• In-class Exam #1, Wednesday, Feb 1 (50 minutes)

•
In-class Exam #1, Wednesday, Feb 1 (50 minutes)
Covers chapters 1-3, HW_A and lecture material.
Formula sheets/notes (2 pages single sided) allowed.
Hand held calculators allowed.
•
HW_B due Friday, Feb 10
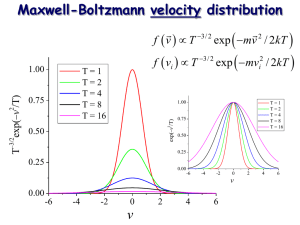
Maxwell-Boltzmann velocity distribution
T
i
T
3/ 2
3/ 2 exp exp
mv
2
/ 2 kT mv
2
/ 2 kT i
1.00
0.75
T = 1
T = 2
T = 4
T = 8
T = 16
1.00
0.75
T = 1
T = 2
T = 4
T = 8
T = 16
0.50
0.50
0.25
0.25
0.00
-6 -4 -2 v
0 2 4 6
0.00
-6 -4 -2 v
0 2 4 6
Maxwell-Boltzmann speed distribution
4
2
m kT
3/ 2 v
2 exp
mv
2
/ 2 kT
Maxwell-Boltzmann speed distribution
4
2
m kT
3/ 2 v
2 exp
mv
2
/ 2 kT
; ( )
N
( ) v m
2 kT
1/ 2
m
v
8 kT
1/ 2
m
v rms
3 kT
1/ 2
;
m
The probability density function
• The random motions of the molecules can be characterized by a probability distribution function.
• Since the velocity directions are uniformly distributed, we can reduce the problem to a speed distribution function which is isotropic.
• Let
f(v)dv be the fractional number of molecules in the speed range from v to
v + dv
.
• A probability distribution function has to satisfy the condition
0
1
•We can then use the distribution function to compute the average behavior of the molecules: v
0
v
2
0
2
v rms
v
2