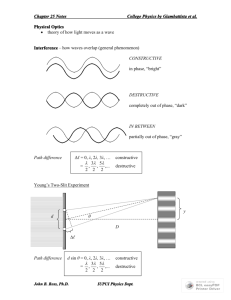
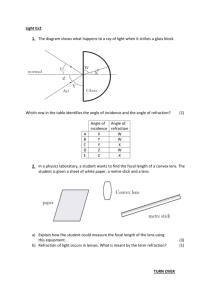
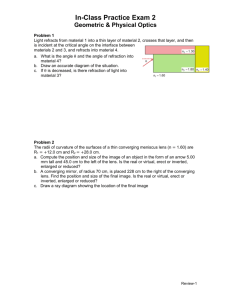
Today’s Lecture Review for Final
advertisement

Today’s Lecture Review for Final Hydrostatic Equilibrium with External Forces: Gravity Force from above – PA Force from below – (P+dP)A Gravitational force (pulling down) dFg = ρgA dh balance PA + ρgA dh = ( P + dP) A ρgA dh = AdP => dP = ρg dh Note that h measures increasing depth. Hydrostatic Equilibrium with Gravity dP = ρg dh This is a differential equation. How do we calculate the actual pressure? We integrate the equation P = P0 + ρgh And end up getting an integration constant, P0. What is this constant about? Mostly pain in the neck!.. In order to find pressure, P, in a given point, we need to know the pressure P0 in some reference point, h0. Then we compare the depth between the two points and calculate P as: Buoyant Force In equilibrium the upward pressure force, Fp, is equal to the weight of the fluid, Fg. If the fluid is replaced by a solid object, the pressure force remains the same. In general the gravitational force changes. The net force on the solid object depends on whether it is more or less dense than the fluid. Archimede’s principle: The buoyant force on an object is equal to the weight of the fluid displaced by the object. Fp = mw g = ρ wVg The buoyant force is applied to the center of gravity of the displaced fluid (center of the submerged volume of the body). Remember it is only the submerged volume (not necessarily the total volume). Archimedes Principle: The buoyant force on an object is equal to the weight of the fluid displaced by the object. The density of ice is ρice=.917gm/cm3 . The density of sea water is ρsea=1.027gm/cm3. What fraction of iceberg is submerged? So almost 90% of the iceberg is submerged, not so good for those on the Titanic! Mass of fluid, Δm1, entering the tube from the left over the time interval Δt By mass conservation, over the time interval Δt, the same mass, Δm2, is exiting the tube from the right Therefore ρ1A1v1 = ρ2A2v2 or ρAv = const. everywhere along a flow tube. This is the “Continuity Equation”. If the fluid is incompressible and its density, ρ, is constant we have vA = const Total Energy Balance 1 2 2 m(v2 − v1 ) + mg ( h2 − h1 ) = P1 A1Δx1 − P2 A2 Δx2 2 A1Δx1 = A2 Δx2 Incompressible fluids – constant density and volume 1 2 1 2 P1 + ρv1 + ρgh1 = P2 + ρv2 + ρgh2 2 2 1 2 P + ρv + ρgh = const 2 Bernoulli’s equation Example of Bernoulli’s Equation Venturi Flowmeter Consider a Venturi flowmeter with cross-sectional areas of A1 and A2 Solve for v1 in terms of the change in pressure. From Bernoulli’s equation and the Continuity equation, Demo of Venturi tube. Heat capacity is an extrinsic parameter (depends on quantity): When you bring two objects together, heat capacity of the system of the two objects becomes the sum of the two individual heat capacities. It is convenient to introduce specific heat, c, an intrinsic parameter, which is heat capacity of a material per unit mass . Specific heat is measured in J/(K⋅kg). ΔQ = cmΔT ΔQ C c= = m mΔT Heat capacity of a water ball is large due to both high specific heat and large mass of the water inside the ball. Heat Conduction ΔT H = −kA Δx A lake with a flat bottom and steep sides has a surface area of 1.5km2 and is 8m deep. In the summer the surface is 30oC while the bottom is 4oC. What is the rate of heat conduction in the lake? Thermal Resistance ΔT H = − kA Δx Analogous to the Ohm’s law: V I= R The current, I = Δq/Δt, amount of charge per unit time, is analogous to the heat-flow rate, H = ΔQ/Δt, “thermal current”. The voltage, V, the factor driving the electric current, is analogous to temperature difference, ΔT, “thermal voltage” . Continuing the analogy: electric resistance, R, is analogous to Thermal resistance is introduced as The electrical conductivity, σ, is analogous to the thermal conductivity, k. Black Body Radiation All matter radiates energy via black-body radiation “Stephan-Boltzmann