Prelab
advertisement

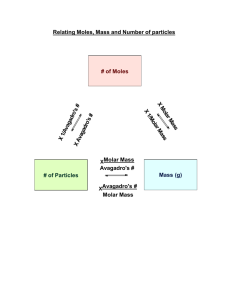
AP Chemistry 2015-2016 Lab: The Effect of Ka Name: Date: Per: Directions: This lab is actually two labs in one. You will be exploring the effect of Ka two factors: amount of base neutralized and reaction rate. Prelab 1. Write a purpose statement that includes 2 hypotheses (one on how a higher Ka affects reaction rate, and one on how a higher Ka affects the amount of base neutralized). 2. Prelab Questions. Answer the following: a. Write the complete chemical reaction between aqueous hydrochloric acid and solid magnesium. b. Write the net ionic equation for (a). c. Write the complete chemical reaction between aqueous acetic acid and solid magnesium. d. Write the net ionic equation for (c). e. The Ka for acetic acid is 1.8 x 10-5. Calculate the number moles of H+ ions in a 20 mL solution of acetic acid at equilibrium. f. HCl is a strong acid. Calculate the number moles of H+ ions in a 20 mL solution of hydrochloric acid. g. Write the complete neutralization reaction that occurs when hydrochloric acid reacts with sodium hydroxide. h. Write the net ionic equation for (g). i. Write the complete neutralization reaction that occurs when acetic acid reacts with sodium hydroxide. j. Write the net ionic equation for (i). 3. Flow chart the lab procedure. 4. Create 2 data tables for the data you will be collecting throughout this lab. You should create one data table for the qualitative observations in the rate lab, and another table for the quantitative data you are collecting in the NaOH lab. Materials 1.0 M acetic acid 1.0 M hydrochloric acid 2 - 10 mL graduated cylinders 2 - 100 mL beakers 2 - 125 mL flasks 2 - 50 graduated cylinders buret pipette solid magnesium phenolphthalein indicator sodium hydroxide (≈ 2 - 3 M) Procedure (note: you will set up the rate reaction, and it will run while you do the NaOH neutralization. You need to create flowcharts for BOTH procedures, separately). Effect on Rate 1. With a graduated cylinder and dropper, place 20 mL of 1 M HCl into a 100 mL beaker labeled HCl. 2. With new glassware, repeat this process with 1M acetic acid. 3. Obtain two 0.30 - 0.35 g pieces of magnesium ribbon. 4. If desired, start your video camera. Place the magnesium ribbons in the corresponding beakers at the same time. Record the time. 5. Record observations of the reactions at the initial time. 6. Record time and observations approximately 10 minutes after step 4. 7. Record time and observations approximately 30 minutes after step 4. 8. Record time and observations near the end of the period. 9. Cleanup – beaker waste must be dumped in the large waste beaker on the front teacher table. Rinse all glassware thoroughly. Effect on NaOH 1. With a graduated cylinder and dropper, place 10 mL of 1 M HCl into a 125 mL flask labeled HCl. 2. With new glassware, repeat this process with 1M acetic acid. 3. Add 3 drops of phenolphthalein to each flask. Swirl. 4. Load a buret with sodium hydroxide, bleed, and record the starting value. 5. Use a buret to add sodium hydroxide dropwise to the flask. As the sodium hydroxide is added, you should see a pink color that quickly goes away as it is first added. As more sodium hydroxide is added the pink color will persist for a longer period of time. Occasionally rinse the inside of the flask with a bit of distilled water. 6. As the “end point” is approached, the sodium hydroxide should be added very slowly while swirling the flask. 7. When the pink color from the phenolphthalein remains despite swirling, record the volume of NaOH added from the buret. 8. Repeat steps 4 through 7 for the acetic acid. Analysis 1. How do the moles in pre-lab questions 3 and 4 compare? 2. Which acid/magnesium reaction seemed most vigorous? Explain. 3. How did the moles of NaOH consumed in each reaction compare? 4. What does that say about the moles of H+ ionized from each acid? 5. How do the moles in prelab questions 3 & 4 compare? 6. Explain how hydrochloric acid could neutralize the same amount of OH- ions when they started with different amounts of H+ ions? Use LeChatelier’s principle to explain this. Conclusion Your conclusion should be in paragraph form and must include thorough directions of the following: Validity of your hypotheses – were you correct? How do you know? If you were incorrect, what did you learn? Provide evidence from the lab. Summary of how the value of Ka affects a reaction Error analysis – if you did not get the expected results, what went wrong? Be specific. Lab report due Wednesday, November 18th