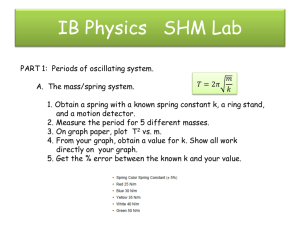
Document 10915487
advertisement

THE DEVELOPMENT AND ANALYSIS OF A
VENTRICULAR FIBRILLATION DETECTOR
by
Scott David Greenwald
B.S.E.,
Duke University
(1982)
SUBMITTED IN PARTIAL FULFILLMENT
OF THE REQUIREMENTS FOR THE
DEGREE OF
MASTER OF SCIENCE
IN ELECTRICAL ENGINEERING AND COMPUTER SCIENCE
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
May 1986
Scott David Greenwald
The author hereby grants to M.I.T. permission to reproduce and to
distribute copies of this thesis document in whole or in part.
Signature redacted
Signature of Author__
Department of Electrical Engineering and Computer Science
May 22, 1986
Signature redacted
Certified b y.
S. redacted,
Signature
Accepted by(
A-
Archives
-Z
I~'
~
,. ark
Thesis Supervisor
novzr
Arthur C. Smith
Students
Chairman, Departmental Committee on Graduate
MASSACHUSETTS INSTITU1E
OF TECHNOLOGY
JUL 2 31986
I IFpA RiES
-
Room 14-0551
77 Massachusetts Avenue
f*-
MiT
Libraries
Document Services
Cambridge, MA 02139
Ph: 617.253.2800
Email: docs@mit.edu
http://Iibraries.mit.edu/docs
DISCLAIMER OF QUALITY
Due to the condition of the original material, there are unavoidable
flaws in this reproduction. We have made every effort possible to
provide you with the best copy available. If you are dissatisfied with
this product and find it unusable, please contact Document Services as
soon as possible.
Thank you.
Some pages in the original document contain pictures,
graphics, or text that is illegible.
-
- 2
DEVELOPMENT AND ANALYSIS OF A
VENTRICULAR FIBRILLATION DETECTOR
by
SCOTT DAVID GREENWALD
Submitted to the Department of Electrical Engineering and Computer Science
in partial fulfillment of the requirements
for the Degree of Master of Science
May 22, 1986
ABSTRACT
Three detection schemes were developed in order to discriminate
ventricular tachycardia, flutter, and fibrillation from electrode motion
noise.
The first detection scheme (Detector 0) had been described in
the literature and was implemented in this study as a reference for two
novel detectors under development (Detectors 1 and 2.) Detectors 1 and 2
estimated the power spectrum of 4-second segments of digitized ECG with
a second-order autoregressive (AR(2)) process. The two coefficients of
the AR(2) model were used as the features to distinguish between the
rhythm classes.
For each of the four classes, the features were assumed to be Gaussian
distributed. Thus the conditional distributions of the features for each
class were dependent only on two parameters, the mean vector and the
covariance matrix.
Detectors 1 and 2 differed in their estimation of
these two parameters.
Detector 1 estimated the mean vector and covariance matrix over all the features.
Detector 2 estimated these parameters by first
calculating the parameters for each patient in the database and then averaging these patient-specific parameters.
Two cost functions (B1 and B2) were used to evaluate the detectors. The
detectors were optimized with respect to gross sensitivity (Bi) and the
sum of gross sensitivity and positive predictivity (B2).
The results show that all three detection schemes were equivalent with
respect to B1. Detector 2 performed slightly better than Detector 1 and
much better than Detector 0 with respect to B2.
Thesis Supervisor : Dr. Roger G. Mark, M.D., Ph.D.
Title: Matsushita Associate Professor of
Electrical Engineering in Medicine
-
- 3
Acknowledgements
where
I know where to begin, it's just tough finding
want
to
thank
so
at
the
time.
I
end.
many people who have helped me over the past years.
Not so much for this thesis.
on
to
I
This just happens to be what I was working
want to thank them for their guidance through my
struggle to figure out what MIT was all about, to
understand
relation-
ships, and to understand self.
I now happen to be where I wanted to be when I
ever,
I
didn't get here the way I planned.
started
out;
how-
I don't think that I would
have started had I known a priori what mountains where ahead of me.
because
of
the
special
people mentioned in this all too brief "thank
you", I transended the terrain.
I first
a
But
That is , WE transended the terrain.
want to thank Prof. Roger Mark (Roger) for his guidance
as
thesis supervisor and as the achetype of dedication, persistance,
and
forgiveness.
Always constructive and giving.
The only
person
I
know
that puts self last. Always.
Clearly, without a doubt, I am in greatest debt to
Imagine someone with all the answers and no egotisim.
Paul
time for one more question.
thanks
for
his
guidance
(And there were quite a
with
He always had the
few.)
I
him
owe
this work, for my budding professional
career with Computers in Cardiology, for teaching me
statistics and ....
Albrecht.
C
and
Venix
and
calm
amid
I am honored to be his friend.
Dr. David Israel, M.D.
the BMEC computer confusion.
Soon to be a Ph.D.
Remarkably
True patience with his work and others.
I
-
- 4
thank him for his help in making the computer a less formidible machine.
I
I appreciate the constant encouragement from Jeff Madwed.
to
MIT
came
Unfortunately, a thesis is a marathon
as an academic sprinter.
(or two.) Thanks, Jeff, for helping me keep the pace.
Wolfram Jarisch played vital roles in launching my thesis.
fine
statistician.
music... .structured
George
and
knows
exact.
ECG
and
George Moody
I want to thank those people who got me started.
like
analysis
Wolfram is a
knew
Bach
Phil Devlin, Joe Meitus, Diane Perry,
and the Arrhythmia Lab Staff deserve my
gratitude
for
their
help
in
establishing the Malignant-Arrhythmia Database.
I want to thank those people who got me finished.
Ferguson
Paul
and
Gaal
Imre
for
their
I will
remember
that
help with using NROFF,
delightfully simple word processing language with a mind of its own.
sincere appreciation to Gloria McAvenia, Terry Parekh,
(and
and Keiko Oh for their administrative support
(financially)
indebted
to
the
My
Patty Cunningham,
friendship.)
I
am
Kleberg Foundation who has kindly sup-
ported my MEMP fellowship and saw this work to completion.
I want to thank those
indebted
to
of MEMP.
to
A'lady. A scholar.
the
staff
in
between.
Physics student.
don't
You can survive anything given you
A special thanks to Debbie Burstein
alone.
me
helped
every Medical Engineering/Medical
group of INDIVIDUALS.
also
people who
I
am
What a
do
it
who revitalized the spirit
She balances them
well.
I
am
indebted
and students of the Biomedical Engineering Center.
Thanks.
And most importantly,
I want to thank Dad, Mom, Brian and Lisa.
If
I
had
to
-
- 5
choose my family again, it
hundred miles away but always here.
would be them.
I love you.
Four
-
- 6
Table Of Contents
TITLE PAGE........................................................
1
ABSTRACT..........................................................
2
ACKNOWLEDGEMENTS..................................................
3
TABLE OF CONTENTS.................................................
6
1.0 INTRODUCTION..................................................
8
1.1 Relevant Cardiac Physiology and Electrocardiography.......
1.2 The VF Detection/Artifact Rejection Problem
for Arrhythmia Detectors..................................
1.3 Formulation of the Detection Problem......................
8
15
18
2.0 BACKGROUND....................................................
28
2.1 Historical Ventricular Fibrillation Detection.............
2.1.1 Power Spectral Detection Methods....................
2.1.1.1 Relative Power About the Spectral Peak Detector
(Fixed Bandwidth).............................
2.1.1.2 Relative Power About the Spectral Peak Detector
(Varied Bandwidth).............................
2.1.1.3 Relative Power in Spectral Bands Detector......
2.1.2 Time Domain Detection Methods.........................
2.1.2.1 Shifted Waveform and Addition Detector........
2.1.2.2 Peak/Trough Series Detector...................
2.1.2.3 Amplitude Histogram Detector...................
2.2 Discriminating Malignant Arrhythmias and Noise
Using Autoregressive Modeling..............................
2.2.1 Introduction........................................
2.2.2 Spectral Resonance and Q.............................
2.2.3 Continuous-Time and Discrete-Time Relationships.....
2.2.4 Autoregressive Modeling.............................
3.0 METHODS......... .............................................
29
30
30
35
39
45
45
49
56
59
60
63
67
70
79
3.1 Database Development.................................... o.79
3.1.1 Creation of the Database............................ 83
3.1.2 Malignant-Arrhythmia Section......................... 83
3.1.3 Noise Section...................................... 85
3.2 Implementation of a Reference Detector.....................
86
3.2.1 Digest of the Detection Scheme...................... 86
3.2.2 Analysis of the Detector............................
86
3.3 Implementation of an Autoregressive Model Detector........
92
3.3.1 Digest of the Detection Scheme....................... 92
3.3.2 Analysis of the Detector............................. 94
-
- 7
3.4 Detector Evaluation Methodology........................... 107
3.4.1 Detector Performance Curves......................... 107
3.4.2 Estimating Confidence Limits for
Arrhythmia Performance Measures..................... 115
4.0 RESULTS OF THE DETECTION SCHEMES.............................. 126
4.1 Results for the Reference Detector........................ 126
4.2 Results for the Autoregressive Model Detectors............ 126
5.0 DISCUSSION................................................... 212
5.1 Discussion of the Reference Detector Results.............. 214
5.2 Discussion of the Results of the
Autoregressive Model Detectors........................... 221
6.0 CONCLUSIONS.................................................. 231
REFERENCES....................................................... 238
-
- 8
Chapter 1
1.
INTRODUCTION
1.1.
RELEVANT CARDIAC PHYSIOLOGY AND ELECTROCARDIOGRAPHY
Figure 1.1.1 shows a picture of the heart and diagrams its
tion
system.
right.
ling
The
heart is
conduc-
functionally two pumps separated left
from
Each pump is composed of two chambers, an atrium (the top
tank"),
and
a
"fil-
ventricle (the main ejecting unit at the bottom.)
These two pumps act in series to oxygenate the blood by pumping blood to
the
lungs
(right
heart) and to pump the oxygen-rich blood to the body
(left heart.)
The origin of the heart beat is in a concentrated
located
in
group
of
cells
the left atrium (sino-atrial node (SAN).) These cells, gen-
erate action potentials periodically which initiate electrial wavefronta
which
spread across the atria.
Because the SA node initiates the heart
beat, the SA node is called the primary pacemaker of the heart.
pacemaker
The SAN
rate is modulated by the nervous system through both parasym-
pathetic and sympathetic innervation.
Tracts of muscle cells preferentially conduct this action potential
throughout
the
heart.
These tracts are outlined in figure 1.1.1.
conduction pathways in the atria link the primary pacemaker site
atrial
node)
with
(sino-
a secondary pacemaker located between the atria and
the ventricles (the atrio-ventricular node (AVN).) The AV node
autogenic
The
but fires at a slower rate than the SA node and is
the impinging action potiential from the SA node.
is
also
"reset" by
-
- 9
The heart muscle (myocardium) contracts in response to
the
electrial
potential
across
the
cellular
changes
membranes
mechanical coupling.) Thus the resultant spread of
in
(electro-
electrical
activity
across the atria causes them to contract.
The atria are electrically
through
the
AV node.
isolated
from
the
ventricles
except
Thus ventricular contraction is initiated by the
SAN after the action potential has passed through the AV node.
The
AVN
acts as a delay allowing the atria to contract and "top off" the ventricles before they eject the blood from the heart.
The ventricular conduction system
through
the
Bundles
of
His,
branches into the myocardium.
synchronized
order
at
the
AVN,
continues
and ends with the Purkinje network that
The entire conduction
system
ensures
of atrial-then-ventricular contraction.
the electro-mechanical
depolarization.
begins
coupling,
contraction
Following contraction,
is
coupled
a
Because of
to
cellular
cells return to their polarized
resting potentials during muscular relaxation.
The electrical activity of the heart is clinically observed with an
electrocardiogram
(ECG)
monitoring
system.
The composite electrical
activity of the heart cells produce potentials at
body.
Monitoring
the
surface
of
the
potentials between different electrodes taped to the
chest or limbs produce the electrocardiogram.
Figure 1.1.2
normal
form
shows a segment of an electrocardiogram
individual.
a
nearly
correspond
to
The
periodic
(ECG)
of
a
figure shows a series of discrete beats which
signal.
physiologically
Different
significant
portions
events.
of
each
beat
(Refer to figure
10
-
-
1.1.3.) In broad terms, the P wave corresponds to atrial depolarization,
the
QRS
complex
corresponds
to ventricular depolarization, and the T
wave corresponds to ventricular repolarization.
occurs
during
the
QRS
complex
but
Atrial
repolarization
is masked by the large potential
changes of ventricular depolarization.
If there is insufficient blood flow to the heart muscle,
the
ven-
tricular tissue may become irritable.
The result is that the ventricles
may initiate a
the
mechanism.
beat
independent
of
normal
The resultant beat, which originated in an "ectopic focus",
is called a "premature ventricular complex" (PVC)
1.1.4.
pacemaker-conduction
and is
shown in figure
This figure shows wide, premature ventricular beats interspersed
with narrow, normal sinus beats.
Ectopic ventricular pacemakers may generate series of
than
isolated
beats.
A
series
of
three
PVCs
rather
or more in duration at an
equivalent rate of 100 per minute is called ventricular tachycardia
is
shown
in
figure 1.1.5.
If the ventricular rate increases signifi-
cantly (250-300 bpm), then the ventricular complexes begin
pose
and
mask
and
to
superim-
out the smaller amplitude portions of beat complex. The
resultant ECG looks sinusoidal (as shown in figure 1.1.6) and is
ventricular flutter (VFL).
called
(Ventricular flutter is defined here as high
grade VT with sinusoidal morpholgy.) Although
ventricular
flutter
may
subside back to a lower grade of VT, it frequently rapidly progresses to
ventricular fibrillation (VF).
Ventricular fibrillation, as shown in figure 1.1.7,
isolated
ventricular complexes.
foci have developed,
is
devoid
of
At this stage, a number of ventricular
each competing for the mechanical
control
of
the
heart.
11
-
-
During VF, because there are a number of isolated foci, the ven-
tricles contract chaotically in an unsynchronized
heart
pump
Death
ensues
is
uncoordinated,
as
the
brain
little
blood
deteriorates
fashion.
Since
the
is ejected to the body.
from
lack
of
oxygen
and
nutrients.
Thus it
is clinically imperative to detect when a patient is in VFL
and VF in order to intervene and save the patient's life.
VT, VFL, and VF are potentially lethal
cardiac
Because rapid
arrhythmias,
they
collectively calles "malignant arrhythmias."
Superior veno cova ---------------
CONDUCTING STRUCTURES
- --- ------SA NODE
Right atrium ---------.
Tricuspid valve --------
-
-
Coronary sinus
----
Right ventricle ------------
Interventricular
----
---
-----
COMMON
BUNDLE
- ------ LEFT BUNDLE BRANCH
--
RiGHT BUNDLE BRANCH
---------- ANTERIOR DIVISION OF
LEFT BUNDLE BRANCH
septum
Left ventricle --------- --- -- -
DIVISION OF
LEFT BUNDLE BRANCH
-
L c
-POSTERIOR
.onducting system of the human heart, showing anatomical features of the heart
(labels at left) and the conducting structures (labels at right). (Modified from Bennrnghoff:
Lehrbuch der Anatomie des Menschen, 1944. J. F. Lehmanns Verlag, Munich.)
Figure 1.1.1 Diagram of the Human Heart and its Conduction System.
are
Figure 1.1.2 Normal Sinus Rhythm.
-
- 12
-
- 13
I I
SI I I I
I
_7m7--
10041
SECONO
16!
I I
I
:1
!I
d
U I
II
p
S-T
I SGMENT
tEGMpENT!
I~~~t I i ;
L~~I*hi~.N
u
NTERVANTE
I
-i
ll~
i I lIII IIU
Il
P-R
ADULTS
NORMAl
RANGES
INTERVAL
0.18
TO
0R$
INTERVAl
0.20 SECOND
0.07
TO
0,10
RATE
O-T INTERVAL
60
0.33 TO 0.43 SECOND
70
80
SECOND
0-7
.. ......
.-
-NT
COUNT NUMBER
3
5:
0.16 SECOND
0.12 TO 0.14 SECOND
0
11 TO 0.13 SECOND
0.10 TO 0.11 SECOND
0.32 SECOND
S2:!
TO
0.13 TO 0.15 SECOND
0.31 TO 0.41 SECOND
0.29 TO 0.38 SECOND
SECOND
TO 0.01
0.28
TO 0.36 SECOND
SECOND 0.6
0.32 90
TO
0.5
0
0,1
T 0.0 SCON 0.7 T
100
0.27 TO 0.35 SECOND
I ADLTS0. S
120
70
ChILDREN 0.15 TO 0.18 SECOND
0.25
CALCULATION
OF RATE
S-T SEGMENT
0.14
0.06
_
TO
0.07 SECOND
I ....
.
I I
I-T1
INTFRVA
- _
VA-
OF R-R INTERVALS Q3
MULTIPLY 3.5 BY 20 TO
GIVE
N 3 SECONOS
(15
TImi SPACES
OF 0.2 SECOND EAOE
RATE PER MINUTE (70 IN THIS CASO
Figure 1.1.3 Terminology Associated with a Single, Normal Beat.
-
- 14
Figure 1.1.4 Premature Ventricular Complexes
(PVCs)
Normal Beats.
Figure 1.1.5 A Short Burst of Ventricular Tachycardia.
Interspersed
with
15
-
-
Figure 1.1.6 Ventricular Flutter.
Figure 1.1.7 Ventricular Fibrillation.
1.2.
THE VF
DETECTION/ARTIFACT
REJECTION
PROBLEM
FOR
ARRHYTHMIA
DETECTORS
Because of the clinical relevance of malignant arrhythmias, immediate
detection
of high grade VT,VFL,
so important to detect these episodes,
and VF is imperative.
monitoring
machines
Since it
is
frequently
have
a
high
tions.) It
rate of false alarms
is the purpose of this
-
- 16
(alarms due to false positive detecsection
to
describe
the
different
types of false alarms.
Most arrhythmia monitors classify beats in the electrocardiogram by
detecting
separate
beats
as isolated events, extracting features, and
classifying the beats based on the particular beat features.
systems
which
isolate
Monitoring
beats to classify the electrocardiogram perform
poorly on rhythm disturbances such as high grade VT, VFL, and VF.
Because VFL or VF
beat
detection
is not a periodic signal of discrete events, the
algorithm
three types of mistakes.
alert
the staff.
may
cause the monitoring machine to produce
Two of the mistakes
are
amplitude
VF
is
serious.
