume of NaOH used in each titration by the mass of the KHPh sample for each titration. Divide the larger ratio by the smaller ratio. If the resulting number is greater than 1.03, then do a third trial. Part B. Measurement of Acetic Acid in Vinegar 1. Use the analytical balance to obtain the mass of a clean, dry 250 mL Erlenmeyer flask. It is okay if the flask is a little wet inside as long as it is only distilled water. It must be dry on the outside. 2. Add ~15 mL of vinegar to a clean, dry 50 mL beaker. Pre-rinse a 5.00 mL pipet with a small quantity of vinegar. Pipet a 5.00 mL sample of vinegar into the flask. Reweigh the flask containing the 5.00 mL sample. 3. To the vinegar sample in the 250 mL Erlenmeyer flask, add approximately 100 mL of distilled water and then two or three drops of phenolphthalein indicator. 4. Refill your buret with the NaOH solution. 5. Titrate the vinegar solution with the NaOH solution to a very pale pink endpoint. 6. Repeat steps 1 – 5. If two titrations are not within +/- 0.50 mL of each other, repeat the titration. . Clean up: 1. Rinse all waste solutions down the drain. 2. Wash all glassware. Invert the buret and mount it in the buret clamp on the ring stand, with the stopcock open to allow it to drain. Leave the other washed glassware upright. Clean up your area. E15-2 Name___________________________ Section___________________________ Partner___________________________ Date_____________________________ DATA SECTION Experiment 15 INCLUDE THE APPROPRIATE SIGNIFICANT FIGURES. Part A. Standardization of the Sodium Hydroxide Solution Sample 1 Sample 2 Sample 3 Sample 1 Sample 2 Sample 3 Mass of 250 mL flask + KHPh (g) Mass of 250 mL flask (g) Mass of KHPh (g) Initial buret reading of NaOH (mL) Final buret reading of NaOH (mL) Volume of NaOH used (mL) Need 2 good titrations within 3%. Part B. Measurement of Acetic Acid in Vinegar Mass of 250 mL flask (g) + 5.00 mL Vinegar (g) Mass of 250 mL flask(g) Mass of 5.00 mL Vinegar (g) Initial buret reading of NaOH (mL) Final buret reading of NaOH (mL) Volume of NaOH used (mL) Need 2 good titrations within +/- 0.50 mL E15-3 DATA TREATMENT Experiment 15 INCLUDE THE APPROPRIATE SIGNIFICANT FIGURES. Part A. Standardization of the Sodium Hydroxide Solution (A.1) Calculate the moles of KHPh for: Sample 1: Sample 2: Sample 3: (A.2) Realizing you titrated to the equivalence point, use your calculations from Part A to calculate the moles of NaOH for: Sample 1: Sample 2: Sample 3: (A.3) Calculate the concentration (M) of the NaOH solution for each sample, and then obtain the average value: Sample 1: Sample 2: Sample 3: AVERAGE: E15-4 DATA TREATMENT continued Experiment 15 Part B. Measurement of Acetic Acid in Vinegar (B.1) Use your average value of the NaOH molarity to calculate the moles of NaOH for: Sample 1: Sample 2: Sample 3: (B.2) Realizing you titrated to the equivalence point, calculate the moles of acetic acid in the 5.00 mL sample of vinegar: Sample 1: Sample 2: Sample 3: (B.3) Calculate the mass % of acetic acid in vinegar, and then obtain the average value: Sample 1: Sample 2: Sample 3: AVERAGE: E15-5 QUESTIONS Experiment 15 1. How accurately does the 100 mL of water used to dissolve the KHPh in the standardization of the NaOH solution need to be measured? Explain. 2. When transferring the KHPh in Part A, if some of the KHPh missed the opening to the Erlenmeyer flask and fell onto the weighting pan and stayed, how would the calculated molarity of the NaOH solution compare to the actual value (i.e., is the calculated concentration more, less or the same as the actual value)? Explain. 3. During the titration of KHP in part A of this experiment, you obtain a dark pink endpoint (instead of a pale pink endpoint). Will this result in the calculated molarity of the NaOH solution being higher, lower or the same as the actual molarity? Explain your answer 4. How does obtaining a dark pink endpoint (instead of a pale pink endpoint) in the titration in Part B, affect the calculated mass % of acetic acid in vinegar compared to the actual value (what it should be)? That is, is the calculated mass % greater than, less than or equal to the actual value? Explain. E15-6 Name ___________________________ Section ___________________________ Date _____________________________ PRE-LAB EXERCISES Experiment 15 INCLUDE THE APPROPRIATE SIGNIFICANT FIGURES. 1. Write the balanced molecular equation for the neutralization of acetic acid (CH3COOH) with sodium hydroxide. 2. Before the sodium hydroxide can be used in this experiment, it must be standardized (i.e. the concentration must be measured accurately). This lab will use C6H4(COOH)COOK (KHPh) to standardize the NaOH solution. Write the balanced molecular equation for the neutralization of KHPh with sodium hydroxide. 3. Calculate the concentration of the sodium hydroxide solution (in M) if it takes 25.00 mL of sodium hydroxide solution to titrate 0.5000 g of KHPh (MM = 204.22 g/mol). 4. Calculate the mass % of acetic acid in vinegar if you found 0.00400 mol of acetic acid in 5.000 mL of vinegar (density = 1.01 g/mL). Mass % of component X = E15-7 mass of X total mass of solution (containing X) 100