Law” Blackbody Spectra for Different Temperatures Wien’s law states that the peak in the intensity of blackbody radiation is inversely proportional to the temperature, λmax = a / T. Defining x = hc/λkBT, we found that xmax= 4.965. From this we can solve for the wavelength that corresponds to the maximum in the intensity distribution: For our Sun the wavelength for the maximum intensity is ~502nm. Hence the surface temperature of our Sun is found from: In general the intensity distribution of the black body radiation from a star yields the surface temperature of that star! V = NkT / P The ideal gas law Doubling the temperature, number of molecules, pressure? P = NkT / V Keeping the volume and the number of particles constant, but doubling the temperature? N is normally very big, while k is a very small number… k=1.38X10-23J/K N = nN A NA = 6.022×1023 – Avogadro number, number of molecules in 1 mole of a substance; n is the number of moles in the gas. PV = nN A kT = nRT R = 8.314 J/mol⋅K universal gas constant Ideal Gas - example What is the volume occupied by 1.00 mol of an ideal gas at standard temperature and pressure (STP), where T=0oC and P=101.3kPa=1.013x105Pa (1atm)? The ideal gas law An Avogadro's number of gas molecules occupies 22.4L at STP! Kinetic theory of the ideal gas. From the Kinetic Theory of gases From the ideal gas law The average kinetic energy is Physical meaning of the absolute temperature is a measure of the average kinetic energy of a molecule. The rms speed of a molecule – thermal speed: First law of thermodynamics ΔU = Q − W change in the internal energy of the system net heat transferred to the system work done by the system The change in the internal energy of a system depends only on the net heat transferred to the system and the net work done by the system, and is independent of the particular processes involved. The equation is deceptively simple… One of the forms of the general law of conservation of energy. BUT! Be careful about the sign conventions. Positive Q is heat transferred to the system. Positive W is work done by the system. Work done and heat transferred – Work done by the gas our major concerns! W = ∫ dW = ∫ PdV P – (varying) pressure of the gas; dV – differential volume change ΔW = PΔ V P Always the Ideal Gas Law V Area under curve on a P-V diagram Review of our Thermodynamic Processes… ΔU = Q − W PV = nRT W = ∫ PdV Adiabatic processes – no heat transfer. Q=0 ΔU = −W Positive work, W, is done by the expanding gas at expense of reduction of its internal energy… Since there is no heat supplied from the outside to replenish the gas energy the temperature declines. Work can be expressed in terms of -ΔΤ or -Δ(PV) Adiabatic processes – no heat transfer. Q=0 ΔU = −W We now know the work done by an ideal gas during an adiabatic expansion, but how do pressure and temperature behave? From the ideal gas law: From the 1st law: As an exercise for the student Specific Heats of an Ideal Gas From kinetic theory we showed the average (translational) kinetic energy per molecule is: For n moles the internal energy due to KE is: This means we can find Cv : For inert gases we have: Cyclic processes What is so special about them? The system returns to the same point (state) P What can we say about change ΔU, Q and W? Since the system returns to the same state and V U is a function of state only, Therefore by the 1st law we have A B W =Q ΔU = 0 ΔU = Q − W That is the system gets heat Q from the outside and does an equal amount of work W. Or the other way around! Both Q and W may be negative! What simple thermodynamic process are cyclic processes similar to? In what are they different? Carnot Cycle What is the net work and efficiency of a Carnot cycle? Wtotal = WAB + WCD A B D C Since ThVBγ−1=TcVCγ-1 and ThVAγ-1=TcVDγ-1 Qc Tc = Qh Th Efficiency We have VB / VA = VC / VD and W Qh − Qc Th − Tc = = e= Qh Qh Th Entropy Consider the Carnot Cycle where we found If we change the definition of Qc so that it is the heat added vs heat rejected then Any