If
the
small ("fine VF"), the beat detector will fail to
isolate a beat complex and thus ring an asystole alarm (i.e.,
that
which
life.
type of false alarm is not clinically
of
alarms
The third mistake is a missed VF episode (false nega-
tive), a mistake that endangers a patient's life.
The first
false
an
alarm
the heart has stopped beating.) This false alarm caused the system
to default so that the patient
would
receive
immediate
attention
as
he/she would require anyway.
The second type of false alarm is due to noise confusing the detection
algorithm.
Because
isolated event detection systems have diffi-
culty with undulating waveforms and rhythm disturbances,
certain
types
of noise elicit false positive alarms.
Electrode motion artifact is produced when a patient moves or
turbs
the
electrodes
attached
to
his/her
skin.
Electrode
dismotion
artifact (EM noise) may cause two
electrocardiogram.
-
- 17
misclassifications
of
the
First, if the noise glitches are significanty large,
the beat detector may classify the glitches as some form
tricular
complex.
Second,
if
of
wide
the
beat
detector
to detect a beat and therefore sound an asystole alarm.
take, although benign to a healthly patient
electrodes,
is
significant
ven-
the artifact masks out the electrocar-
diogram without producing distinct glitches,
fail
observed
because it
who
simply
would
This mis-
moved
his/her
diminishes the clinical staff's
confidence in the utility of the machine.
An excessive
rate
of
false
positives may cause the staff to respond less efficiently to alarms than
desired.
respond
If a VF episode occured and caused an
alarm,
the
too late to successfully resuscitate the patient.
a missed event, not due to the detecton system, but rather
staff
Here we have
due
to
effect of excessive false postives on the staff/machine system.
izing the staff/machine response is discussed in
section
may
the
(Optim-
3.4.1.)
This
research focused on correcting this type of false alarm due to noise.
The third type of error is false negative
episode.
If
the
peaks
rejection
of VFL are significant,
trigger off every fourth or fifth peak and report
wide,
of
a
VFL/VF
the beat detector may
that
a
sequence
of
bizarre complexes had occurred, but not that anything immediately
significant
had
passed.
Like
the
false
negative
due
to
the
staff/machine system, this missed event may endanger a patient's life.
The objective of this research was to develop
algorithm
that
would
work
in
a
rhythm
detection
parallel to a primary beat classifier.
This parallel processor would discriminate between the collective set of
high grade VT, VFL,and VF from noise artifacts.
1.3.
-
18
-
FORMULATION OF THE DETECTION PROBLEM
This section formulates the general detection
duces
problem
and
intro-
terminology used in the design and analysis of detection schemes.
Figure 1.3.1 shows a block diagram description of the general
detection
problem.
OBSERVATION
00-
DETECTOR
DECISION
Figure 1.3.1 Block diagram of the general detector.
A detector may observe one of many different classes of
In
this
study,
for
input
signals.
example, the detector may observe an episode from
either of the following four classes : 1) Ventricular Tachycardia
2)
Ventricular
Flutter
electode motion artifact
one
class
are
similar enough
classes.
not
(VFL), 3) Ventricular Fibrillation (VF), or 4)
(NOISE).
identical,
within
each
The episodes
(observations) from
class
that
it
can
distinguish
between
(This is not always the case since the detector will make misThe
reasons
particular detection schemes make certain mistakes are presented in
the discussion sections (chapter 5).) The output of the detector for
input
any
but the detector assumes that they are
takes.) The types of detection errors are discussed below.
why
(VT),
observation
is
the
decision
of
an
the class type from which the
19
-
-
observation came.
The following illustrative example describes
discriminating between two classes.
the
binary
case
of
Let the null hypothesis be that the
observation was electrode motion arifact (i.e, class NOISE.) Denote this
by H0 = N.
hypothesis
Then the alternative hypothesis must be that the
observation was not artifact (i.e., the observation was from
the
classes
Denote this
alternative
N
or
V.
if
an
observation
belonged
to
Since observations may be from one of two classes, and
the detector may assign an observation to either of those
the
hypothesis
V.
=
The detector's task is to decide
class
of
VT,VFL, or VF.) Let the three malignant arrhythmia classes
be combined into the one class, V.
by Hi
either
two
classes,
detector may make four different types of decisions over the course
of observing all its input episodes.
That is, the detector may
1) correctly decide that an observation was
from
class
V
(true
positive detection (tp)),
2) incorrectly decide that an observation was from class V
(false
positive detection (fp)),
3) correctly decide that an observation was
from
class
N
(true
(fase
nega-
negative rejection (tn) or correct rejection), or
4) incorrectly decide that an observation was from N
tive rejection (fn)).
(This terminolgy is clear if one assumes that the detector is interested
in
detecting
the
serious
arrhythmia
events
and rejecting the noisy
-
- 20
artifact.)
One can begin to evaluate the performance of a detector by
ing
record-
the number of each of the four types of decisions that the detector
makes for a set of observations (i.e., a test
database.)
To
interpret
the detector performance, these numbers are ordered in a decision matrix
which is frequently called a "confusion matrix." Figure
confusion
matrix
for
1.3.2
shows
a
the binary decision problem under consideration.
The number of events of a particular type of decision are denoted by the
captital letters of the decision type.
Once the results of a detctor have been compiled, one
its
efficacy
can
examine
by defining and evaluating detector performance measures.
This section presents three measures for evaluating a detector
sensi-
tivity, specificity, and positive predictivity.
The data in a confusion matrix describes how well a
formed
over
a
particular database.
will
make.
postive
decisions
that
a
(These measures allow one to compare the perfor-
mance of different detectors.) These probabilities are estimated by
false postive rate (FPR) and true positve rate
confusion matrix data.
per-
It is often important to estimate
the probabilities of false postive and true
detector
detector
the
(TPR) calculated from the
Specifically,
TPR
=
FPR =
The true positive rate is
also
TP + FN
FP + TN
called
the
detector to detecting observations of class V.
"sensitivity"(SE)
of
the
-
- 21
TRUTH
N
V
TP
FP
N
FN
TN
ALGORITHM
Figure 1.3.2 Binary Confusion Matrix. The horizontal axis labels the
true class types of the observations.
The vertical axis labels the assigned class types
(decisions) for the observations.
Each element in
the matrix contains the number of observations from class j assigned to
class i
(where (i,j)
is the location of an element in the matrix.) The
sum of the elements in any column(j) is the total number of observations
in the database of that particular class(j). The sum of the elements in
any row(i) is the total number of observations
that were assigned to
that particular class(i).
TP number of true positive decisions.
FP
number of false positive decisions.
TN number of true negative rejections (correct rejections).
FN number of false negative rejections.
The "specificity"(SP) of a detector is a
how
measure
which
describes
well the detector ignores observations from class N (i.e.,
how well
it correctly rejects noise.) It is defined by
SP = --FP + TN
The FPR is related to the specificity by
FPR = 1 - SP
To understand which performance measures are
important,
one
must
consider
-
- 22
hospital staff respond to alarms generated by monitoring
that
machines. The machine will sound an alarm for only the
positive
cases.
nor
can
detector
the
However, the monitor will sound
create an alarm if it misses a V event.
an alarm if noise confuses the detector into
deciding
observation
the
These false positives create a problem with the hospital
was a V event.
If the FPR is excessive,
itoring
false
That is, there is no reason to notify the nurse if the
patient is moving and creating motion artifact,
staff.
and
true
machine
and
may
the staff looses confidence is the mon-
therefore
to its alarms less effi-
respond
ciently. A useful measure of the influence of the false positives on the
staff/machine
system
is the positive predictive accuracy
(PPA)
defined
by
PPA
=
TP
TP + FP
A detector which
The PPA is also called the positive predictivity (+P).
maxamized these three measures (SE=100%, SP=100%, PPA=100%) would always
make the correct decision.
In the design of a detector, one can frequently tweak the detection
so
algorithm
that one can trade off percentage points between the dif-
ferent performance measures.
For example, one can always make a
detec-
tor 100% sensitive to V events simply forcing the detector to call every
observation a V event.
have a 0% specificity.
therefore
with a 0% sensitivity
between
This detector
N
and
V
and
100%
classes.
would
setting
have
no
and
TN
Conversely, one could make a detector
specificity
Obviously
by
reversing
the
roles
there are detector settings in
between which would yield non-zero performance measures.
Because different detector
settings
yield
different
numbers
of
TP,FP,TN,
and
FN
decisions,
-
- 23
detector settings are often set based on
assigning costs of making each of the four decisions.
Specifically, the
four costs would be assigned to the decision types, and the setting that
minimized the expected cost of the detector would be selected.
lowing
discussion
The fol-
shows the derivation of the detector setting used to
minimize the expected cost of a detector for the binary case.
Recall that there are two hypotheses (classes)
H0
=
N
H1 = V
There is an a priori probability that the
these
events, namely, P(H )
=
detector
will
see
each
of
a priori probability that the observation
was N, P(H 1 ) = a priori probability that the observation was V.
A detector makes its
observation.
It
decision based on extracting features from the
compares
these
features
with
some
information it
learned from a learning database of episode samples of each class
(Different
detectors
extract
different
features
numbers of features) so the discussion can not
be
type.
(and even different
made
more
specific
until specific detection schemes are discussed in sections 2.2, 3.2, and
3.3.)
As an example, consider the case where the
feature
on
the
threshold (,q).
tued
extracts
one
(x) from the observation (say average amplitude of the episode.)
This detector then will decide what
based
detector
value
of
this
class
the
observation
came
from
single feature (x) with respect to some
For convenience, let the feature be
Gaussian
distribu-
under both hypotheses. Let p(xIN) denote the conditional probabil-
-
- 24
ity density function of feature x given that the feature came from class
N.
Likewise let p(x|V) denote the conditional probability density func-
tion of feature x given that the feature came from class V.
A
picture
of this example is diagrammed in figure 1.3.3.
p(xIV)
p(xIN)
x
D0
.-
DECIDE N
M
D1
DECIDE V
1
Figure 1.3.3 Binary Decision Problem. The conditional distributions of
the feature (x) are shown with respect to both hypotheses H0 = N and
H1 = V.
The decision region where the detector decides class i is
denoted by Di on either side of the threshold 1. Changing the threshold
alters the (non-overlapping) decision regions and thus alters the detector results.
The problem is to determine where to set the threshold
the
desired results.
to
achieve
In this case, we are interested in minimizing the
expected cost of the detector given that we have assigned costs to
of the four decision types.
each
Let C
class
i
-
- 25
denote the cost of deciding that the observation
given that it
really was from class j.
was
from
Then the expected cost
the detector is given by,
E(C)
2C
=
P(xH. IH )P(H.)
(1.3.1)
i=Oj=O
where P(xeH IH) is the probability of
the
feature
x
came
from
H.
when
that
the
observation
in reality it came from H
.
with
deciding
P(xeH IH) depends upon the decision region D. and is given by,
P(xeH
HI ) = J, p(xIH )dx
Substituting the definitions for P(xH IH ) into equation 1.3.1 and
realizing
that
the
decision regions D
and D
are non-overlapping and
together make up the x-axis gives
E(C) = C00 P(H ) + C 0 1 P(H1 ) +
fD
(CO-C00)P(HO)p(xlHO)
1
(1.3.2)
(C01 -C1 1 )P(HI)p(xIH1 )] dx
This equation reduces to
E(C)
=
C 0 0 P(H 0 ) + C0 1 P(H1 ) + (Clo-C00)P(H0)PF - (C01 C 1)P(Hl)PD
where PF and PD are the probabilities of false and true
postive
detec-
Equation 1.3.2 shows that the expected cost is minimized by
minim-
tions respectively.
izing
the
integrand and therefore by assigning each observation to the
decision region D
when
(C1 0 -C0 0 )P(HO)p(xIH0 ) -
(C0 1 -Cj 1 )P(H1 )p(xIH1 ) (0.
Equation 1.3.3 may be rearranged to yield
the
optimal
(minimal
(1.3.3)
cost)
-
- 26
decision threshold for the detector as
"t
)
p(x|Hl) >
(C1 0 -C 0 0 )P(H0
p(xIH0 )0 (C01-C11)P(H) =
The expression on the left in equation 1.3.4 is called
ratio"
because
it
is
the
"likelihood
a ratio of two probability (likelihood) density
functions and is denoted by L(x).
The likelihood ratio criterion degenerates into two other
for specific cost and
criteria
a priori probabilities : 1) the Minimum Probabil-
ity of Error (also called the Maximum A Posterior: (MAP)) criterion, and
2) the Maximum Likelihood (ML)
criterion.
If the costs of making a correct decision are zero, and
of
incorrect
decisions
are equal
(i.e.,
the
costs
= C.., Cii =0), then L(x)
C
becomes
-
P(HO)
P(H1
)
p(xIH1 )
p(xIH0 )
Dividing Eqn. 1.3.5 on both sides by p(x) , using Baye's rule, and rearranging gives
P(H Ix)
=
P(H Ix)
(1.3.6)
which says to assign the observation to the class with the higher a posterior probability.
This is the Maximum A Posteriori Probability detec-
tion criterion.
If 1) the costs of making a corect decision are zero, 2) the
making
an
assume that the
P(H1)),
error
a
are
priori
equal
(as with the MAP detector), and 3) we
probabilities
then rearranging Eqn. 1.3.4 gives
are
equal
(i.e.,
P(H 0
)
of
costs
-
- 27
p(xIH1 )
p(xIHO).
=
(1.3.7)
This is the Maximum Likelihood detection criterion.
The ML detector criterion is
detection
readily
applied
to
multiple
problems since the detector decides to assign the observation
to that class with the highest conditional probability density.
detection
criterion
The
ML
was used in this study as a landmark from which to
compare other detectors which were optimized with respect to
(benefit)
class
functions.
(Refer
some
cost
to section 3.4.1 for a discussion of the
benefit functions used in this study.)
As discussed earlier, there is a trade off between the FPR and TPR.
A
graph
which shows this trade off by plotting the TPR against the FPR
as a function of -qis called a Receiver Operating Characteristic
Figure 1.3.4 shows a ROC.
hold for a detector.
assigned
the
Each point along the curve specifies a thres-
The selected threshold is a function of the
decisions
as
costs
described above or by maximizing a benefit
function with respect to threshold
3.4.1.)
(ROC).
setting
(as
described
in
section
-
- 28
ROC
100
D Z SE
0
PF ~ 1-sP
100
Figure
1.3.4
Receiver Operating
Characteristic
(ROC).
The ROC
describes the trade off between the TPR and FPR as a function of threshold q along the curve. Any one point along the curve specifies a detector.
Chapter 2
2.
BACKGROUND
This chapter describes the previous work done in VF detection,
presents the theory for two novel autoregressive detection schemes.
and
2.1.
HISTORICAL VENTRICULAR FIBRILLATION DETECTION
critical literature search, the state of research
In an initial
VT,
-
- 29
VFL, and VF detection was established.
In particular, papers in the
field were examined for database sources, detection schemes, and
tor
response
to
artifact.
A
digest
in
of
the
detec-
research is
pertinent
presented and followed by a comparison of the detection schemes.
The reviewed articles discussed detection schemes as well
analysis
of VT, VFL, and VF characteristics.
as
data
These papers were divided
into the sections indicated below.
Data Analysis schemes
1. Multitransformation[1,2]
2. Autocorrelation[3]
Detection schemes
1.
Relative
Power
About
the
Spectral
Peak
(
Detector
Fixed
Bandwidth )[4]
2. Relative
Power
About
the
Spectral
Peak
Detector
(
Varied
Bandwidth )[5]
3. Relative Power in Spectral Bands Detector[6]
4. Shifted Waveform and Addition Detector[7]
5. Peak-Trough Series Detector[8]
6. Amplitude Histogram Detector[9]
The detection methods are discussed in this
section.
For
indepth
-
- 30
analysis of the characteristics of VF refer to [1-31.
2.1.1.
the
POWER SPECTEAL DETECTION METHODS
The first
three detection schemes to be discussed were motivated by
spectral
characteristics
the malignant arrhythmias frequently
standard
measure
of
the
The power spectra of
of VT,VFL, and VF.
contain
breadth
of
this
a
principal
peak.
spectral peak is
its
One
Q as
defined by
Q =o
Af
where f 0 is the frequency of the spectral peak and Af is the half
bandwidth.
power
Nygards, Nolle, and Forster tried to estimate the shape (Q)
and properties of the power spectra with a few parameters. These parameters
were to be used to discriminate among ECG classes.
These spectral
characterization methods are described below.
2.1.1.1.
RELATIVE
POWER
ABOUT
THE
SPECTRAL
PEAK
DETECTOR
(FIXED BANDWIDTH)
Detection Principles
A frequency domain approach to detecting VT and
first
by Nygards[4]
VF
was
because the power spectra of VT/VF is dissimilar to
spectra for most other ECG rhythms. Because the power spectra
is
narrowly
attempted
bandlimited
about
a
of
single high-Q peak, while other ECG
waveforms are more broadband, a measure of the Q was estimated in
to
descriminate
graphic events.
these
VT/VF
order
malignant arrhythmias from other electrocardio-
-
- 31
VT and VF were descriminated from other ECG
through
the
and
artifact
following steps : 1) selecting input ECG segments as VT/VF
candidates, 2) estimating the power spectrum for
waveform,
rhythms
3)
calculating
each
VT/VF
candidate
the ratio of power in a fixed bandwidth cen-
tered about the spectral peak relative to the total power in the segment
(i.e.,
estimating
the
Q ) , and 4) classifying the candidate waveform
via a rule table based on this ratio,
the heart rate, and whether or not
a QRS complex was observed.
Candidate VT/VF waveforms for step (1)
were selected from the
pre-
five seconds of input if : 1) less than three normal or supraven-
vious
tricular beats were detected, 2) no neighboring QRS complexes with
mal
morphology
or
timing
were
detected, 3) the average signal power
exceeded a threshold, and 4) no major artifacts were
baseline
drift,
or
high
nor-
derivatives ) .
signal processing, feature extraction,
observed
(
e.g.,
Steps (2) through (4)
and classification methods
-
the
are
-
discussed below.
Digest Of The Detection Method
Digital Signal Processing
The total power of the candidate signal was computed
domain.
in
the
Stable power spectral estimates of the input were calculated by
averaging power spectral estimates of overlapping sections of the
segment.
Specifically,
the
power
spectrum
of
input
3.84 second candidate
waveforms were estimated from five 1.28 second overlapping
this
time
segments
of
input. Each 1.28 second segment was zero-padded to 5.12 seconds in
order to enhance the appearance of the spectral estimate. Because VT and
VF
were
principally
-
- 32
bandlimited under 10 Hz, power spectral estimates
were calculated for input segments effectively sampled at 25Hz.
