Derivation of Compton’s Equation MORE CHAPTER 3, #1

10
MORE CHAPTER 3, #1
Derivation of
Compton’s Equation
Let
1
and
2
be the wavelengths of the incident and scattered x rays, respectively, as shown in Figure 3-18. The corresponding momenta are p
1
=
E
1 c
= hf
1 c
= h
1 and p
2
=
E c
2 = hf
2 c
= h
2 using f c . Since Compton used the K line of molybdenum ( 0.0711 nm; see
Figure 3-15 b ), the energy of the incident x ray (17.4 keV) is much greater than the binding energy of the valence electrons in the carbon-scattering block (about 11 eV); therefore, the carbon electrons can be considered to be free.
Conservation of momentum gives p
1
= p
2
+ p e or p 2 e
= p 2
1
+ p 2
2
2 p
1
# p
2
= p 2
1
+ p 2
2
2 p
1 p
2
cos 3-26 where p e
is the momentum of the electron after the collision and is the scattering angle of the photon, measured as shown in Figure 3-18. The energy of the electron before the collision is simply its rest energy E sion, the energy of the electron is
1
E 2
0
+ p 2 e c 2
0 mc 2
2 1
>
2 .
(see Chapter 2). After the colli-
E
1
= hf
1 p
1
= h /
λ
1 m
φ p e
=
1
–– c
θ p
2
= h /
λ
2
E
2
= hf
2
2
– E
0
2
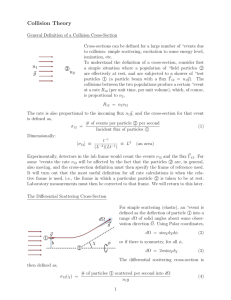
FIGURE 3-18 The scattering of x rays can be treated as a collision of a photon of initial momentum h /
1
and a free electron. Using conservation of momentum and energy, the momentum of the scattered photon h / be related to the initial momentum, the
2
can electron mass, and the scattering angle.
The resulting Compton equation for the change in the wavelength of the x ray is
Equation 3-25.
Conservation of energy gives p
1 c + E
0
= p
2 c + 1
E 2
0
+ p 2 e c 2 2 1
>
2
Transposing the term p
2 c and squaring, we obtain
E 2
0
+ c 2 1 p
1
p
2
2 2 + 2 cE
0
1 p
1
p
2
2 = E 2
0
+ p 2 e c 2 or p 2 e
= p 2
1
+ p 2
2
-
2 p
1 p
2
+
2 E
0
1 p
1 c
p
2
2
3-27
Eliminating p 2 e
between Equations 3-26 and 3-27, we obtain
E
0
1 p
1 c
p
2
2
= p
1 p
2
1
1
cos
2
Multiplying each term by hc
> p
1 p
2
E
0
and using = h
> p , we obtain Compton’s equation :
2
-
1
= hc
E
0
1
1
cos
2 = hc mc
2
1
1
cos
2 or
2
-
1
= h mc
1
1
cos
2
3-25
More Chapter 3 11