(1)
advertisement
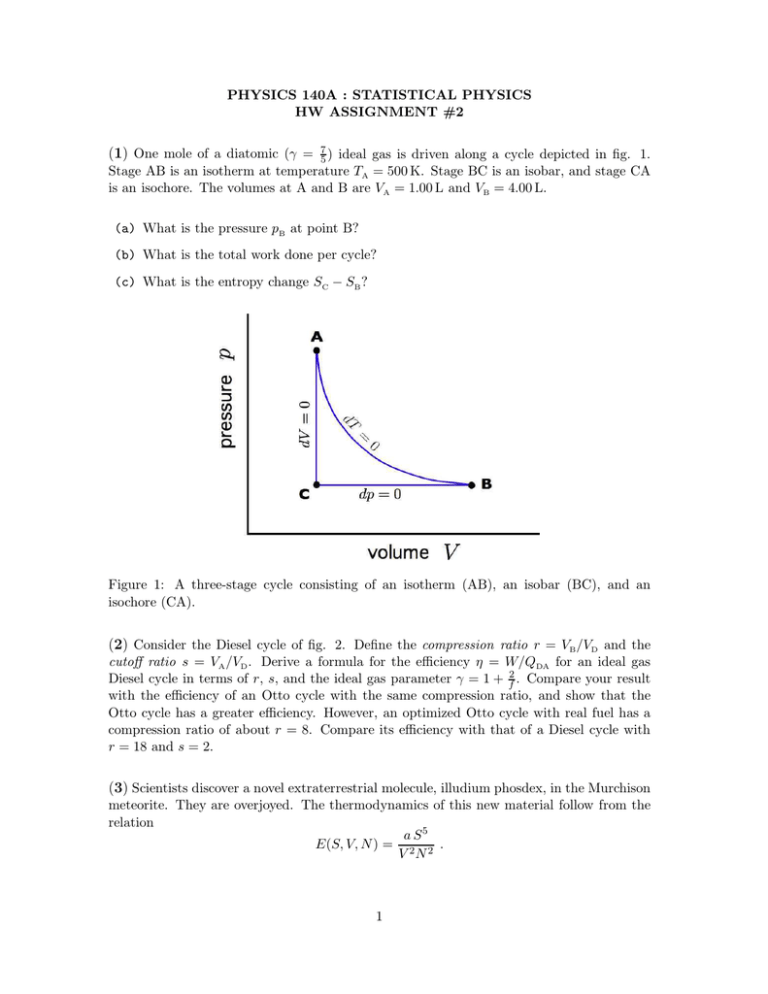
PHYSICS 140A : STATISTICAL PHYSICS HW ASSIGNMENT #2 (1) One mole of a diatomic (γ = 75 ) ideal gas is driven along a cycle depicted in fig. 1. Stage AB is an isotherm at temperature TA = 500 K. Stage BC is an isobar, and stage CA is an isochore. The volumes at A and B are VA = 1.00 L and VB = 4.00 L. (a) What is the pressure pB at point B? (b) What is the total work done per cycle? (c) What is the entropy change SC − SB ? Figure 1: A three-stage cycle consisting of an isotherm (AB), an isobar (BC), and an isochore (CA). (2) Consider the Diesel cycle of fig. 2. Define the compression ratio r = VB /VD and the cutoff ratio s = VA /VD . Derive a formula for the efficiency η = W/QDA for an ideal gas Diesel cycle in terms of r, s, and the ideal gas parameter γ = 1 + f2 . Compare your result with the efficiency of an Otto cycle with the same compression ratio, and show that the Otto cycle has a greater efficiency. However, an optimized Otto cycle with real fuel has a compression ratio of about r = 8. Compare its efficiency with that of a Diesel cycle with r = 18 and s = 2. (3) Scientists discover a novel extraterrestrial molecule, illudium phosdex, in the Murchison meteorite. They are overjoyed. The thermodynamics of this new material follow from the relation a S5 E(S, V, N ) = 2 2 . V N 1 Figure 2: A Diesel cycle consists of two adiabats, an isobar, and an isochore. (a) Let a = 1048 in MKS units. What are the MKS units of the constant a? (b) Derive the analog of the ideal gas law for this system – an equation of state relating p, T , N , and V . (c) How much work is required to isothermally expand 3.00 moles of illudium phosdex from Vi = 2.00 m3 to Vf = 3.00 m3 at a temperature of T = 300 K? Recall NA = 6.02 × 1023 . (d) At a pressure of p = 1.00 × 105 Pa, a quantity of illudium phosdex is placed in a sealed chamber. The chamber is thermally insulated so that no heat is exchanged between sample and environment. Additional pressure is applied and the change in volume is recorded. What is the measured compressibility κ = −V −1 ∂V /∂p? (4) Consider the relation N 1+α exp E(S, V, N ) = ε0 Vα αS N kB , where ε0 and α are constants. (a) Show that this system obeys the ideal gas law. (b) Find the adiabatic equation of state in terms of p and V . (c) Find the molar heat capacities cV and cp . 2 (5) Challenging! Consider the modified ideal gas Carnot cycle in fig. 3. The upper isotherm AB is broken into two separate stages. AA′ is an adiabatic free expansion, and all other stages are quasistatic. Define the parameter x= VA ′ − V A . VB − V A When x = 0, the entire cycle is reversible, and when x = 1, the entire upper isotherm is taken up by the adiabatic free expansion. Also define the expansion ratio r= VB . VA (a) Show that VA VC = VB VD . (b) What is the work W done per cycle? Express your answer in terms of the temperatures T1 and T2 , the ratios x and r, and the number of moles of gas ν. (c) Suppose VA = 1.00 L and VB = 10.0 L. Suppose further that T1 = 50◦ C and T2 = 400◦ C. At what value of VA′ would the work done per cycle vanish? (d) The efficiency of this cycle is defined as η = W/QA′ B . Derive an expression for η(x, r, T1 /T2 ). Figure 3: In this modified Carnot cycle, the upper isotherm is itself broken into two stages. AA′ is an adiabatic free expansion, and A′ B is a quasistatic isotherm. Stages BC, CD, and DA are all quasistatic. 3