Feature Extraction
The features of interest are the estimate of the
spectrum,
the
heart
rate,
and
the
observation
Q
of
of
a
the
power
QRS complex.
Unspecified portions of the ECG monitoring algorithm provide an estimate
of
the heart rate and set a flag for the existance of a QRS peak. The Q
was estimated via the following method.
First, the frequency (F) corresponding to the
spectrum
within 1.7 and 9.0 Hz was established.
peak
Second,
of
the
power
the power in a
bandwith of 2/3F to 4/3F was then calculated. Last, the algorithm determined
nal.
the
ratio (R)
of this power to the total power of the input sig-
With these features established,
the detection scheme advanced
to
the classification phase.
Classification
The candidate waveform was
classified
feature values to the decision table below.
by
mapping
(See 2.1.1.)
the
estimated
-
- 33
Table 2.1.1. VT and VF Descrimination Rules
Relative
Power of
spectraj
Peak (R.)
Heart
Rate
(beats
per
minute)
>= 85%
< 240/min
>= 240/minIVF
QRS complexes
identified ?
-VT
< 85%
>= 65%
< 65%
65
no
yes
-_
Undefined
_______L
-
L
Diagnosis
1 R denotes the ratio of power in a bandwidth 2/3 F to 4/3 F
relative to the total signal power.
Summary of Detector Performance Results
The performance measures
sensitivity-specificity
used
evaluation
were
of
not
based
arrhythmia
Nygards[4] presented results in two other manners :
false
positives
on
the
standard
detection
schemes.
1)
the
number
of
per 1000 patient hours, and 2) the ratio of true posi-
tives to false positives.
The descrimination results for Nygards Test Set
table 2.1.2.
are
presented
in
-
- 34
Table 2.1.2 Computer classification of VT and VF using Nygards' descrimination algorithm
VF
F
16
6
VT
119
_
I
_
__ _
21
1 17 1
I
j other
IL..........i
I
5
114
1
I
VF Sensitivity
72.7%I
VF Positive Predictivity
17.4%
lumber False VF Alarms
000 patient monitoring
hours
3.6
Ratio of True VF Alarms
to False VF Alarms
1:4.75
I
/
I
VI
VT
VF
_______-[-__,V
*
Computer classification
True condition
IF|SR+high
artifact
AF*
Po
* AF is atrial fibrillation or flutter
** SR is sinus rhythm
Discussion Of The Detector Results
The sources of false VF alarms were
1) motion artifact,
2) loose electrodes,
3) atrial flutter or fibrillation
(frequently occurring with bundle branch block),
4) sinus rhythm with large P or T waves and small QRS
complexes, and
5) wide ventricular waveforms.
Nygards explained his results by the fact that he worked with
ited
Test
lim-
Set, and therefore needed to use wide criteria to detect VF.
He suggested that a better descrimination between VT
and
VF
could
be
made
through
an
-
- 35
analysis of the time variation of the power spectrum.
(This suggestion is followed by Herbschleb. [2] ) In addition, he stated
that
electrode impedance could be monitored to reduce false alarms
the
due to motion artifact. Last, he suggested that a scheme which considers
the
power
in
the harmonics may help delineate VF from normal rhythms.
(Forster[6] pursued this approach.)
2.1.1.2.
RELATIVE
POWER
ABOUT
THE
SPECTRAL
PEAK
DETECTOR
(VARIED BANDWIDTH)
Detection Principles
Nolle [5] discriminated
utilizing
records.
bandwidth
VT/VF from artifact and other waveforms by
Nygard's estimate of the Q of the power spectrum of candidate
This
(Wi)
estimator
about
calculates
the
fixed
(2/3F
of
power
in
a
Nygards calculated R using
to 4/3F) bandwidth and the total signal power; however,
Nolle calculated R as a ratio of the powers in two different
where
(R)
the frequency corresponding to the spectral peak
(F) to the power in a larger bandwidth (W2).
a
ratio
bandwidths
the larger, outer bandwidth contained the smaller, inner one.
He
investigated the changes in percent true and false positive VT/VF detection as functions of :
1) different inner bandwidths centered about F,
2) different fixed inner bandwidths
(i.e, not centered about F), and
3) different outer bandwidths.
For each selected pair of inner and outer bandwidths, Nolle
established
receiver operating curves to evaluate the detector's performance.
-
- 36
Digital Signal Processing
The database used for this study was collected from input ECG
nals
which
alarms.
had
caused
the
monitoring
computer
The database was therefore a biased
artifacts.
rich
in
VF-like
Sixty-one VT/VF records from 49 patients (11VT and 50VF) and
the
original
database
The first 4.096 seconds following the onset of each VT/VF episode
(or the onset of artifact) comprised
coded
to produce multiple
sample,
148 artifact records from 69 patients comprised
pool.
sig-
signals
were
stored
data compression scheme.
the
final
database.
The
alarm
in data-compressed form via the Aztec [10]
The reconstructed records had been effectively
sampled at 250 Hz and zero padded when necessary to produce 4.096-second
segments.
The FFT was applied off-line to estimate the
power
spectrum
of each of these segments.
Feature Extraction
The frequency of the peak power component
the
power
(F)
in different bandwidths was calculated.
was
identified
and
The feature used to
discriminated between artifact and VT/VF was the ratio R of the power in
the
inner
bandwidth
divided
by
power
in the outer bandwidth. Nolle
tested different combinations of inner and outer bandwidths to calculate
R as shown in table 2.1.3.
-
- 37
Table 2.1.3 : Inner And Outer Bandwidth Pairs
Used For The Calculation Of The Power Ratio R
|
Centered About F*
INNER BANDWIDTH W1
L_
OUTER BANDWIDTH W2
Not Centered About F
0.25 - 3.91
Not Centered About F
1.5 -24
0.25 -24
- 9.75
1
*
F is
BANDWIDTH USED
0.90 F -1.1
0.85 F -1.15
0.80 F -1.2
0.75 F - 1.25
0.70 F - 1.3
0.65 F - 1.35
0.60 F
1.4
0.55 F - 1.45
0.50 F
1.5
0.45 F -1.55
0.40 F -1.6
0.35 F -1.65
0.30 F - 1.7
0.25 F - 1.75
I1.5
----I
i
the frequency of the peak power component.
Nolle presented only the results for different detectors made
same
(Hz)
F
F
F
F
F
F
F
F
F
F
F
F
F
F
outer
bandwidths.
bandwidth
(1.5Hz
to
24
The inner bandwidths were
Hz)
and
centered
with
about
with
different
the
peak
the
inner
fre-
quency, F, and incremented in steps from (.9 F - 1.1 F) to (.25 F - 1.75
F).
Each change in inner bandwidth corresponded to the design of a dif-
ferent detector.
Classification
Artifact was discriminated from VT/VF based on the value of R
culated
for
the record.
cal-
If R was greater than a threshold T, then the
input was classified as VT/VF. Otherwise it
was labeled
artifact.
The
threshold was varied from 0 (corresponding to all power outside W1) to 1
-
- 38
(corresponding to a WI band limited signal) in order to produce receiver
operating
curves.
The
receiver
operating
curve
for
Nolle's
best
selected detector is shown in figure 2.1.4.
100
z
0
U
S50-
U
LLI
0
U
05
0
FALSE POSITIVES
10
[%)
Figure 2.1.4. A receiver operating curve shows the proportions of
records
correctly
classified
VT/VF
versus the false positive proportions of
artifact records as the detector threshold (T) is varied
from
zero
to
one. The detector bandwidth is 70% in this example.
Results Of The Detection Method
The descrimination results for the selected detector with an
bandwidth
of 70% F on this database are shown in table 2.1.5.
formance measures used were the number of true
positive
inner
The per-
detections
of
-
- 39
VT/VF and the number of false positive classifications of artifact.
Table 2.1.5 : Detector Performance 1
% TP 2
Threshold (T)
% FP 3
.36
100%
86%
.73
93%
19%
8%
.93
___
----------
0
0%
1 Detector architecture
)
)
Inner bandwidth ( .65 F - 1.35 F ) Hz
Outer bandwidth 1.5 -24 Hz
2
Detection
VT/VF
(i.e.,
3 TP = True Positive
FP = False Positive (i.e., Artifact Misclassification
Discussion of the Detector Results
This single-feature classification scheme had difficulty in detecting
5%
of the VT/VF records because of their low R values due to power
in higher harmonics.
Although the first four-second segment of a
VT/VF
or artifact episode comprised the database, Nolle stated that subsequent
segments of most of these "difficult" VT/VF
episodes
did
have
suffi-
ciently high R values so that the detector could properly classify them.
He concluded that had he used
scheme,
he
longer
segments
in
his
classification
may have been able to discriminnate better.
In contrast to
presenting the detector's sources of false negatives, Nolle did not discuss
which
artifact
types
were
particularly
difficult to correctly
reject.
2.1.1.3.
RELATIVE POWER IN SPECTRAL BANDS DETECTOR
-
- 40
Detection Principles
Forster[6] applied two different frequency domain
schemes
to
discriminate
extrac-
VF from other cardiac rhythms.
The two
detection schemes described below differ in
scheme
(
the
first
detection
1 ) was a simplified version of the second scheme
Detector
Detector 2 ).
that
Forster did not combine VT and VF as a single class
(
tion
feature
like
Nygards and Nolle, but later tested the VF detector with VT to determine
its response.
rhythms
Both
beginning
detection
with
the
schemes
discriminated
VF
from
other
following steps : 1) estimating the power
between
lower, middle, and upper frequency bands,
( See figure 2.1.6.
)
spectrum for each of the candidate waveforms, 2) establishing boundaries
and 3) calculating the ratio (R) of power in the middle band ("VF-Band")
to the power in the lower band.
Detector 1 classified its input by com-
paring the calculated ratio to a threshold.
Detector 2 used the ratio R in addition to : 1) examining the lower
frequency
band for significant spectral structure, and 2) examining the
upper frequency band for significant spectral structure
Detector
2
and
harmonics.
classified the candidate waveform via a rule table based on
-
- 41
the ratio R and the information in the upper and lower frequency
fuOO
I
Vt
e4CftI
bands.
I
IsI
iI 'CHeoa .an
MCy Band
I
I
~
I
I
~\
I
I
C
I
I
I
I
I
Uj
I
~/
C
U
jD
20
Frequency(HZ)
Figure 2.1.6. The lower, middle ('VF-Band'),
and upper
power spectrum are indicated for a VF sample spectrum.
bands
of
the
Digest of the Detection Method
Digital Signal Processing
The database was composed of records from patients under
for
cardiac
arrest
or other life-threatening events.
Each record was
recorded through stainless steel defibrillation paddles or
silver
chloride electrodes.
was
ments.
used
to
via
silver-
The continuous-time records were digitized
at 40 Hz following anti-alias filtering up through 16 Hz.
FFT
treatment
A
128-point
estimate the power spectrum of 3.2 second input seg-
This regimen resulted in spectral resolution of .31 Hz over
the
-
- 42
20 Hz bandlimited spectrum.
Feature Extraction
The power spectrum was divided into three sections
quency
band
a
low
from 0 to 3.5 Hz, a middle frequency ("VF-Band") band from
3.5 Hz to 8.0 Hz, and a high frequency band from 8.0 Hz to 20
low
and
Hz.
The
high band boundaries were adjusted so that the major frequency
components were in one of the bands.
tion
fre-
were
The features used for
discrimina-
the power ratio R and the two (unspecified) estimates of the
significance of the spectral structure in the low and high bands.
Classification
Detector 1 classified the input by comparing the
candidate
waveform
to
a
threshold.
ratio
R
examined
the
If R exceeded 1.1, the input was
classified as VF. Otherwise the input was declared undefined.
2
of
Detector
the upper and lower bands because other cardiac rhythms had
power in these regions and thus could better discriminate between inputs
with this added information.
For example, normal sinus rhythm had power
in the low frequency band, while both supraventricular
tachycardia
abnormalities
had power in the
in
depolarization
and
repolarization
"VF-Band." Detector 2 classified the candidate waveform by
estimated
2.1.7.)
feature
values
to
the
decision
table
below.
mapping
and
the
(See table
-
- 43
Table 2.1.7. VF Discrimination Rules
|
PowerRatio (R)
< 1.
Power Spctral Content
---------F
---
I
Undefined
_Noise OnlyVF
S>.i
Significant Structure
or Harmonics
Undefined
1 R denotes the ratio of power in the middle frequency band
the power in the lower frequency band.
divided
by
Summary of Detector Performance Results
Forster selected 141 VF records and 135 other records to constitute
his
database.
Data segments comprising the VF portion of the database
were collected at various times following cardiac arrest and
tion.
resuscita-
Records with various rhythm types completed the database as shown
in table 2.1.8.
Table 2.1.8
.
-
- 44
Database Composition
Number of Records 1
Rhythm Type
|1
Ventricular Fibrillation
Normal Sinus Rhythm
Normal Sinus Rhythm with
Atrial Premature Beats
or Ventricular Premature
Beats or Abnormal Depolarization and Repolarization
Atrial Arrhythmias with
Atrial Flutter or Atrial
Tachycardia or Abnormal
Depolarization and Repolarization
141
32
32
45
26
Wide QRS or Narrow QRS
rhythms
or
Electrode
Motion Artifact
Records were 3.2 seconds long.
The performance measures used were
predictive
accuracy.
sensitivity,
and
Forster presented only his final results for the
decision criteria displayed in table 2.1.7.
results
specificity,
Table 2.1.9 summarizes
for both detectors on this database.
results of testing Detector 1 with
18
the
Forster also reported the
episodes
of
VT.
correctly rejected 13 episodes but misclassified 5 as VF.
The
detector
Thus Detector
l's specificity to VT was 72%.
Table 2.1.9
Detectors 1 and 2 Performance Results
Detector L% Sensitivity
1
91
2__
T
73
% Specificity__
73
9999
% Predicitve Accuracy]
78
-
- 45
Discussion of the Detector Results
Because Forster presented only his final
detector
results
rather
than receiver operating curves for the detector, the results do not show
the compromise between sensitivity and specificity as a function of
threshold
value for either detector.
Forster used the threshold of 1.1
to compare the two different detection strategies.
using
more
information
(i.e.,
the
He
showed
that
by
structure of the outer bands ) he
the
could greatly enhance
specificity
detection
critical to a patient's survival, the sensitivity
of
VF
is
measure is paramount to
Detector
1
is
and
specificity
predictive
and
accuracy.
predictive
Because
accuracy.
Hence,
superior to Detector 2 based on the sensitivity perfor-
mance metric.
The results of Detector l's VT
detector
had
discrimination
indicate
that
the
difficulty in rejecting VT. Forster states that the heart
rate of the 5 misclassified VT episodes was greater than 200
beats
per
minute, and thus the spectra were similar to those of VF.
2.1.2.
TIME DOMAIN DETECTION METHODS
The three previous detectors used spectral properties to detect VF.
The
last
three
methods
describe detection rules based on time-domain
features.
2.1.2.1.
SHIFTED WAVEFORM AND ADDITION DETECTOR
Detection Principles
A time-domain feature extraction method was investigated by
because
it
was
computationally
less
intensive
than
the
Kuo[7]
presented
-
- 46
frequency domain methods.
The algorithm was implemeted
monitoring
design
system.
The
on
an
HP
EKG
philosophy was based on the fact that
VT/VF is often sinusoidal in morphology. Because the sum of
a
sinusoid
and itself shifted by half a period is zero, VF could be detected if the
sum of itself and a shifted copy were small.
algorithm
which
1)
selects
candidate
VF was
classified
4)
adds
half
amplitude
the
last second there were neither a normal QRS,
baseline shifts. The
mean
to
the
The features E and A were used to classify VF.
Candidate waveforms for step (1) were selected from
within
the
the shifted copy to the original input to obtain the
sum E, and 5) calculates the ratio A of the waveform
normal QRS height.
an
VF waveforms, 2) estimates the
ECG's mean period, 3) shifts a copy of the input record
period,
by
estimation
of
the
mean
period,
the
input
if
paced beat, or
E,
and
A
is
presented below.
Digest of the Detection Method
Digital Signal Processing
Preprocessing, sample rate, and digital signal
processing
of
the
of
the
recorded waveforms was not presented.
Feature Extraction
The feature employed in this detection scheme was the
residual
amplitudes
given
sum
by E. The sum E was calculated following an
estimate of the mean period (T) given by equation 2.1
,
=
=
-
v(j)
2n
T
.v(j)-v(j-l)
J=O
(2.1)
where T is
points
ple.
in
-
- 47
the number of sample points in one period, N is the number of
3 seconds of data, and v(j) is the amplitude of the jth sam-
If the mean frequency ( 1/T ) was between 2 and 9 Hz,
date waveform was still
considered a possible VF epsiode.
the
candi-
The sum E was
calculated by equation 2.2.
SIv (j) +v (j-T/ 2)
E =(2.2)
V)
1+1 v(j-T/2)
j=0
where M is the number of samples in two seconds.
The second feature was the ratio of the candidate
tude
to
the
earlier
selected because it
sinusoidal
normal
QRS complex amplitude.
was noted that low
morphology.
amplitude
VF
waveform
ampli-
This feature was
did
not
exhibit
The E and A estimates were passed to the clas-
sification portion of the detector.
Classification
The input was classified by mapping the estimated feature values to
the classification table 2.1.10.
-
- 48
Table 2.1.10. Classification Rules For VF
Diagnosis
A 1E2
S(.63
VF
>= .63
Undefined
< .41
VF
>= .41
Undefined
-
< 1/3
-
>= 1/3
1 A : The ratio of waveform amplitude to the normal QRS height.
2 E : Sum of the input waveform with a 180 degree phase-shifted
copy.
Summary of Detector Performance
Results
The algorithm was implemented on a system used to monitor
patients.
While observing 70 patients over 148 patient days, the detec-
tor generated 11 VF alarms. Eight were true
were
other
rhythms.
reported.
The
predictive accuracy
hours,
and
4)
the
VF
epsisodes
while
three
Each of the true VF episodes was detected within
four seconds following the onset of the
were
hospital
episode.
No
false
negatives
performance measures used were 1) sensitivity, 2)
,3) the number of false positives per
1000
patient
ratio of true to false positive VF detection.
descrimiantion results for this detector are shown in table 2.1.11.
The
-
- 49
. Computer Classification Of VF
Using Kuo's Descrimination Algorithm
True Condition
AF 1
Other 2
|
Computer Classification
L
1
8
VF
2
100
VF Positive Predictivity
61.5
%
VF Sensitivity
Number True VF Alarms
1000 patient monitoring
hours
Number False VF Alarms
%
Table 2.1.11
/
2.25
1000
patient monitoring
0.85
hours
Ratio of True VF Alarms
to False VF Alarms
- - - - - -
_-_
1:0.3751
I
- --
1 AF is atrial fibrillation or flutter
2 Other false positives were due to rapid changes in ECG morphology
of thin to wide QRS complexes.