closed reversible cycle can be made up of incrementally small isothermal and adiabatic cycles. This leads to This means that the integral representing the change in entropy, is path independent! Summary of the First Law of Thermodynamics First Law of Thermodynamics Thermodynamic processes, Quasi-static - work is given by Isothermal – constant temperature Isochoric – constant volume Isobaric – constant pressure Adiabatic – no heat transfer Equipartition theorem – 1/(2kT) average molecular energy for each degree of freedom y x λ - equation of a harmonic wave ω = 2π / T = 2π f ω = kv k = 2π / λ y( x, t ) = Ay cos(kx − ωt ) = Ay cos(kx − kvt) = = Ay cos[k ( x − vt)] A crest corresponds to a point, where The general wave equation: Velocity on a string: Wave intensity is the wave power per unit area P I= A Measured in J W or 2 2 s⋅m m For a plane wave the intensity remains constant. For a spherical wave it decreases with the distance, r, from the source, like P I= 2 4πr Does it mean that the power is lost as the wave propagates? Speed of Sound in a Gas v= B ρ Can we calculate the bulk modulus of elasticity, B, of a gas? What process should we assume? Sound waves are propagating quickly. No time for heat exchange! ⇒ Adiabatic process. For an adiabatic expansion: Hence: With the result: PV γ = c = const Speed of Sound in Air γP v= ρ Air is essentially 79% nitrogen (N2) and 21% oxygen (O2). The masses of nitrogen and oxygen molecules are 28au 32au respectively. At one atmosphere of pressure and room temperature, 20oC, one mole of gas under these conditions occupies V=22.4x293/273L=24L, and the density is: For a diatomic molecule γ =1.4 and In air the speed of sound must satisfy Measuring sound intensity in decibels, dB I 0 = 10 −12 W/m I = I 0 ⋅ 10 β / 10 ⎛I ⎞ β = 10 log ⎜⎜ ⎟⎟ ⎝ I0 ⎠ 2 - reference value, corresponding to threshold of sensitivity of a normal ear at about optimal frequency An example: TV is turned down from 75 dB to 72 dB, -3dB. How does its sound intensity change? General Expression for Doppler Shift For a moving observer: For a moving source: Both of these expressions are included in the general relationship: Here uo is the velocity of the observer and us is the velocity of the source. It is usually best to use physical intuition to determine the signs. The Doppler shift results in an increase in the frequency when the object and the source are approaching each other with an increase in velocity. Police Radar – sends frequency f. The car moving toward the radar (moving observer) at a speed u receives a frequency: v+u f '= f v It reflects the received frequency f’, but it is a source moving toward the radar, so that the radar receives a frequency f' 1+ u / v = f f ''= 1− u / v 1− u / v If u/v is small, which is typical for electromagnetic waves (v = light speed). f ' ' = f (1 + 2u / v) The frequency of the received signal is higher by a fraction 2u v Frequency and Wavelength at Refraction. • As light travels from one medium to another, its frequency does not change – Both the wave speed and the wavelength do change – The wavefronts do not pile up, nor are created or destroyed at the boundary, so ƒ must stay the same 1 2 1 2 1 2 f = f v λ = v λ λ1 v1 c / n1 n2 = = = λ2 v2 c / n2 n1 So we see that Snell’s Law • In terms of indices of refraction the law of refraction becomes • Sine of angle of incidence (refraction) is inversely proportional to the index of refraction of the medium Snell’s law of refraction is written in a form symmetric to the incident and refracted beams: http://www.physics.uoguelph.ca/applets/Intro_physics/refraction/LightRefract.html Displaced Laser Beam l Find the displacement of a laser beam that is approaching a 5cm thick layer of ice with an angle of incidence of θ1 = 30o when ice has an index of refraction of n=1.3. Defining the diagonal through the slab as l we find: From Snell’s law: Hence the displacement is: Critical Angle • A particular angle of incidence will result in an angle of refraction of 90° – This angle of incidence is called