Discussion of the Detector Results
The sources of false alarms were
ECG
changes
to
small
or
: 1) atrial flutter,
wide QRS complexes.
and 2) sudden
The perfect sensitvity
result is encouraging that such a detection scheme works well.
However,
the small database size weakens the significance of the report.
2.1.2.2.
PEAK/TROUGH SERIES DETECTOR
Detection Principles
Brekelmans developed a time domain scheme to discriminate among VT,
VFL,
VF,
asystole,
and
other
rhythms.
A two-tier detection scheme
50
-
-
encompassing a primary detector with a parallel
detector
and implemented on a patient monitoring system.
pemor v
DVOrtIM opere
?t
designed
(See figure 2.1.12.)
Orre due?.ner
Grid ok '>c105s,11.0- on
vats
tor i P
OCer
was
trepshoi COmDuled OurIn noem "'
suOf'On
otrms res.uitr on ?rom Successive
Dost"Ine f1tuati
ns
Figure 2.1.12. The primary and parallel detector arrangement.
The primary and parallel detector played
discrimination of the electrocardiogram.
different
roles
correlation ) techniques.
It's
matching
role was to classify VT and asystole.
A Feature Extractor and a Peak-Trough
parallel detector shown in 2.1.13.
the
The primary detector estimated
the RR intervals and classified the QRS complexes via template
(
in
Integrator
(PTI)
comprised
the
-
- 51
ECG
FEATURE
inflp.A I
";Kt rUt(~
-
Dr IIj]1or
de'ecen
EXTRACTOR
__0
Figure 2.1.13.
tor (PTI).
Integra-
different
baseline
differentiated
between
due to noise, VFL, or VF by extracting duration and ampli-
tude features from the
detector
level
Feature Extractor and Model of the Peak-Trough
The parallel detector
disturbances
v
passed
undulating
information
In
input.
regarding
addition,
the
primary
QRS morphology to the parallel
detector to alter the VF/VFL alarm threshold (detection level modulation
switch)
and the input switching logic of the PTI (fibrillation switch).
With the information from the primary detector,
parallel
the
detector
classified VFL and VF.
The Peak-Trough Integrator
internal
state.
consisted
of
an
Two switches modulated the input.
input,
output,
and
The possible inputs
were a constant K if the fibrillation switch was closed and/or the duration
feature
if
the
prominence
switch was closed.
switch was closed if either : 1) there was no
QRS
The fibrillation
within
the
last
2
seconds, or 2) there was a large change in the first derivative over the
-
- 52
past 3 seconds compared with the previous 15 seconds.
prominence
switch
was
closed
(unspecified) threshold.
if
In addition,
the
the amplitude feature exceeded some
If the feature was less
than
the
threshold,
the input was assumed to be small-amplitude noise.
The
level,
triggering
the
internal
VF/VFL alarms.
state
variable,
was
responsible
for
The current level, given that the output was
a delayed input, was calculated using the recursive formula of a
moving
average filter given by equations 2.3.
level(n)
output(n)
level(n-1) + input(n) - output(n)
=
=
input(n-N)
(2.3)
where N = 200.
The level was reset to zero if either : 1) the duration feature exceeded
a
threshold,
(i.e., the input zero-crossing rate was too high and thus
the input was assumed to be interference), or 2)
found
normal
the
primary
detector
QRS complexes (in which case the parallel detector should
not decide VF or VFL.) When the level exceeded the threshold set by
the
modulation switch, a VFL or VF alarm would sound.
The
detection
level
switch selected the H(igh) threshold as long as there were no
important
changes in the QRS amplitude or width, or in the RR interval.
If impor-
tant changes had occurred, the
When
the
level
switch was closed,
switch
selected
the
exceeded the current threshold and if
then the diagnosis was VF.
Digest of 'the Detection Method
L(ow)
threshold.
the fibrillation
Otherwise it was VFL.
-
- 53
Digital Signal Processing
No mention was made regarding the preprocessing of the input ECG.
Feature Extraction
The primary
presented.
detector's
However,
QRS
techniques
were
not
Brekelmans[8] did describe the feature extraction
routine of the parallel processor.
prominence
classification
(amplitude),
were
Two features, the wave duration
extracted from the input.
and
Figure 2.1.14
illustrates the derivation of these two features.
M
I In
P
M
~c~iol
Pt.
P
P1
Y2 ----
3
.A2 xJ
x4,
Figure 2.1.14. Derivation of the wavelet parameters. a) waveform with
two peaks (I and III) and one trough (II). b) the three separate elements.
c) measurements on each element.
Figure 2.1.14b shows the input of figure 2.1.14a decomposed
dual
peaks
and
troughs.
to
The four turning points (p1,p2,p3,and p4) of
each individual wavelet were selected from the first derivative
input.
indivi-
of
the
These points were used to derive five parameters (Ml to M5) for
-
- 54
each wavelet as shown in figure 2.1.14c, where :
= duration of the leading slope
M
2 ~
M2 = duration of the trough (or peak)
X1'
X2
x3
M3 = duration of the trailing slope
:
M4 = amplitude of the leading slope
y, - y 2 ,and
M 5 = amplitude of the trailing slope
4
-
x3
'
y4 - y 3
Two features, F
and F2, were calculated from these
five parameters.
F
=
wave duration
: M
F2
=
prominence
: Abs(M 4 ) + Abs(M 5
)
The feature F
and
+ M2 + M 3
troughs
was an input to the PTI and weighted the number of
heavily.
The
feature
F2
selected
F
peaks
as an input and
estimated the importance of the baseline activity relative to the normal
complex amplitudes.
Classification
The PTI level and the fibrillation switch position were
the decision table 2.1.15 below to classify VF and VFL.
by (unspecified) criteria by the primary
was
detected
by
the
detector.
mapped
to
VT was detected
Finally,
asystole
primary detector when the QRS classifier had not
identified a beat within the last three seconds.
-
- 55
Table 2.1.15. Classification Rules for VFL and VF
i
Current PTI Level
Exceed The Threshol d?
|
Fibrillation
Switch Closed ?
-wtc -- l------
Yes
Yes
No
No
Diagnosis
VF
Undefined
VFL
Undefined
INo
Yes
Summary of Detector Performance Results
Brekelmans collected a database of 14 VT,VFL, and VF episodes.
database
was evenly divided to form a learning set and a test set.
test episodes were correctly classified. That is, there were
episodes
or
false
positives.
no
The
All
missed
In addition, the average detection time
was three seconds with a maximum of five seconds.
The results are
sum-
marized in table 2.1.16.
Sensitivity
100%
F
11
100
100 %
I
Predictive AccuracyJ
100%
|
100
100
Specificity
100%
100 %
100 %
%
_Rhyth
VT
VFL
VS
VT, VFL , and VF Discrimination Results 1
%
Table 2.1.16.
%
|
Seven episodes comprised the test database.
Discussion of the Detector Results
The
strongly
perfect
supports
sensitivity,
specificity,
and
predictive
Brekelmans' ad hoc approach to classification.
ever, the limited size of the database weakens the significance
result.
accuracy
of
Howthe
2.1.2.3.
-
- 56
AMPLITUDE HISTOGRAM DETECTOR
Detection Principles
by
This detection system developed
amplitude
histogram
isoelectric segments.
these
rhythms
to
Langer[9]
used
the
signal's
between rhythms with and without
discriminate
Because VFL and
VF
lack
isoelectric
segments,
were detected (as VFL/VF) by the lack of a large peak of
the amplitude histogram corresponding to the baseline.
Digest of the Detection Method
Signal Processing
The detection system was designed for an implantable defibrillator.
An intracardiac electrode provided input to the monitoring system.
input signal was band-pass filtered to remove power from the ST
(i.e,
amplitude
histogram
of
Hz.)
contrast,
figure
The
algorithm
then
estimated
(i.e,
the
the filtered signal. Figure 2.1.17 illustrates
filtered normal sinus rhythm with its
In
segment
the lower breakpoint was 15 Hz), and to remove interference
the upper breakpoint was 100
This
2.1.18
attendant amplitude histogram.
shows
corresponding amplitude histogram.
an example of filtered VFL and its
-
- 57
[,I)j 1&0
7
I
I
f
I
CI
'-~~
I ~~Jk ~
_
___________________________________
I
--
cj
p
,r t
Figure 2.1.17. Filtered normal sinus rhythm recorded from an intracardiac electrode (left) and its
corresponding amplitude histogram (right).
The labeled points in the figures indicate the same events.
Note the
large peak centered about the baseline, X 0 0
Aso
ktI
-
- 58
L'
(X)
0
10
Ti
Amp I tu d
'
Figure 2.1.18. Filtered ventricular fibrillation recorded from an intracardiac electrode (left) and its corresponding amplitude histogram
(right). Note the absence of a peak centered about the baseline, X
0
The clear difference between histogram peaks about the baseline for different
waveforms
was
used
to
discriminate
between VFL/VF and other
inputs.
Feature Extraction
The single feature used to discriminate VFL/VF
was
the
peak
in the amplitude histogram.
and
other
rhythms
Langer [9] did not specify
which features of the peak were critical to his detection routine (e.g.,
height,
steepness,
or
width.)
The estimation of the peak feature was
passed to the classification stage of the detection scheme.
-
- 59
Classification
The input was classified by comparing the baseline peak feature
an
threshold.
unspecified
threshold,
fined.
If
the feature estimate did not exceed the
Otherwise the diagnosis was
the diagnosis was VFL/VF.
This
decision
to
unde-
determined that the safe, non-stimulating
rule
state was the failure mode of the defibrillator.
Results of the Detection System
The defibrillator was chronically
implanted
in
an
test
animal.
Langer did not describe a database nor an evaluation procedure; however,
his results stated that the system did well but misinterpreted sustained
ventricular
rhythms
greater than or equal to 350 bpm. However, because
these clinically rare rhythms would
also
countershock,
require
these
false positive shocks were not unfortunate.
Discussion of the Detector Results
Langer was enthusiastic about the approach as a
VFL/VF
with
intracardiac electrodes.
monitoring
systems,
the detection scheme may not be
that
other
ECG
waveforms
lack
plague
effective in
discriminating artifact from VFL/VF using surface electrodes.
tion,
detect
Since intracardaic monitoring is
not susceptible to motion artifact and other noise sources
ECG
to
method
In
addi-
isoelectric potentials (e.g., atrial
flutter) and may induce false positives using only
this
single-feature
classification scheme.
2.2.
DISCRIMINATING MALIGNANT ARRHYTHMIAS AND
GRESSIVE MODELING
NOISE
USING
AUTORE-
-
- 60
INTRODUCTION
2.2.1.
Previous chapters discussed the motivation for and the mechanics of
detectors which discriminate the ECG based on the shape of the ECG power
spectrum.
spectral
In particular, Nolle's method of estimating the Q of the main
lobe
via
ratios
of
chapter investigates another
estimates
power was used to classify VT/VF.
spectral
technique
the power spectrum via an autoregressive model.
an approach for discriminating ECG
principal
descrimination
reasons.
and VF are well
records,
was
This
which
Modeling,
implemented
for
as
two
First, quasi-sinusoidal waveforms such as VT, VFL,
represented
by
autoregressive
models.
Second,
the
autoregressive modeling technique has been well developed in the literature.
Because autoregressive
literature
is
modeling
methods
are
established,
the
rich with tests for model identification and adequacy as
well as methods for interpretating modeling results.
The selection of the autoregressive
discriminating
ECG
Figure 2.2.1 shows an
tricular flutter and its power spectrum.
band
modeling
around .25
be
Hz.
designed
example
of
ven-
Figure 2.2.2 illustates the time series and power
near 4 Hz.
electrode
for
The power is located in a nar-
spectrum for an example of electrode motion artifact.
flutter,
technique
signals was motivated by examining power spectra of
ventricular flutter and noise.
row
(AR)
motion
In contrast
artifact has a narrowband spectrum centered
These examples suggest that a discrimination scheme
dependent
lobe of the spectra or its
upon
with
may
either the relative area beneath the main
location
along
the
frequency
features are estimated by autoregressive modeling.
axis.
Such
l; iI
-
- 61
VENTRICULAR FLUTTER
THPE 605
CHANINEL
0
;
START 416579
FILTERED
NORMALIZED
2
ECG
1 6
008
CLASS
2.4
POWER
3
2
4.0
SPECTRUM
1.2
0 9
0.6K
o0
h
0
4
6
8
10
Figure 2.2.1 Example of a four-second segment of venticular flutter
(top) and its attendant power spectrum (bottom). The power spectrum
displays the single principal peak characteristic of VFL.
-
- 62
ELECTRODE MOTION' ARTIFACT
TAPE
0
CHANNEL
0
START
FILTERED
9041
CLASS
0
ECG
1,5
1.0
-1O ;j5
08
16
NORMALIZED
2 4
POWER
32
4.0
SPECTRUM
0.7
0 43
0
24
0
2
41
Figure 2.2.2 Example of a four-second segment of electrode motion noise
(top) and its attendant power spectrum (bottom). The power spectrum
displays a lower frequency principal peak.
2.2.2.
-
- 63
SPECTRAL RESONANCE AND Q
processes.
In this thesis we have been concerned with modeling
In
particular, we have some time series y[n] which is a sampled sequence of
the continous time signal y(t), that is, y[n] = y(nT), where
period between samples.
T
is
the
The time series y[n] is the process of interest
and we wish to model it by estimating its power spectra.
spectra
An equivalent approach to modeling the signal power
would
be to model a system (i.e, a filter ) which produces the process y(t) at
its output given a white noise input as described in figure 2.2.3.
71(t)
y(t)
H(f)
Figure 2.2.3 System function model
filtering a white noise process.
of
creating
a
process
random
The spectral characteristics of the system fuction of the
by
would
filter
of noise is constant.
That is
,
be identical to the transform of the signal process because the spectrum
H(jw) = Y(jw)
where H(jw) is the frequency
response
of
the
system
H(s),
function
s = jw, and Y(jw) is the Fourier Transform of the process y(t).
Peaks in the Fourier Transform (i.e, frequency
tion)
correspond
to
poles
in
representa-
domain
the system function.
The single-sided
spectra of figures 2.2.1 and 2.2.2 contain a single principal
positive
peak
for
frequencies. Thus the complete symmetric spectra would contain
two peaks and could be modeled by a filter with two poles.
form of a two-pole system is given by
The
general
-
- 64
A(S-Szl) (S~"z2)
(S-s P (s-sp 2
)
H~s,
5
where K is a real constant, and s
'z2'
and
The frequency response of the filter
poles of the system function.
p1, and sp2
are
the
is derived from the system function by setting s = jw, i.e, H(jW).
zeroes
The
magnitude and phase of the frequency response are
2
SjW-sz
I
H(jw)
= K
2
II jW-s
=1
arg( H(jw)
)
arg(jw-szi
=
~.
i
arg(jw-s
).
I
_
Because the spectrum is estimated with an all-pole model, the spectrum
is modeled without explictly defining the zeroes.
the system function is zero at s
H(s)
=
=-
With two poles,
and is given by
K___
The denominator can be expanded in the form
s2 + 26w s + WO
where
p1 = 5 p2
,0+
Figure 2.2.4 shows the pole-zero plot for the filter.
The
time
domain
signal y(t) which is the inverse Fourier Transform of Y(jw) = H(jw) is a
damped sinusoid of the form
y(t) = Ce
0 tcos[(w0
_'2)t +
el,
where C and G are determined from the initial conditions.
ing
factor,
As the
damp-
4, increases from 0 to 1, the poles move on a semicircular
-
- 65
s-plane
s P1
x
0'
T 0\I
t2
tO)0
x
sp2
Figure 2.2.4 Pole-zero plot of a two pole system function.
locus in the s-plane as shown in figure 2.2.5.
s-plane
= 0
(_ x__
r0
= 1
=0
Figure 2.2.5 Pole trajectory of a two-pole system function as a function
of the damping factor 4.
If 4 = 0, y(t) is a nondecaying sinusoid of frequency wo0 . Thus, the term
w0 is called the undamped natural (radian) frequency[11].
-
- 66
The frequency response is given by
K --H(jw) = (jW-Sp
)(jW-Sp2)
*
If the poles are close to the jw axis as depicted in figure 2.2.6, then
'
Oj
sp1 =p2~~
s-plane
X
jW
Z 2jw
0
X
Figure 2.2.6 S-plane description of a resonant system (i.e.,
near the jw axis.
For frequencies near wO0 , the effect of the distant pole is
stant
so
that
(jo-sp2
~ j2w 0 .
Thus
poles
two
nearly
con-
the frequency response in this
H(jw)
~
-
+
K
)
region reflects the local pole and may be approximated by
~- (j + (x ")(2jo)
which is the same as
H(jw)
~
Htaw)
1(00
Y
Let Af be the half-power bandwidth of the spectra
and
f0
be
the
undamped
natural
-
- 67
Define Q =
frequency.
to describe the breadth of
the peak relative to the resonant frequency.
broad
peak,
while
a
Thus a small Q
large Q implies a narrow peak.
with nonzero f 0 , a two-pole model will
yield
the
implies
a
Then for spectra
universal
resonance
curve given in figure 2.2.7 and defined by
H(jw)
Note that Q =
twice its
-
H(jw)
|~f-f
1 + j2Q1f0j
which is the magnitude of the complex pole divided by
r eal part.
IH
w)
71
half-power bandwidth =
7C
45-
S
(j 2r
)
,32
-450
I
-45*
-90*
Figure 2.2.7 Universal Resonance Curve.
2.2.3.
CONTINOUS-TIME AND DISCRETE-TIME RELATIONSHIPS
The previous discussion focused on relating the the
Fourier
Transform
(
i.e,
shape
of
the
the power spectrum ) to the poles in the s-
-
- 68
plane for a contiuous-time signal y(t).
notion
of
estimating
the
of the spectra with a parameter Q and
peak
related the estimate Q to the pole locations of the model
tion.
This
section
the
That discussion introduced
func-
system
investigates the relationship between continuous-
time and discrete-time signals and their frequency representations.
A continuous-time
Fourier
Transform
the FT squared.
data
segment
(FT).
of
flutter
would
have
a
continuous
The power spectrum, P(f), is the magnitude of
Because flutter is bandlimited (to bandwidth B Hz), the
may be sampled above the Nyquist rate (2B samples/sec) so
segment
that the discrete-time Fourier Transform (DTFT) of the
segment
The discrete-time power spectrum,
does not contain aliased frequencies.
DTFT.
P(O), is the square of the magnitude of the
is discrete,
sampled
the
Because
time-
F(O) is continuous and periodic with period
domain
signal
1[12].
The dimensionless frequency variable, 0, is frequency in Hz norto
malized
rate (i.e., O=fT , where 1/T is the sampling
sampling
the
rate.) Because the flutter segment is
magnitude
of
the
DTFT
is
even.
real (rather
than
Thus, because the DTFT is even and
periodic, the spectral information is completely contained
half
period
quency,
1/2.
of the DTFT.
the
complex),
By convention,
in
any
one
the abscissa (normalized fre-
0) of the DTFT is plotted for the first half period, from
0
to
This range of normalized frequency coresponds to the range of fre-
quency in Hz that varies from DC to 1/2 the sampling
These
frequency.
facts conclude that the relation between spectra for continuous and sampled signals is given by P(f) =
the sampling rate.
(fT) for
If| < 1
scale
f
=
1/T
is
Because the FT of an ECG segment and the DTFT of the
sampled segment are equivalant over the range
izontal
where
factor,
the
frequency
Ifk
I 2 except for
a
hor-
'axis of the DTFT power spectrum
-
- 69
.
plotted in this thesis is scaled by f
DTFT identical over the range
This scaling makes the FT
and
if
IfI(-f and thus easy to interpert.
2
Because the spectrum is calculated on the computer rather than with
closed
form
equations, the spectra is estimated with a finite ( rather
than infinite ) number of frequency components.
discrete
Another transform , the
Fourier transform (DFT), samples the continuous, periodic DTFT
at N equally spaced samples of the first
the DFT of x[n]
period only.
, the sampled segment.
Let
X(k)
denote
The relationship between the DFT
and DTFT is given by
X(k) =
I(k)
N
for the N frequency components 0 < k < N-1.
of
the
DFT
is
from k = 0 to k = N-1, while the DTFT frequency varies
from 0=0 to 9 = 1/2.
plots
The plots of the power spectra in this thesis
of the DFT (i.e.,
are
discrete frequency values ) with the magnitudes
of each discrete component connected.
specified
range"
Thus the "frequency
This linear interpolation between
frequencies accounts for the jagged nature of the power spec-
tum.
The DFT is used to estimate the FT of a continous-time signal.
relation
between
The
the DFT of the finite discrete sequence and the FT of
the continous sequence is given by
X(k) = I( N ) = X(f=kf
Ns
for 0 < f < f
.
Since the ECG was sampled at f
= 250 Hz for N
points, the frequency resolution of the power spectrum is if~
=
1024
.25 Hz.
The DTFT is a useful technique for comparing continous-time spectra
with
discrete-time
spectra.
However,
discrete time systems are more
-
- 70
readily designed and analyzed with the Z
transform.
The
relationship
between the Z-transform and DTFT power spectral representations is given
by
P(N)
where z = ej2no.
P(z)
=
(2.3)
Thus, the DTFT Y(9) is the (scaled) Z transform
power
spectra evaluated on the unit circle.
2.2.4.
AUTOREGRESSIVE MODELING
Because the envelope of the power spectra of VT, VFL, VF, and noise
are
approximated
two poles, this discussion is limited to two-pole
by
of
The Z transform
modeling for estimating power spectra.
a
two-pole
model is given by
I
F(z)
where z = ej2n.
=
|
I(i-pIz
I
1
G
)(1-p
1
2 z~ )I
2
(2.4)
|
The inverse Z transform of the system related to
P(z)
is a second order difference equation given by
y[n] = a 1ly[n-1] + a 2 y[n-2] + i[n]
where y[n] is the digitized electrocardiogram and -[n]
sian
(i.e, n[n] ~ N(O,a 2 ).
process
itself, equation 2.5 is
is a white
gaus-
Because y[n] is twice regressed on
second-order
autoregressive
The
process.
and a2 are strictly determined from the poles p, and p
2
.
coefficients a
a
(2.5)
The relationship between the pole locations and the corresponding
coefficient
values is discussed in detail in Appendix 7.2.
ence equation 2.5 completely determines the DTFT power
given by
alpha
The differ-
spectra
and
is
-
- 71
2
2a2
(
2
2
1 +1 a
for 0 (= 0 <=1/2.
2
R
2
+ a2
+
-
2
(2.6)
aI(1-a 2 )cos2nO - 2a 2 cos4n
Thus, the envelope of the power spectra is completely
determined by the values of the two coefficients from the autoregressive
equation 2.5.
tra
are
In particular, the breadth and peak location of the spec-
determined from a1 and a2 .
Substituting equations 2.3 and 2.4
in equation 2.5 yields 1) the relationship between
the
poles
and
the
alpha coefficients, and 2) the relationship between the gain, G, and the
variance of the noise process, a2 .
The detailed
relation
between
the
spectral shape and coefficient values is discussed in Appendix 7.2.
The
technique for estimating a1 and a 2 from a DTFT of a ECG segment is
cussed
a2
in
detail in section 3.1.
coefficients
envelopes
of
is
to
describe
noise/artifact
dis-
The purpose of estimating the a, and
the
differences
between
and malignant arrhythmias.
spectral
These coeffi-
cients are the features used to discriminate between ECG inputs and
are
the basis for classifying the rhythm disturbances.
The coefficients a1 and a2 are the two estimates
envelope.
They
describe
breadth of the spectrum.
which
the
of
the
spectral
frequency of the peak component and the
These coeffients form a feature
vector,
is used to discriminate between noise and malignant rhythms.
distribution of the feature
vectors
for
all
segments
spans
a
a
The
two-
dimensional feature space.
To introduce the feature space and its
power
spectrum,
figure
2.2.8
relationship to modeling the
shows the correspondance
plane and the feature space representaions of a two pole
displayed time series and power spectrum.
The
pole
between the zmodel
of
the
locations
of
the
-
- 72
PELATIONSHIPS AMONG FOUR DATA REPFESENTATIONS
DATA SA1PLE
POI!ER SPECTRUM
19
-
CI.0
6
0.064
2
0.048
0.032
-e
0.016-
--6
0 0
0 8
1 6
2.4
3
2
4
0
Z PLAIIE
.2
yWr.
1~~
a2y[n-21
Impha2
Cay~n--11 +
I5
.3
4
.5
+ 711n]
alphaI
-.
-. 75
-1
25 -75
-. 25
.25
.75
1
25
Figure 2.2.8 Relationships among four ways of representing a data segment : (a) time series, (b) power spectra, (c) z-plane pole-zero plot,
and (d) two-dimensional autoregressive feature space.
-
- 73
two-pole model of the power spectrum are indicated
plane.
The
angular
as
X's
in
frequency
Thus for a greater angular displacement, the peak of the spectra
is at a higher frequency.
gin
z-
displacement of the pole from the real axis indi-
cates the position of the peak of the DTFT spectrum along the
axis.
the
indicates
The radial location of the pole from the ori-
the breadth or Q of the spectra.
Thus as pole locations
move closer to the unit circle, the spectrum becomes narrower.
The two-pole model is represented
equation
by
a
second
difference
as described in the lower right quadrant of figure 2.2.8.
coefficients a1 and a 2 are the two features used to
series.
order
The
describe
the
The
time
domain of the coefficients, the feature space, is bounded
by a triangle where aI varies from -2 to 2, and a2 varies from -1 to 1.
The nonlinear mapping of the poles from the z-plane
to
the
alpha
coefficeints in the feature space are given by the following equations :
= RE Z1 +Z2]
a
a2
where Z
=
(2.7)
-Z1 Z2
and Z2 are the two poles of the
(2.8)
model.
The
above
equations
were derived by equating the z-transform of equation 2.5 and to the general form of a second-order system,
(1-p 1 z~1 )(1-p
Equation 2.8 shows that a 2
of
the product of the poles.
,
-.
2
1
)
_
H(z) =
the ordinate variable, is the negative
Since the magnitude of the poles are less
than one, the product of the poles will be less
than
their
magnitude.
Thus, a 2 amplifies the displacement of the pole from the unit circle and
-
- 74
describes
the
width
a 2 = .975 x .975 = .95
of
the
With
.
spectrum.
relation
to
In
the
this
example,
original
signal, a 2
describes the damping of the time series.
Equation 2.7 describes that a1 , the variable along the abscissa
the
space,
feature
If the
is the sum of the real parts of the poles.
poles are complex conjugates as in the example in figure 8, a1 is
the
projection
of
a1 = 2 x .25 = .5 .
the
poles
along
the real axis.
in
twice
In this example,
Thus, for complex poles and a fixed a 2 , a1 is
pro-
portional to the spectral peak location along the frequency axis.
mapping
These equations indicate that the nonlinear
plane
to
the
z-
feature space would be quite sensitive to shifts in pole
Thus it
locations.
the
from
would be visually easier to distinguish the forms of
the time series by inspecting the feature space rather than the z-plane.
As an example,
figure 2.2.9 compares the actual and estimated spec-
tra of the flutter example given in figure 2.2.1.
I( fs) in the bandlimited region 0 < f < 6 Hz.
Figure
2.2.9a
Figure 2.2.9b and 2.2.9c
shows the estimated power spectrum and the pole-zero diagram
transform
that
corresponds
to
the estimated spectrum.
shows the feature space for this example.
that
shows
of
the
Z
Figure 2.2.9d
From this diagram, it
is seen
a spectral model with conjugate poles located near the unit circle
produce high-Q spectra like
corresponds
to
having
the
those
in
figure
2.2.1.
This
situation
feature located near the lower edge of the
feature space.
However, the spectra of flutter and noise are rarely
ineated.
clearly
del-
Figure 2.2.10 shows that the power spectrum for a second exam-
-
- 75
VENTRICULAR FLUTTER
HrhW 1
E
k
L
EVf
.
ToF
CL
41 E
FEP
S
2
I PECTRUM
0 024
-U
-
aiFEtTI!FE SPACE
3
cl 1 6
.. F.0
F.-.CF
-252
2.2.9
The
~
1
5-1-
4
6
4
6
8
6.0
P
-1
Figure
605.
1
4
5 0
5 1 1
.5 2
four representations of a VFL data segment from tape
-
- 76
ple of flutter has a broad bandwidth and that its main peak
near 2 Hz.
This figure indicates that the distribution
of
power is weighted heavily for lower frequencies ( i.e., less than
noise
but that there is a significant peak at 2 Hz (
1.5 Hz ),
region
where
VF
normally
only
be
similar.
A
in
the
descrimination
rule
on the location of the peak component of the spectrum would
correctly classify the first pair of examples but
Likewise,
i.e.,
has most of its power.) These examples show
that flutter and noise peaks can
a
not
the
last
pair.
descrimination rule based on the Q of the main lobe of the
power spectrum (e.g., Nolle's method
first
located
The power spectrum of a second example of motion artifact is
shown in figure 2.2.11.
based
is
)
would
correctly
classify
the
Correct classification
pair of examples but fail with the latter.
is possible by estimating both of these features via AR modeling.
Autoregressive modeling provides estimates of
location
and
both
a2
component
sets
of
examples.
In
AR modeling estimates these features more efficiently than the
computationally intensive spectral area methods.
and
peak
breadth of the spectral envelope (i.e., not just the main
lobe ) which together correctly classify
addition,
the
were
These estimates of
a1
the features used to classify the different ECG segments.
Thus AR modeling was implemented because the technique well described in
the
tion.
literature
and
appropriate for providing features for discrimina-
-
- 77
VENTRICULAR FLUTTER
TMPE 427
CHANNEL 0
START
308193
CLASS 2
FILTERED ECG
1
u
HIPMs
6
2.4
3.2
4
0
I ED POWER SPECTRUM
i
I IIi~
fl
.i
0
0 0
4
6
8
10
Figure 2.2.10 Time series (top) and power spectra (bottom) of a second
example of VFL from tape 427.
-
- 78
ELECTRODE MOTION ARTIFACT
TAPE 0
;CHAtIEL 0 i START 2034
CLASS 4
FILTEPED ECG
13
[K. ~AA
-0.1
2.4
3.2
lbF19MAL IZED PO6WER SPECTRUM
IMI
I I II
4
0
I
A
3'
0 J
el
44
I
81
j,\
Figure 2.2.11 Time series (top) and power spectra (bottom) of a second
example of electrode motion artifact.
-
- 79
Chapter 3
3.
METHODS
3.1.
DATABASE DEVELOPMENT
A database of noise and ventricular
arrhythmias
order to develop and test each detection scheme.
was
compiled
in
The database consisted
of examples of ECG events in each of the event classes that the detector
needed
to discriminate.
In particular, the database consisted of exam-
ples of ventricular tachycardia, flutter,
motion
artifact.
Because
the
rhythm
fibrillation,
and
electrode
detector under development was
designed to work in
parallel
rhythms
of sequences of isolated events (e.g., normal sinus
consisting
with
a
beat-by-beat
detector,
cardiac
rhythm ) were not included in the database. It was assumed that the pribeat-by-beat
mary
processor
would
classify
all
such isolated-event
rhythms.
The database was partitioned into two sections : 1) a learning
of
data
for
the
development of a detector,
evaluation of a detector.
each
class
of events.
the data by
Depending
calculating
upon
the
and 2) a test set for the
The learning set provided ECG
examples
For
the
feature
strategy
example,
of
the
values
for
all
the
if
from
examples.
discriminating algorithm of the
be
dif-
the detector models the ECG signal for each
class of events as with the autoregressive detector,
values
from
The feature extraction algorithm could train on
detection scheme, the use of these sample feature values would
ferent.
set
then
the
feature
the learning set may be used to estimate parameters (e.g.,
-
- 80
p and
for each event class.
fixed
by
For example, the decision regions in the
autoregressive
space are fixed by the elliptical contours of the Gaussian pro-
bability distribution assumed for each class of features.
if
are
the estimated values of the model parameters and by the deci-
sion criteria.
feature
In this case, boundries in the feature space
the
detector
power detector,
does
In
contrast,
not model each event class as with the relative
then the sample feature values from the learning set are
used to set expected boundries of the features for each event class.
In
this case, boundries between classes may be "geremandered" in
to
optimize
the
detector
order
performance statistics on the learning set. The
assumption in this design is that a
detector
optimally
tuned
to
the
learning set will also perform well on the test set.
The test set portion of the database is used to evaluate the detector.
The detector extracts feature values from each sample event in the
test set and classifies it
according to the
from
Performance criteria are subsequently used to
the
learning
set.
class
boundries
evaluate the detector's ability to classify events from
the
developed
test
set.
These performance measures are used to compare different detection strategies using the same database.
The selection and
issues.
The
use
of
a
database
raise
four
first issue addreses how well the database represents the
general population.
The designer is concerned that the
examples
event
for
controversial
each
class
is
sufficient.
number
estimate feature values for each.class.
ECG
For purposes of this
study, we assumed that the database was sufficiently large
cantly
of
to
signifi-
In addition, we assumed
-
- 81
that the examples were a maximum
likelihood
estimate
of
the
general
population distribution of events.
The second issue deals with how to
design
use
the
during
the
of the detector. If the detector were to be used in environments
where the a priori probablity of cardiac events
the
database
Intensive
different
(e.g.,
Care Unit versus the Emergency Ward, ) then the designer
would need to consider how to adjust the decision
porate
were
this knowledge.
boundries
to
incor-
Because the detector in this study was designed
for general application, the decision boundries reflect
the
fact
that
all event classes were weighted with equal a priori probabilities.
The third issue involves the selection and use of performance measures
to
evaluate
the detector.
The performance criteria describe the
ability of the algorithm to reduce costs incurred by the patient, hospital,
and
society.
(The selection of detector performance measures and
the consideration of patient cost is discussed in section
formance
criteria
are
useful
tested on the same database.
mance
bases.
are
to
3.4.1.)
Per-
compare different detection schemes
Incorrect conclusions arise
when
perfor-
measures are used to evaluate detectors tested on different dataThe error stems from the fact that certain performance
dependent
upon
the
represented in the database.
relative
prevalence
of
the
This discussion assumes that
measures
class
the
types
charac-
teristics of any particular class of events are the same among different
databases.
events
The difference between databases is the different number
of the same class among databases.
measure , Accuracy,
For example,
defined as,
Accuracy = Number of correct decisions
Total number of decisions
of
the performance
-
- 82
is database dependent because the statistic depends upon the
events
all possible classes contained in the database.
of
statistics such as Accuracy cannot be used to compare
over
different
databases.
In
the
results
contrast, statistics which depend upon
can
be
used
to
results of a detector tested on different sized databases.
For example, consider the case where a database consists of two
of
of
Performance
detector
only one class of events are database independent and
compare
number
classes
- A and B - and the detector decides A or B for each example
events
in the test set.
Then the quality
measure,
Sensitivity
for
class
A
events, defined as,
Se
A
=
Number of class A events which were correctly detected
Total number of class A events
depends only upon one class of events in the database and therefore
be
used
to
compare
detector
can
performance on database with comparable
characteristics.
The last controversial issue is the manner in
should
be divided into learning and test sets.
the learning set,
development
of
the
detector.
However,
arrhythmia
small
evaluation.
base
database
the larger
during
the
the larger the test set, the
performance.
Because
ven-
examples such as ventricular flutter are difficult
to obtain, new and larger databases are not
single,
the
Intuitively,
the better the decision boundry estimates
greater one's confidence in the detector's
tricular
which
database
easily
compiled.
Thus
a
must be used for both detector development and
Different philosophies exist on how to partition the
data-
into learning and test sets in order to qualitatively optimize the
use of the database.
Bootstrapping, the method employed in dividing the
database in this study, is described in section 3.4.2.
1.1.1.
83
-
-
CREATION OF THE DATABASE
A database of noise and ventricular arrhythmias was
two
separate
and
fibrillation.
ventricular
of
of
of the database were
the
include
ECG.
subject-generated
the
database
The records in this portion
electrode motion artifact section.
an
tachycardia,
Each event was digitized in the context of
an extended patient ECG recording. The second portion
was
from
The first portion was a malignant ventricular
databases.
arrhythmia section consisting of episodes
flutter,
compiled
samples
noise
which
not
did
The content and acquisition methods for establishing
the databases are discussed below.
3.1.2.
MALIGNANT-ARRHYTHMIA SECTION
The first portion of the total database was a section comprised
examples
of
ventricular
flutter,
tachycardia,
Twenty-four hour Holter Monitor Recordings from
these
arrhythmias
were
collected
from
and
fibrillation.
patients
sixteen
of
with
the ECG tape libraries of the
Brigham and Woman's Hospital and Beth Israel Hospital in Boston.
These
records were scanned on an Avionics "Dynamic Electrocardioscanner" Model
660A.
Twenty-two thiry-five minute
portions
tapes were digitized via a Digital PDP 11/23.
of
the
twenty-four
hour
Both channels of the ana-
log records were sampled at 250 Hz with a 12 bit ADC with no DC
offset.