the critical angle • For angles of incidence greater than the critical angle, the beam is entirely reflected at the boundary – This ray obeys the Law of Reflection at the boundary • Total internal reflection occurs only when light attempts to move from a medium of higher index of refraction to a medium of lower index of refraction – key to fiber optics Summary for Converging Lens Image real, inverted, reduced (magnified for l < 22f ) Virtual, upright, magnified Lens Magnification – Diverging lens 1 1 1 + = l l' f f 0, ℓ − anything, ℓ ′ 0 and |ℓ ′ | ℓ; M −ℓ ′ /ℓ, M 1 Virtual, upright, reduced To Summarize for both types of lenses: Imagine a camera with a single lens with a focal distance f = 35mm. By how much and in what direction should the lens be moved to move its focus from an object, which is far-far away to an object at a distance of 1.5m? Far-far away means l = ∞, and l’= f = 35mm. New distance l = 1.5m; l’= ? A healthy human eye can clearly see (focus) objects at distances from infinity to about 25cm. How is that achieved? By changing the focal distance of the lens! We always have l’≈ 22mm. Far-far away means l = ∞, and f = l’ ≈ 22mm. An object at l = 25cm means The adaptive lens driven by the eye muscles changes the focal distance of the eye by “only” 9%. But this is quite a lot!.. Can we see any interference without a laser? Approximation used: d << L θ r2 − r1 = d sin θ Constructive (a bright strip) Destructive (a dark strip) r2 − r1 ≈ dy / L for small d sin θ = mλ d sin θ = (m + 1 / 2)λ y/L Approximation used: d << L θ Constructive (a bright strip) r2 − r1 = d sin θ d sin θ = mλ Consider two slits .075mm apart located 1.5m from screen. If the 3rd order bright fringe is 3.8cm from the screen center what is λ? Approximation used: d << L θ Destructive (dark strip) r2 − r1 = d sin θ Consider two slits .075mm apart located 1.5m from screen. Given λ =633nm what is the distance from screen center to the 3rd order dark fringe? Diffraction Limit For Circular Apertures Diffraction imposes a fundamental limit on the ability of optical systems to resolve closely space objects. Rayleigh Criterion Two peaks are can barely be distinguished if the central maximum of one coincides with the first minimum of the other. For a circular aperture (a) Two point sources are far enough apart so that they are well resolved (b) Two point sources are separated enough to barely satisfy Rayleigh Criterion (c) Two point sources are so close that they are not resolved. Resolution of the Eye Assume light of wavelength of λ = 500nm and a pupil diameter of d = 2mm. (a) Estimate the limiting angle of resolution for a human eye. For a circular aperture (b) Find the minimum distance of separation, d, for two objects that are 25cm from the observer. At 25cm objects can not be resolved if they are closer than 80μm! Multiple-Slit Interference and Diffraction Gratings From the diffraction limit with only two slits the intensity pattern is πd sin θ S = 4 S 0 cos ( ) λ 2 What if you require better resolution? Consider multiple slits. For multiple slits The location of the bright fringes still satisfies d sin θ = mλ The location of the dark fringes now satisfy Here N is the number of slits. This means there are N-1 dark fringes between each bright fringe. Resolving Power and Diffraction Gratings For a grating with N slits the criteria for the mth order maximum is The adjacent minimum occurs at Maximum for λ’ = minimum for λ Multiple-Slit Interference and Diffraction Gratings A binary star system has one massive star at rest and another smaller star rotating about it. The hydrogen α line for the rotating star is Doppler shifted from λ=656.272nm to λ=656.215nm. What order spectrum is required for astronomers to resolve these lines when using a grating that is 2.5cm wide with 2000 slits/cm? Since the order must be an integer, the resolution of the two wavelengths is third order, m = 3. Interference in Thin Films Constructive Interference: Destructive Interference: Is the wavelength inside the film and