The amplitudes of the signals were not calibrated.
The twenty-two
existing
software
records were formated in a manner
programs designed for the MIT/BIH database.
records were hand-annotated for the onset of each
ever,
compatible
individual beats were not labeled.
rhythm
with
All the
change;
how-
A summary of the distribution
-
- 84
of tachycardia, flutter, and fibrillation events is shown in table
Table
3.1
also
shows
the
relation
between
Malignant-Arrhythmia Database tape number.
patient
3.1.
number and the
-
- 85
Table 3.1 Distribution of Database Segments Among Patients
No. 4-Second Data Segments
Patient
MalignantArrhythmia
I
Number
Tape No.(s)
1
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
0
51
58
1
0
2
12
0
37
53
10
2
225
4
20
7
TOTAL
482
VF
VFL
VT
|
|
I
|
|
|
j
62
16
12
0
0
8
0
219
54
0
0
5
0
17
3
0
10
0
162
112
39
0
0
0
0
0
0
0
0
0
0
0
396
323
|
|
|
|
|
|
|
|
||
j|
||
||
II
||
||
II
II
||
418,419
420
421,422
423
424
425,605
426
427
428,429,430
602
607
609,610
611
612
614
615
|
|
|
I
Patient ventricular arrhythmia episodes were partitioned
into 4-second segments.
3.1.3.
NOISE SECTION
An artifact database was established by another investigator in the
development of a noise stress test[13].
The artifact database was
created by recording signals from limbs of subjects in lead configurations which minimized the ECG signal.
Twenty-five hours of two-channel
signal were recorded on Avionics model 445 Holter Recorders.
The sig-
nals were digitized at 250 Hz in the same manner described above.
The
noise recordings were visually inspected, and electrode motion artifact
portions were concatenated to form a single 30 minute noise record.
This 30 minute noise record was partitioned into 519 non-overlapping 4-
-
- 86
second data segments to form the noise section of the database.
3.2.
IMPLEMENTATION OF A REFERENCE DETECTOR
3.2.1.
DIGEST OF THE DETECTION SCHEME
The relative-power detector intoduced by Nygards[4] and later
developed by Nolle[5] was implemented to serve as a landmark for comparsion with new detection schemes.
This section describes that implemen-
tation.
Figure 3.2.1 summarizes the relative-power
detector.
algorithm consists of three stages: a preprocessor,
and classifer.
feature extractor,
The preprocessor reads one channel of the digitized ECG
in 4-second segments and high-pass filters
line wander.
The detection
the segment to remove base-
The feature extractor calculates R, an estimate of the
breadth of the principal spectral peak.
The classifier compares the
estimate R with a fixed threshold T and assigns the input observation to
class V (i.e., VTVFL, or VF) if R is greater than or equal to T and to
class N (i.e, electrode motion noise) if less than T.
3.2.2.
ANALYSIS OF THE DETECTION SCHEME
Preprocessor
The first
stage of the detection scheme was a high-pass filter,
preprocessor, which removed low frequency noise form the signal.
database had been sampled at 250 hz, and the digital filter
to emulate a single pole continuous time filter
Hz.
or
The
was designed
with a breakpoint of .3
The HPF is described by the following equations
:
- 87
ALGORITHM SUMMARY
INwuT EcG
1.
PREPROCESSOR
4-SECOND SEGMENTS
HPF
2.
FEATURE
ESTIMATE
EXTRAC7OR
THE SPECTRAL Q
WITH R
3.
CLASSIFIER
COMPARE R
WITH A THRESHOLD T
NOISE
VF
Figure 3.2.1 Relative-Power Detector.
the text.)
y[n] = ay[n-1]
(The algorithm
is
described
in
+ x[n] - x[n-1],
-1
H(z)
=
-Z
1-az
1
The equation of the spectral shape is given by
.
-j2rr#
H(q) =
1-ae-j2no
Figure 3.2.2 illustrates the unit sample response of the digital filter.
-
- 88
HIGH P4'3S
FILTER UIlT SA11PLE RESPONSE
-
1
4J
4
SECOHDS
Figure 3.2.2 Unit sample response of the high pass filter used to remove
baseline wander from the ECG.
The magnitude of the corresponding frequency response is shown in figure
3.2.3.
-
- 89
1.10
7
0
RESPONSE
-
MH*G,.ITJOE OF THE HIGH PASS FILTER FREQUENCY
44-
I
*1~
-I
6
4
8
10
H'
Figure 3.2.3 Magnitude of the frequency response of the high-pass filter
used to remove baseline wander from the ECG.
The filter sufficiently removed low frequency baseline wander but did
not remove linear trends.
Feature Extractor
A single feature is extracted from each filtered data segment via a
two-pass process.
As described in section 2.1, the feature of interest
was R, the relative-power estimate of Q, which describes the breadth of
the spectral resonance curve.
3.2.4.
The calculation of R is shown in figure
The feature R was calculated by dividing the power in an inner
bandwidth (W1) by the power in an outer bandwidth (W2).
Nolle had
investigated a number of variations on selecting the inner and outer
-
- 90
POWER SPECTRUM'.
F
W2
f (Hz)
F
1.5Hz
2
____
_
24Hz
Figure 3.2.4 Calculating the relative power ratio, R, as an estimate of
the spectral resonance curve. The estimate R is calculated by dividing
the power in an inner bandwidth (W1) centered about the spectral peak by
the power in an outer, fixed bandwidth, W2. W2 was fixed from 1.5 to 24
Hz. The width of Wi was proportional to the frequency of the spectral
peak (F).
The coefficient of proportionality was independently varied
to find the optimal detector with respect to two cost functions.
bandwidths.
He found that the optimal detector (with respect to maxim-
izing senstivity) was one which used a fixed outer band (1.5-24Hz),
and
an inner band with a bandwidth proportional to the frequency of the
spectral peak (F)
(i.e.,
the "peak frequency".)
Since we were concerned about implementing a detector which had
been described in the literature, an outer bandwidth of 1.5-24Hz was
selected.
The lower edge of the band was above most of the noise power.
The upper frequency edge was well above the major spectral peaks of
VT,VFL, and VF.
Various inner bandwidths were selected, all proportional to F.
The
91
-
-
constant of proportionality was a percentage of the peak frequency.
This constant is called the inner-bandwidth percentage
(IBP).
The IBPs
tested are listed in table 3.2.5.
Table 3.2.5 Bandwidths Tested in the Relative-Power Detector.
The inner
bandwidths (Wi) were proportional (a percentage) to the principal spectral peak (F). The Inner Bandwidth Percentage (IBP) is the coefficient
of proportionality.
The outer bandwidth (W2) was fixed from 1.5-24Hz.
IBP
40%
50%
60%
70%
80%
90%
92%
94%
96%
98%
100%
110%
120%
1130%
140%
150% 1
BANDWIDTH U
Hz)
0 80
.
F - 1.2 F
0.75 F - 1.25 F
0.70 F - 1.3 F
0.65 F -1.35'F
0.60 F -1.4
F
0.55 F - 1.45 F
0.54 F - 1.46 F
0.53 F - 1.47 F
0.52 F - 1.48 F
0.51 F - 1.49 F
0.50 F - 1.5 F
0.45 F - 1.55 F
0.40 F -1.6
F
0.35 F - 1.65 F
0.30 F - 1.7 F
0.25 F -1.75 F
J
|
_
j
1 F is the frequency of the peak power
component.
Classifier
The detector classified the input observation by comparing R with
some preset threshold, T.
For each IBP, the thresholds were varied to
optimize the cost (benefit) functions which were used to evaluate the
performance of the detector.
The overall optimal detectors were
selected from the best for each IBP.
-
- 92
3.3.
IMPLEMENTATION OF AN AUTOREGRESSIVE MODEL DETECTOR
3.3.1.
DIGEST OF THE DETECTION SCHEME
The AR features described in section 2.3 were used in a detection
scheme to discriminate malignant rhythms from noise.
Figure
3.3.1 sum-
marizes this detection scheme for the binary case of discriminating
between noise and flutter.
This figure serves as a reference point to
introduce and summarize this section.
The detector is comprized of three functional sections -
prepro-
cessor, feature extractor, and classifier. The preprocessor reads in
four-second segments of one channel of the ECG and high-pass filters
data in order to remove baseline-wander noise.
the
As discussed above, the
features used to discriminate between malignant rhythms and noise were
motivated by the fact that the power spectrum is approximated by a
second-order autoregressive process.
The coefficients a1 and a 2 form a
feature vector, a ,which is used to discriminate between malignant
rhythms and noise.
The detector uses a maximum likelihood classifier. The classifier
calculates the conditional probabilities that the input feature vector
was noise or that it was a malignant rhythm.
It then assigns the input
feature vector, a, to the class with the higher probability.
a2 coefficients are Gaussian distributed.
tion depends on only two parameters,
covariance matrix
,
The a1 and
Since the Gaussian distribu-
the mean vector
,
p, and the
I, the detector was designed by separately calculat-
ing these parameters for noise and for malignant rhythms.
The classif-
ier then calculated the conditional probabilites for each input segment
-
- 93
ALGORITHM SUMMARY
Iin'r
,
EkG
41. PREPROCESSOR
4-SECOWD
SEGMENTS
H??
AR(2) MODEL
FEATURE
ExTRACTOR
Yin)- 6gyln-1)
3. CLASSIFIER
VFL
p(
.
,
)
+ a2 Yin-2)
.
+
in)
)
2.
)
ex
VF
NO ISE
1(;--)T
Figure 3.3.1 Summary of the autoregressive detection algorithm.
and assigned it to the appropriate class.
-
- 94
The preceding section described the motivation for estimating the
spectrum with an autoregressive process and the overview of the detection scheme.
The following discussion describes in detail the
mechan-
ics of each of the three detector sections.
Analysis of the Detector
Preprocessor
The preprocessor was the identical one employed with the reference
detector. The high pass filter is described in section 3.2.
Feature Extractor
Section 2.3 motivated the use of autoregressive modeling to
discriminate rhythms.
This section explores two issues relevant to
spectral estimation and feature extraction : 1) theoretical estimation
of the feature values, and 2) the actual algorithm for estimating the
feature values.
Proceeding with an understanding of the relationship between the
power spectrum of a signal and the two-dimensional feature-space
representaion of the time series, we look now at the method for estimating the two features.
Each input ECG segment is modeled by the autoregressive equation,
y[n]
=
aly[n-1] + a 2 y[n-2] + 1q[n]
The problem is to estimate a1 and a 2 for each input.
.
(3.1)
The solution comes
from manipulating the estimated autocovariance function for each input.
-
95
-
The autocovariance function at lag m is defined as the expectation
and is given by
,
of the product of a zero-mean sequence and a shifted replica of itself,
y[m] = E (y[n]-p)(y[n-m]-p)]
where g = E y[n]] is the mean of the process.
(3.2)
If the autocovariance
function is normalized by the variance of the signal (i.e, y[O]), the
result is the autocorrelation coefficient function,
p[n]
=
(3.3)
X$
y[0]
Applying equations 3.2 and 3.3 to the process equation 3.1, we obtain
the following equation which shows that the autocorrelation coefficients
are also an autoregressive process,
p[n] = alp[n-1] +
a2p n-2]
Evaluating this last equation for n = 0 and 1, and realizing that the
p[0] = a 1 p[1] + a 2 p[2]
.
autocovariance is an even function, we obtain the Yule-Walker equations,
p[1] = a P0] + a P[l
2
Solving these two linear equations for a 1 and a 2 ,and recognizing that
= 1, yield the coefficient estimates
a
1
_ 1[1](1
1
-
Thus the estimates of the features a
:
-p[2])
2
p[1]
2
and a 2 are completely determined
from the autocorrelation of the input evaluated at the first
i.e.,
p[]
and p[2].)
two lags,
(
p[]
-
- 96
If the process is wide sense stationary and stable, then there are
three constraints on the coefficients.
These constraints dictate that
the feature space be bounded by a triangle. (See figure 3.3.4.)
1-
L
~I1+
H
&
/-1 t
-1.~
I
~1~
*,
e
~
~LI
'..-'
I
1
I
0
-. 5
ALPHA
.5
1
5
1
Figure 3.3.4 Two-dimensional autoregressive model feature space.
That is
a2 + a
<1
a2 - a
<1
-1
< a2
1
Thus the feature space displayed in figure 3.3.4.
is an isosceles tri-
angle with a base that varies from -2 to 2 and a height from -1 to 1.
In addition, the relationship between the coefficients and complex
poles is given by the following equation,
al + j
-j
2
-4a 2
-
- 97
The poles are real and equal when a2 =
in the feature space.
12
which defines the parabola
If the poles are complex conjugates as in the
example in figure 2.3.8,
less than -a
a2
then the descriminant is positive and a 2 is
. Thus, the area below the parabolic curve corresponds to
conjugate poles which in turn correspond to oscillating time series.
The remaining area in the feature space above the parabola is a nonlinear mapping of the real axis into the z-plane.
This region
corresponds to time series which consist of damped exponentials.
Athough the noise used to develop the model was white Gaussian
noise,
the results show that the noise was slightly correlated
(colored).
function,
Thus, the autocorrelation of noise was not a unit sample
and therefore added to the autocorrelation function of the
y[n] process at lags 1 and 2.
In the case of colored noise, if
the
theoretical a1 and a 2 features were estimated by the autocorrelation
fuction at lags 1 and 2 using the Yule-Walker Equations (i.e,
p[1] and p[2]), then the features would actually model the noise spectrum.
To avoid this noise interference, the autocorrelation function was
evaluated at lags away from the origin (i.e., region of noise correlation.) Recall that the shape of the autocorrelation function envelope
contains the significant signal information.
That is, for any bandlim-
ited signal, the envelope of the function looks the same sampled at any
rate above the Nyquist rate.
Thus, if the signal were sampled suffi-
ciently slow (but above the Nyquist rate) so that noise in adjacent samples was not correlated, then p[1] and p[2] could be used to estimate a
*
and a
2
98
-
-
We effectively decimated the observed signal by a factor of twenty
by using p[20] and p[ 4 0 ] instead of p[1] and p[2] in our estimates of
the features.
Since the noise was correlated for lags less than 10, the
features estimated were those for the sigal process only.
In order to maintain a low variance on the estimates of rho[20 and
p[40] all
points separated by lags of 20 and 40 were used.
The differ-
ence in using p[20] and p[40 from a signal sampled at 250 Hz instead of
p[l] and p[2] from a signal sampled at (250/20) Hz is that the variance
of the estimate of a1 and a2 is reduced by a factor of 20.
Recall that from section 2.2, the lower edge of the triangle in the
feature space coresponds to the unit circle.
The lower right corner
corresponds to DC and the lower left corner corresponds to half the sampling rate.
Since the orginal signal was digitized at 250 Hz, but the
features were effectively estimated for a signal sampled at 250/20 Hz,
the lower left corner of the feature space corresponds to 6.25 Hz.
The next two examples help solidy the relationship among the time
series, power spectrum, and the feature space.
The first example in
figure 3.3.5 shows the sum of two cosines of different amplitudes and
different frequencies.
frequency of 1 Hz.
The first component has an amplitude of 1 and a
The second smaller component has an amplitude of .2
and a frequency of 9 Hz.
The power spectrum shows, as expected, that
96% of the power is due to the larger 1 Hz component with a small contribution by the 9 Hz component.
Because the peak of the spectrum is
located below 3.125 Hz and the input is oscillatory,
a
the feature values,
and a2 lie below the parabola in the lower right quadrant.
in figure 3.3.5 lists the estimate coefficent values.
The table
SUM OF TWO COSINES
99
-
-
A1=1
F1=1
HORMALIZED
SUPERIMPOSED WAVEFORM
1.5
2.0
0.9
1.6
0.3
A2=.2 F2=9
POWER SPECTRUM
fl1.2-
I
'
-1.5
0.0
0.4
'
0-8
0.0.
1.6
FEATURE
2.4
3.2
4.0
-
-!
-
li0.8-
-0.3
0
2
4
6
8
-Aii
1@
SPACE
I
.5FEATURE VALUES,
0
ALPHA1
ALPHA2
-2.5-2-1.5-1 -. 5 0
.5
1.302797
-0.620437
1 1.5 2
Figure 3.3.5
First tutorial example of the relationship among timeseries, power spectrum, and the feature space representations.
100
-
-
The second example shown in figure 3.3.6 is the sum of two cosines
of the same amplitude but slightly different in frequency.
As expected,
the spectrum contain two adjacent peaks. Because the input is oscillatory and the average frequency of the spectrum is above 3.125 Hz, the
feature values lie
in the lower left
quadrant below the parabola.
To demonstrate how the feature extraction method works on samples
from the database, the following two examples are case studies of venThe earlier example of
tricular flutter and electrode motion artifact.
flutter is shown with its actual and modeled power spectrum in figure
3.3.7.
Because the peak of the power spectrum occurs at. a non-zero fre-
quency,
the autoregressive estimate of spectral envelope yields conju-
gate poles.
These poles map to the mark below the parabola in the
feature space in figure 3.3.7.
Figure 3.3.8 shows the earlier example of electrode motion artifact
along with its actual and modeled power spectrum.
Because the actual
spectrum has a great deal power around DC, the model estimated the
envelope with a peak at the origin.
the positive real axis.
The poles of such a model lie along
This condition corresponds to feature values
which lie above the parabola but below the horizontal axis.
Notice that
a2 is nearly zero which implies that a first autoregressive model may
fit
the data.
This observation is intuitively reasonable since the
spectrum may be modeled by placing a single peak at the origin (i.e.,
one pole on the real axis not too near the unit circle.)
This section has investigated the theoretical and empirical methods
of extracting the features from the input ECG.
The next portion of this
chapter describes the method used to classify the input signal using
SUM OF TWO COSINES
SUPERIMPOSED
01=1 F1=4.75 A2=1
WAVEFORM
A
j
-1.2
'
F2=5 25
NORMALIZED POWER SPECTRUM
0.78
2.0
0.4
101
-
-
)AJi0.42
I IIu1
0.14
-2.8
0.0AI
0.
0.8
1 .6
FEATURE
2.4
3-2
4.0
0
If
2
4
.8
10
SPACE
FEATURE VALUES
2LPHA
0
-1.412718
-1
-2.5---1 5--1-.5
0
.5
1 1. 52
Figure 3.3.6 Second tutorial example of the relationship among the
time-series, power spectrum, and feature space representations.
-
- 102
VENTRICULAR FLUTTER
TAPE
CHANIEL 0
427
START 308193 ; CLAP
HCTUL. FOjiER SPECTRUM
FILTERED ECG
0 30 -
2
20
-0
0 16
0 14
00
0 3
16
50~
O,
1.6 2
_
__
1_
_
_
__
_
_
S(
ul 0:.03
0 0
E
0 0
4
3
't r 7
TpII
2
4
0
00
1
4
36
4 8
6 0
FEATURE SPACE
4 4
-
.
0
-2.5-2- 1 5-1-.5
0 .5
1 1.52
Figure 3.3.7 Ventricular flutter example illustrating the autoregressive
model estimate of the power spectrum.
-
- 103
ELECTRODE MOTION ARTIFACT
THPE 0
FILTERED
2.0
CHANIIEL
0
ECG
CLASS 2
H'.TU-AL
SFELIRUII
POWER
-0.25
:.3
.210
-
A
.05
8 -1
0 0
0.8
1 6
2432
iuh
iihEll
Ier CI,
4.0
0 0
1
2
24
3
4.8
FEATURE
SPACE
5- 1-5
0
E.0
'
-0
START 2034
5/
-1 5-
i..ia43. - 0
.
e
-2.5-2-1
.5
52
1 15
Figure 3.3.8 Electrode motion artifact example illustrating the autoregressive model estimate of the power spectrum
-
- 104
these feature values.
Classifier
The third functional portion of the detector shown in figure 3.3.1
is the classifier.
For each data segment, the feature extractor esti-
mates the coefficients a1 and a 2 which form the feature vector,
a =
a2
As an example of the classification rule, consider the binary case of
discriminating between flutter and noise.
The feature vector, E,
belongs to one of two classes, or hypotheses,
: NOISE
H
H
:VFL
The classifier calculates the conditional probability of observing the
feature vector given that it
was flutter and the conditional probability
of observing the feature vector given that it was noise.
If the condi-
tional probability of flutter is greater than that for noise, the classifier assigns the input to flutter, otherwise it assigns it to noise.
Thus, the detector classifies the feature vector by comparing the two
conditional probabilities shown below.
H1
p(aIH1 )
=
H
p(IH0 )
(
(3.4)
a
0
This classification rule is pictorally represented for hypothetical
one-dimentional marginal distributions in figure 3.3.9.
The broader
distribution represents the conditional probabilty of flutter.
The
-
- 105
MAXIMUM LIKELIHOOD CLASSIFICATION
S40
-r
p(a|HO)
0J. 1
)
p(aH1
-0
0
-10
10
20
30
DECIDE
NOISE
DECIDE
VFL
DECIDE
VFL
Figure 3.3.9 Hypothetical Noise and VFL marginal conditional distributions which illustate the maximum likelihood decision rule (Eqn. 3.4)
other represents noise.
The detector classifies the input as flutter in
the region where the flutter distribution exceeds the noise distribution.
In this example, the classifier decides flutter outside the
intersection of the distributions.
The input is declared noise for
feature values between the distribution intersections.
The calculation of the probabilties depends upon the form of the
distribution of the a feature vector.
not be known apriori.
The form of the distribution can-
The discrimination is based on the assumption
that a1 and a2 follow a Gaussian distribution. (This was verified using
the Kolmorgorov-Smirnov Test both for the VFL coefficients [p < 0.025]
and the noise coefficients
106
-
-
[p < .005]).
Under the Gaussian assumption,
the conditional probability distributions of the noise a, and a 2 and the
VFL
a
and a2 are functions of their respective mean vectors and
covariance matricies
p(
a,s,) ) = (2n) 112
exp -
T(ap) (a-
where
a =
[)
r = E[u]
=
E[(a-i)T(a-j)].
The classifier is designed by estimating the mean vectors and covarince
matrices separately for flutter and noise from a database of noise and
flutter.
These parameters completely describe
both conditional proba-
bilities.
Definitions of the Two Autoregressive Detectors
Two different autoregressive detection schemes were implemented.
The schemes differed only in the method of estimating the Gaussian probability parameters.
The first scheme (Type I) estimated the mean vec-
tor and covarince matrix for each class separately by estimating the
parameters over the collective set of features for each class.
This
detector is also called Detector 1 or the AR(2) Gross Covariance Matrix
Detector.
The second scheme (Type II) estimated the parameters by first
calculating the mean vector and covariance matrix for each patient
independently anf then averaging these patient-estimates over all
patients.
This second scheme is also called Detector 2 or the AR(2)
Average Covariance Matrix Detector.
-
- 107
The difference between the detec-
tion schemes is that Detector 1 weights each segment equally while
Detector 2 weights each patient equally.
The second detection scheme
was developed from the first by the philosophy that the detector should
not be tuned to that subpopulation (patient) with the largest number of
segments of in the database for each class.
This chapter has discussed the detection method involving autoregressive modeling of the power spectrum.
The following chapter
describes the results of the implemented detection schemes.
3.4.
DETECTOR EVALUATION METHODOLOGY
Section 1.2 presented the terminology relevant to the basic detection problem in order to interpret the results of previous research in
VF detection.
This section discusses the evaluation methods applied to
the design and analysis of the three types of detection schemes implemented in this study.
The first
section focuses on the performance
measures and cost (benefit) measures used to determine the "optimal"
detectors.
The second section discusses the bootstrap technique.
This
technique provides a means of estimating the variability of the performance and cost measures.
3.4.1.
DETECTOR PERFORMANCE MEASURES
For convenience, the definitions of the performance measures introduced in section 1.2 are summarized below.
classes of VT,VFL, and VF.
artifact.
Sensitivity (SE)
Class V denotes the combined
Class N corresponds to electrode motion
to ventricular events is defined as
SE
=
-
- 108
T
TP + FN
The specificity (SP) of the detector to reject noise is defined as
SP = FP TN
+ TN
The positive predictive accuracy (PPA) is defined as
PPA
-TP
TP + FP
where the following notation is used : TP is the number of correctly
classified class V events, FP is the number of misclassified class V
events, and TN is the number of correctly classified class N events.
In order to evaluate a detector,
vation must be recorded.
the true class of each test obser-
In addition to recording the class type for
each test input, the patient number was also noted.
events were not patient-specific,
noise.
Since the noise
no patient number was associated with
Therefore the sensitivity of the algorithm in detecting each
patient's ventricular events could be calculated.
The average of the
detector's sensitivity to each patient's events is called "average sensitivity" (aSE).
On the other hand, the sensitivity of the detector to
all the events taken as a whole is called "gross sensitivity" (gSE).
Since noise was not assigned to patients, only a gross positive predictive accuracy could be determined (gPPA).
Restated, average senstivity is the fraction of ventricular events
that are correctly classified for an average patient.
Gross sensitivity
is the fraction of total ventricular events which were correctly classified.
Gross positive predictivity is the fraction of the events classi-
fied as ventricular events that were actually ventricular events.
The
distinction between the performance measures is that average statistics
109
-
-
weight each patient equally while gross statistics weight each event
equally.
The trade-off between the performance measures is often illustrated
in two curves.
The first curve, the Receiver Operating Characteristic
curve (ROC), displays the trade-off between gross sensitivity and the
gross false positive rate (1-gSP) as a function of threshold.
Another
curve, the System Operating Curve (SOC), displays the trade-off between
gross sensitivity and gross positive predictive accuarcy.
these curves are shown in figure 3.4.1.
Examples of
This figure provides an illus-
trative example of how ROC and SOC curves can be used to. select the
better of two detection schemes.
Recall that every point along an ROC
curve corresponds to a single detector.
(Different points along the same
ROC curve actually correspond to the same detection algorithm with different threshold (1)
settings.
different performance,
specific detector.
But since a different threshold yields a
I consider each threshold setting to create a
Thinking in this fashion makes the analysis of the
results clearer.)
Let the dotted and solid lines in figure 3.4.1 correspond to different detection schemes A and B.
For example, let A and B be the
autoregressive Type II and relative-power schemes respectively.
portion of the figure shows,
The top
that for any fixed FPR, the sensitivity of
scheme A is superior to that for B.
Thus, in general,
the detection
scheme with the highest ROC curve (sharper "knee") is superior to all
others with respect to these performance measures.
(Note that a ROC
curve that is a line connecting the lower left and upper right points of
the graph could represent a receiver which makes a decision by flipping
-
- 110
ROC :
/
100 T
/
PZSE
0
P
SOC :
~ I-SP
100
100"
SE
0
PPA
100
Receiver
Figure 3.4.1 Hypothetical Detector Characteristic Curves.
Operating Characteristic curves (ROC) (top) and the System Operating
Characteristic curves (SOC) (bottom). Detection scheme A (dotted line)
is superior to detection scheme B (solid line) at all thresholds.
an unbiased coin.
That is,
PD
F for all threshold settings.
Thus,
it is expected that any detection scheme that uses information obtained
from the observations would have an ROC curve above this equal-
111
-
-
probability line.)
The bottom portion of figure 3.4.1 shows the SOC curves for both A
and B detection schemes.
For any fixed gross positive predictive accu-
racy, the gross sensitivity of scheme A is superior to scheme B.
Again,
in general, the detection scheme with the sharpest "knee" would be the
superior scheme with respect to these performance measures.
The method discussed above works well to distinguish between different detection schemes as long as the ROC or SOC curves do not cross.
Figure 3.4.2 shows the case when the ROC and SOC curves of different
detection schemes cross.
Clearly, that detector which is better depends
upon the threshold setting.
The problem of deciding which detection
scheme is better is resolved by introducing cost functions.
As discussed in section 1.2, the detector threshold may be selected
by minimizing the expected cost of the detector after assigning cost
weights to the different decision types.
In this case the likelihood
ratio selects the threshold to minimize the following cost function
+
Cost(iO = C0 0 P(H0 ) + C 0 1 P(H1 )
(C1 0 - COO)P(HO)PF(7) - (C0 1 - Cll)P(Hl)PD(n)
The minimal cost of each detection scheme
to select the overall optimal detector.
(A versus B) could be compared
(That is, optimal with respect
to minimal cost.)
In this study, we introduce two new cost functions based on the
gross sensitivity and gross positive predictivity performance measures.
Because we wish to maximize rather than minimize the two cost functions,
we intoduce the term "benefit" functions (measures) as the tools used to
- 112
-
F
-
ROC
100
PD
SE
0
SOC :
P
100
SE
0
PPA
100
Figure 3.4.2 Hypothetical Detector Characteristic Curves. Receiver
Operating Characteristic curves (ROC) (top) and the System Operating
Characteristic curves (SOC) (bottom). Detection scheme A (dotted line)
is superior to detection scheme B (solid line) only for thresholds were
the dotted curve is higher than the solid one.
select optimal detectors.
The benefit measures were selected in order to maximize the
-
- 113
performance of the detector from the patient's point of view (i.e.,
optimally sensitive to malignant ventricular arrhythmias.) Both benefit
measures tune the detector to achieve the same goal (i.e., most efficiacous patient care) but make different assumptions based on the monitoring machine's environment.
If one assumes that every VF alarm is immediately serviced,
then
the patient's best performance criteria would be to maximize sensitivity.
(i.e.,
Thus,
the first
B1 = gSE.)
performance measure
(Bi) is gross sensitivity
This benefit function is directly related to the
minimal cost function discussed above by weighting missed ventricular
events by infinity (i.e.,C 01= C.)
The second benefit function also maximizes alarm service but is
motivated by the fact that the hospital staff must work in conjunction
with the monitoring machine.
The following is a hypothetical situation
which prompted the defining of the second benefit measure.
Consider a
detection scheme which is 100% sensitive to ventricular events by
assigning every observation to class V.
This detector would have a very
high false alarm rate and therefore a terribly low PPA.
Since ,in gen-
eral, there are more noise disturbances than actual ventricular arrhythmia events,
the staff would be servicing nearly all false alarms.
The
staff confidence in the machine would deteriorate and (hypothetically)
their alarm-servicing time would increase.
The machine may act so
poorly that the staff response is so long as to miss a true ventricular
episode and the patient may expire.
is
On the other hand if
the detector
tuned to have no false alarms, the detector would miss many ventricu-
lar episodes.
Thus more patients would die.
-
- 114
This example hypothesizes that the trade-off between false alarm
rate and senstivity influences the alarm servicing time (i.e.,
care efficacy.)
However, it
is not the FPR that is
patient
important to alarm
servicing but rather the percent of alarms which are false positives.
An estimate of this percentage is related to the PPA.
The higher the
PPA, the lower the percentage of false alarms among all alarms.
Suppose that we model the staff/machine service response as a
linear sum of the gross sensitivity and positive predictivity (i.e., B2
= gSE + gPPA.) That is, in terms of patient care efficacy, an increase
in one percent gPPA is worth the decrease of one percent of gSE.
Thus,
in this environment, we want to find the detector that maximizes B2, the
second benefit measure.
The term System Operating Characteristic (SOC)
curve was coined because it expresses the trade-off relationship between
gPPA and gSE that is of interest to the staff/machine system.
Note that the traditional assignment of costs to detector decisions
could not produce the benefit measure B2.
This is because we needed to
assign a cost to the observations that were noise given that the detector decided they were ventricular events.
That is, we needed to assign
a cost to a reality given a decision, instead of assigning the cost of a
decision given a reality.
In summary, this section has introduced two benefit measures (BI
gSE and
=
B2 = gSE + gPPA) which optimize patient care efficacy given two
different assumptions about how the alarms are serviced.
The detection
schemes implemented in this study are optimized with respect to these
two measures.
These optimal detectors will be denoted by optimalB1 and
optimalB 2 which indicate optimal detectors with respect to benefit
-
- 115
measures 1 and 2 respectively.
In addition to presenting results for the optimal detectors, the ML
detector results will also be presented.
The ML detector has a fixed
threshold among all detection schemes and therefore shows how different
detection schemes perform for the same fixed threshold.
3.4.2.
ESTIMATING CONFIDENCE LIMITS FOR ARRHYTHMIA PERFORMANCE
MEASURES
The previous section described the benefit measures used to evalutate detector performance (i.e., sensitivity, specificity, predictive
accuracy, and the B1, B2 benefit measures.) This section presents a
computer-intensive method for estimating the variance of these performance metrics.
In the past, the limited size of ECG databases created two problems
in the development of arrhythmia detectors.
First, the developer had to
solve the trade-off dilemma of partitioning the database into 1) a
Learning Set for the design of the detector, and 2) a Test Set for the
evaluation of the detector.
This classic "designer's dilemma" arises
because a large Learning Set yields a better design, while a large Test
Set yields a better estimate of detector performance.
Second, because
evaluating the detector over one Test Set yields only one estimate of
the detector performance measures,
the developer could not estimate the
confidence limits about the detector performance measures.
Comparison
between different detectors was difficult without an estimate of the
variablility of the detector performance measures.
The estimation of confidence limits requires knowledge of the
-
- 116
underlying distribution of the data, knowledge which can be verified
only with very large samples.
Since it has not been feasible to create
significantly larger arrhythmia databases, there has been a strong
interest in identifying robust statistics based on small samples, and
establishing confidence limits for them.
The statistical bootstrap, developed by Efron[14], is a method to
solve these two problems.
The bootstrap is a resampling techique which
"creates" new databases from the original database.
formation of
By emulating the
of multiple databases, the developer has "unlimited" data
to both design and test the detector.
The evaluation of the detector
over many Test Sets yields many estimates of detector merformance measures.
The variability of the performance measures may then be calcu-
lated from the distribution of these estimates.
The Bootstrap
The bootstrap is a statistical technique which allows one to estimate the distribution of any statistic, e(X,...,XN), no matter how complicated, from a set of observations {X.,i=1,...,N).
Monte Carlo approach in which the statistic
e is
It utilizes a
repeatedly calculated
on subsets drawn from the original observation set {X.,i=1,...,N}.
The
bootstrap procedure can be divided into two steps:
1) Choose at random and with replacement N elements from the original
observation set,
{Xi,i=1,...,N}.
tions is {X*,i=1,...,N}.
bootstrap replication.
The new, hypothetical set of observa-
This new sample (database) is called a
-
- 117
That is, calculate 8(X*,,XN
hypothetical set of observations.
Steps (1) and (2) are repeated many times.
'
2) Calculate the statistics (performance measures) using the new
The estimates of 6 from
step (2) are used to from an estimate of the distribution of
e.
Once
the empirical distribution is known, one can calculate the confidence
intervals directly.
These steps are sumarized in figure 3.4.3.
BOOTSTRAP SUMMARY
X5
-
B.S.
:
X2
X2
SAMPLE
ORIGINAL OBSERVATION
SET
XN - X 8
. E.u) . .. . .. . . .
X,
STATISTICAL
R*
X2
DISTRIBUTION
X4
X4
B.S.
2
SAMPLE
XN
0(5
5...),
X9
XN
N
8; X' ..... ,;)
0. 4
0. 2
ELEMENTS ARE
RANDOMLY SELECTED
WITH REPLACEMENT
75
-
1
B. S.
XN
-
80
es
90
95
X3
X7
X2
10000('S.
y
I
Figure 3.4.3 Illustration of the Bootstrap. The original observations
are placed in an hypothetical bin. New observation sets are created
from the original N obseravtions by randomly sampling with replacement N
times from the bin of original observations. This procedure is repeated
e is calculatmany (10,000) times. Since each estimate of the statistic
ed for each of the 10,000 observation sets, an empirical distribution of
The confidence limits of the statistic
can be calculated.
the statistic
may be read directly from the distribution.
100
-
- 118
The bootstrap does not make any assumptions regarding the underlying distribution of the original dataset; it does, however, assume that
the original dataset is a maximum likelihood estimate of the true population (i.e., the original sample well represents the distribution.) The
bootstrap does not provide any new information about the underlying distribution, nor does it remove inherent biases obtained through the
selection of the original sample.
Two features of the bootstrap are important.
First, the new
datasets (databases) are created the same size as the original dataset
Second, the sampling is done with replacement (i.e., multiple copies of
observations can occur in the replicated dataset.)
Tutorial Example of the Bootstrap
Figure 3.4.4 depicts the classic designer's dilemma.
The statistical bootstrap is a computer-intensive resampling technique which emulates the formation of new databases from an original
database. The method is best described by an example in which two detectors were designed to discriminate between noise and VFL only.
(This
example is a portion of the results described in section 4.2.) The database was composed of two sections, a noise portion and a malignant
arrhythmia portion.
The arrhythmia portion consisted of nine patients,
with a total of 396 4-second segments of VFL.
database consisted of 519 4-second segments.
The noise portion of the
These noise segments were
samples from an independently developed noise database and were not ECG
artifact[13].
The noise was put into one group and not assigned to par-
ticular patients because it was felt that noise was not a patient-
119
-
-
THE DESIGNER'S DILEMMA
DATABASE
DEVELOPMENT
EVALUATION
SENSITIVITY ( SE
POSITIVE PREDICTIVITY ( +P
PROBLEM
How
)
I
)
STATISTICS
RELIABLE ARE THE PERFORMANCE STATISTICS ?
Figure 3.4.4 The Designer's Dilemma. This picture illustates the dilemma of dividing a small database between a Learning Set for the development of a detector and a Test Set for the evaluation of the detector.
The right side illustates that for any one partition of the data, only
one set of estimates of the performance measure are created.
The question that arises is how to calculate the reliability of these performance statistics.
specific event.
The listing of the database is shown in table 3.4.5.
The performance of the detector was evaluated based on the classification of the four-second ECG segments.
The performance was reported
in the form of the average VFL sensitivity, gross VFL sensitivity, and
gross VFL positive predictivity.
The definition of these measures are
redefined in table 3.4.6.
In this example,
the bootstrap was applied in paired iterations,
first to create a Learning Set to design the detector, and second to
create a Test Set to evaluate the detector.
This procedure of
bootstrapping both a Learning Set and a Test set is called a "double
Number of ECG Segments
Source
Patient
Patient
Patient
Patient
Patient
Patient
Patient
Patient
Patient
TOTAL
-
- 120
1
2
3
4
5
6
7
8
9
62
16
12
8
219
54
5
17
3
396
Table 3.4.5 Ventricular flutter (VFL) and noise database.
The database
consisted of four-second ECG segments taken from Holter tapes.
Examples
of ventricular flutter were taken from nine patients.
The number of
segments from each patient is shown.
Noise was put into one group since
it was felt
felt that noise should not be considered a patient-specific
ECG event.
9 =
TP + FN
-
Gr Se =
+ FN.
Gr +P=
TP
TP + FP
statistics.
Average sensiTable 3.4.6 Definition of the performance
Se) is the fraction of the VFL segments that are correctly
tivity
(Av
classified for an average patient. Gross sensitivity
(Gr Se) is the
fraction of total VFL segments that were correctly classified.
Gross
positive predictivity (Gr +P) is the fraction of segments
that were
classified as VFL which were actually VFL.
TP, FN, TN, and FP are defined as the total number of of correctly classified VFL,
misclassified
VFL, correctly classified noise, and misclassified noise respectively;
TPi and FN represent the TP and FN for an individual patient. The distinction between the performance measures is that the average statistics
weight each patient equally while gross statisitos weight each VFL segment equally.
bootstrap." The double bootstrap was iteratered many times.
3.4.7 summarizes the double bootstrap method.
Figure
-
- 121
DOUBLE BOOTSTRAP METHOD
DEVELOPMENT
VENTRICULAR
FLUTTER
CALCULATE
396
EVALUATION
NOISE
PERFORMANCE
MEASURE
X
SAMPLING WITH
REPLACEMENT
Figure 3.4.7 The Double Boostrap Method.
The original database samples
are place in hypothetical bins.
A Learning Set is created by randomly
sampling with replacement 396 times from the VFL bin and 519 times from
the noise bin. This new database is used to design the detector (by estimating the mean vector and covariance matrices for noise and VFL
separately.)
A second bootstrapped database is created by randomly sampling with replacement in the same manner to create a Test Database for
metric is evaluated from the
detector evaluation.
The performance
results of the detector over the Test Set.
Two detectors were designed to discriminate between VFL and Noise
(Detectors I and II.) The boostrapped performance measures for the two
detectors are shown in figures 3.4.8 and 3.4.9 respectively.
PERFOREANCE NEASURES
:
-
- 122
NL DETECTOR I
AVERAGE SENSITIVITY
(VFL VS. N ONLY)
GR05S SENSITIVITY
03-
0W
-
76
0
I
32
s3
591
100
-
It
76
32
8
9f
100
E1055 FOS PEEDICTIVITV
.5-
91t
.3
SE
ER SE
ER #P
.2
NEAR
5% LIN
964
16.0
956.8
ai9
2.8
54.1
0'
70
756
3!
so
94
10
Figure 3.4.8 Bootstrapped Performance Measures for Detector I. The perfor 5000 iterations of the double
formance measures were calculated
statistics
bootstrap.
The mean and 5% minimum expected performance
determined from these statistics is also shown.
-
- 123
PERFORNANCE NEASURES : NL DETECTOR 2
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.5
.5-
art
a.It
474
96
2
s
9
(VFL VS. N ORLY)
10
10
16
82
18
94
Ito
615 F05 PIEDICTIVITV
.2
176
1E
2
as99
NEAR
5% LIN
AV SE
g0.
figs
ER SE
UR *r
81.2
55.5
8.4
50.1
19
Figure 3.4.9 Bootstrapped Performance Measures for Detector
II.
The
performance measures were calculated for 5000 iterations of the double
bootstrap.
The mean and 5% minimum expected
performance
statistics
determined from these statistics is also shown.
Table 3.4.10 summarizes the estimated confidence limits for the two
detectors.
-
- 124
Table 3.4.10 Performance Statistics for Detectors 1 and 2.
Ave. Sensitivity
1
DETECTOR 1
%Mean
5
__
5%
ltros
IETECT___
Positive
5
an
95%
86.0 1
88.7
t
an
f
194.71
96.8
I
I
Gross SensitivitylP"diCtivi
7.91 90.61 93.0 1182.81
ML
ML
t
II
-
Detector Type
t5%
i
=
=
98.4
I
8 4.51 90.8-[97.9 1181.41 87.2 195.5 j00.1 1-95.9
198.4
Figure 3.4.8 shows the empirical distributions of the gross and average
VFL sensitivity and gross VFL positive predictivity.
These distribu-
tions allow one to establish upper and lower confidence limits for each
of the measures.
By estimating these limits, the bootstrap provides a
means of assessing the minimum expected performance of the detector on
another database chosen using the same criteria employed to select the
original database.
By estimating the distribution of the performance metrics,
the
bootstrap allows one to make a more intelligent comparison between the
two detectors.
Consider the statistics shown in table 3.4.10.
The mean
statisitos seem to imply that Detector I performs better than detector
II.
However, the 5% and 95% confidence limits on the means show that
the difference in performance would not achive statistical significance.
In the absence of the bootstrap,
one might have inappropriately con-
cluded that Detector I is better than Detector II.
The wide (and over-
lapping) confidence limits on the distributions indicate that the database is not adequate to allow one to determine which detector is better.
This example illustrates the utility of the bootstrap in assessing
arrhythmia detector performance and in aiding in the comparison of
different detectors.
-
- 125
The bootstrap is used in this work to create dis-
tributions of the benefit measures as well as performance measures.
The
"best" detectors with respect to the benefit measures are determined by
examining these distributions.
-
- 126
Chapter 4
4.
RESULTS FOR THE DETECTION SCHEMES
This chapter presents the results for the three detection schemes
described earlier.
The results for the reference detector are presented
as a standard in the first
section.
The results for both autoregressive
detection schemes are presented in the second section.
4.1.
RESULTS FOR THE REFERENCE DETECTOR
-
- 127
PEAK FREBUENCY DISTRIBUTION
VFL
VT
.6
.6
. 36
.35SIR-
.12
.12
*
1.3
2.6
3.9
5.2
6.5
0
1.3
.6-
.98
.l8-
. 35
.36-
.12
.12-
1.3
.6 3.3
3.9
5,2
6.5
5.2
6.5
NOISE
VFIB
.6
0
2.6
5.2
6.5
0
1.3
2.6
3.9
Figure 4.1.1 Distribution of the frequency of the main power spectral
peak for VT,VFL,VF, and Noise.
These hsitograms show the percent of
segments within a specific frequency bin (Hz).
-
- 128
PONER RATIO (R) DISTRIBUTION
:
IBP = 40
VFL
VT
.6-
.6
.*
4fl
12
NOISE
VF
v6
n6
.36-f.12
612-
.Is-
I
.E
.4
a6
.o
I
1
,2
.4
v6
so
Figure 4.1.2 Power ratio (R) distribution of VT,VFL,VF, and NOISE for
4-second data segments. The inner bandwidth percentage is 40%.
These
hsitograms show the percent of segments within a specific range of R.
I
-
- 129
=
POWER RATIO (R) DISTRIBUTION : IBP
YFL
VT
.6-
.98-
.qs-
I2
.12-
*
-
.6
TFh
0
.
.
-
I
0
I-.
as
96
.2
VF
I
I
.11
1.
-
.6
.8
NOISE
.6
-
.5
.40.4
.48
v36-
I
0 .2
I
I
5
s
I
5
a s I
.9
.E
1
*1
0 1hi'
I
1
Ti
jf
III'
.2
.F
.5
~vr
.
I
912-
Figure 4.1.3 Power ratio (R) distribution of VT,VFL,VF, and NOISE for
4-second data segments. The inner bandwidth percentage is 96%. These
hsitograms show the percent of segments within a specific range of R.
I
-
- 130
=
POVER RATIO IRI DISTRIBUTION : IBP
150
VFL
VT
.6'
.6'
.8-
.9*
.35-
.35
31-
.120
.2
0
.q
.6
91-
hil
flK
eI
0
.a
.2
.
.6
.9
3--
VF
NOISE
.6
-
.5
.6
36-
.1212AU
I
0
.
I
.2
all
~~-FF
- - - -9
F. T
9.
*6
A
T
I
.
.2
T
.
8ug
I
I
66
I
11
.9
Figure 4.1.4 Power ratio (R) distribution of VT,VFL,VF, and NOISE for
4-second data segments. The inner bandwidth percentage is 150%. These
hsitograms show the percent of segments within a specific range of R.
1
-
- 131
FEATURE HISTOGRANS FOR CLA5SE5 V AND N
:3P
=40
g 11
p
p(RIN)
p(RIV)
0
U
-
*
T
*022
0
LU
0
.2
UMnd
k$dInflJd
M~Tn
.
-
0
.A
,6
..
,r8
POWER RATIO I11
Figure 4.1.5 Conditional distributions of R for an inner bandwidth perThe distributions are equal for R=.51.
centage of 40%.
-
- 132
FEATURE HISTOERANS FOR CLASSES V AND N : INP
=96
.1a
.081
P
R
p(RIV)
p(R
IN)
-
06
A
L
6014-
*
I
T
y
a
i
0
*v1.
x Pfld
.2
~Miaff
.5
.8
I
POMER RATIO IRI
Figure 4.1.6 Conditional distributions of R for an inner bandwidth percentage of 96%. The distributions are equal for R=.60.
-
- 133
FEATURE HISTOGRANS FOR CLASSES V AND N
: lop =150
us
p(RIV)
.08
p(R IN)
p
A
0
A
I
.06.0
T
y
I
0
~IEIidi II
0
.2
.4
~m.MAIa~inL~ILUflW.LhJ4IIIJ
.6
.e
I
I
PONER RATIO gEl
Figure 4.1.7 Conditional distributions of R for an inner bandwidth percentage of 150%. The distributions are equal for R=.71.
tWHIML DEEFIT
-
- 134
EASES AS A FUCTION OF IBP
Dt : aSE
86-
38
48
58
68
78
82
810
188
118 128
138
148
158
+ gSD
K3AXIKI (PA
2W8
112
t8424
,
+
&
118
128
138
+
176
1,
&
-b
184
16838
48
58
68
78
88
90
188
148
158
Figure 4.1.8 Benefit measures as a function of inner bandwidth percentage (IBP). KEY : (+) corresponds to the maximum value of each benefit
(-) corresponds to the
measure over all detectors with a fixed IBP.
value of each benefit measure for the Maximum Likelihood detector for
that IBP. The first benefit measure (Bi) is the gross sensitivity (gSE)
of the detector to VT,VFL,and VF (top). The second benefit measure (B2)
is the sum of gross sensitivity and gross positive predictivity
(gSE+gPPA) (bottom). The maximum and ML values are equal for detectors
with inner bandwidth percentages of 40,70,120, and 150%.
-
- 135
Table 4.1.9 Maximimum Likelihood and Optimal Detectors as a Function of
Inner Bandwidth Percent Over the Original Database (thresholds and benefit measures)
T1
B1
2,6
T
T1
B2
ma
max.
B1
B2j
4L
-
Inner
Bandwidth
Percent
Max. Likelihood Detector
Detector
-
Optimal
40
.17
100(69.8)
.511
175.7
I .51
80.6
175.7
50
.181
100(69.8)
.50
180.2
1.55
81.7
179.0
60
.231
100(69.9)
.51
183.1
11.55
88.4
184.5
70
.23'
100(69.9)
.52
184.6
.55
88.4
184.6
80
.241
100(69.9)
.57
I 185.1
.60 |
87.1
184.0
90
.271
100(70.0)
.57 | 186.3
.601
88.7
185.1
92
.271
100(70.0)
.57
186.4
.601
89.0
185.3
94
.271
100(70.0)
.58j
186.5
.601
89.6
185.7
96
.271
100(70.0)
.58
186.83
,I
90.0 4
186.25
98
.27J 100(70.0)2
.581 186.83
90.0 4
186.25
100
.271 100(70.0)2
.701
110
.271 100(70.0)2
120
.27
130
.27 |
.21
1I
182.6
.66
86.5
179.4
183.0
.66
87.3
180.0
I 100(69.9)I .70 | 184.6
.701
86.6
184.6
I
.70|
86.9
183.7
.72
85.9
183.6
.71
87.6
183.8
100(69.9)
140
.281
100(69.9)
150
.28
100(69.8)
I .70
.71
I 184.2
I .71 I 184.1
.71
183.8
II
1 Detector threshold setting.
2 All detectors are optimal with respect to Benefit Measure
1.
3 Optimal detector with respect to Benefit Measure 2.
4 Best ML detector with respect to Benefit Measure 1.
5 Best ML detector with respect to Benefit Measure 2.
6 The Gross Positive Predictivity (gPPA) is listed parenthetically.
-
DETECTOR OPERATING CHARACTERISTICS : IBP
GSE VS. I-GSP
=
40
GSE VS. THRESHOLD
too,
10
80
tB2= t
-13
60
60&A-
20
0
0
0
20
9Q
60
80
100
0
.2
.1
.6
I
.8
lIG5E VS. 6PPA
100-1
t
EPPA VS. THRESHOLD
t
'BZF tMi
t
1-
B1
-
50
60-
9020---I
0*I
20
2
01
-
1II, iI
- 136
90
60
80
I0
0
.2
.94
1 1 W
-I
.6
.8
F
I
U
Figure 4.1.10 Detector Characteristics. : Relative Power Detector with an
Inner Bandwidth Percentage of 40%. Standard receiver operating characteristic
(ROC) (i.e.,
true positive rate vs false positive rate as
estimated by the gross sensitivity (gSE) versus 1 - gross specificity
(1-gSP)) (upper left). System Operating Characteristic (SOC)
( gross
sensitivity versus the gross positive predictive accuracy (gPPA)) (lower
left). Gross sensitivity versus threshold (upper right).
Gross positive predictivity versus threshold (lower right).
-
- 137
=
DETECTOR OPERATING CHARACTERISTICS : IIP
GSE VS. I-ESP
96
GSE VS. THRESHOLD
lot-
oil
tM
6040-
20S
U
0
20
90
0
p
G5E VS. GPPA
I
.
I
I
.1
.6
.8
1
6PPA VS. THRESHOLD
t
100-
,
.2
100
tB1
tB2
60-
60-
60-
'to
2:1
0
20
90
60
of
A .1
100
WI
0
.
me
I
I
.
I
.
86
.I
so
I
I
Figure 4.1.11 Detector Characteristics. : Relative Power Detector with an
Inner Bandwidth Percentage of 96%. Standard receiver operating characteristic
(ROC) (i.e.,
true positive rate vs false positive rate as
estimated by the gross sensitivity (gSE) versus 1 - gross specificity
(1-gSP)) (upper left). System Operating Characteristic (SOC)
( gross
sensitivity versus the gross positive predictive accuracy (gPPA)) (lower
left). Gross sensitivity versus threshold (upper right).
Gross positive predictivity versus threshold (lower right).
-
- 138
DETECTOR OPERATING CHARACTERISTICS
:
IBP
=
150
G5E V5a THRESHOLD
GSE VS. 1-ESP
100
tt
B1
60
50-
90
90
-
20. .,
,Eo20
O
-
20
ED
90
10
80
-SE VS. 6PPA
tB2-tM
100
.2
100
.9
.
.s
I
GPPA VS. THRESHOLD
t
10
tBl
80a
90
60-
60-
90
90
20
20
o
0
90
61
90
100
0
.12
A1
e6
to9
Figure 4.1.12 Detector Characteristics : Relative Power Detector with an
Inner Bandwidth Percentage of 150%. Standard receiver operating characteristic (ROC) (i.e., true positive rate vs false positive rate as
estimated by the gross sensitivity (gSE) versus 1 - gross specificity
(1-gSP)) (upper left). System Operating Characteristic
(SOC) ( gross
sensitivity versus the gross positive predictive accuracy (gPPA)) (lower
left). Gross sensitivity versus threshold (upper right).
Gross positive predictivity versus threshold (lower right).
-
- 139
BENEFIT NEASURES AS A FUNCTION OF THRESHOLD
8i:
96%
SE
tIB2
ta
go.
IPB
tM
60
,0
.2
.6
.9
.8
32: GSEIEPPA
lo...
..
160
t
B2
tML
B1
120*
.2
.4
.5
.5
Figure 4.1.13 Benefit measures as a function of threhsold setting for
the relative-power detector (IBP=96%).
BI = gross sensitivity (gSE)
(top). B2 = gross sensitivity + gross positive predictive
accuarcy
(gPPA) (bottom).
The curves indicate that the benefit measures are
robust with respect to their optimal settings.
-
- 140
BOOSTRAPPED DETECTOR CHARACTERISTICS (IBP=961
GSE VS. THRESHOLD
GSE VS. I-GSP
100
,
100
80
50-
20
10*
0
206
20
1
qO
60
80
0
100
.2
.1
.6
.9
1
I-
5PPA VS. THRESHOLD
ESE VS. GPPA
I
100
100-
90go-
80-
6010
50-
40
i
20
40
60
80
0 I
0
100
- I
.2
I I I I
.9 .6
I I
.8
I
i
a
Figure 4.1.14 Ten Bootstrapped Detector Characteristic Curves for the
relative-power detector (IBP=96%).
Receiver Operating Characteristic
curves (ROC) (top left). System Operating Characteristic curves (SOC)
(bottom left). Gross sensitivity versus threshold (upper right). Gross
positive predictive accuracy versus threshold
(lower right).
The
bootstrapped curves indicate the how robust the detection scheme is with
respect to different databases.
-
- 141
BOOTSTRAPPEO PERFORNANCE NEASURES
(DET 0) (IBP:961 (T=.60)
EROSS SENSITIVITY
AVERAGE SENSITIVITY
16
.8-
.9-1
.14
65
12
79
86
93
100
65
12
i9
86
93
100
EROSS P05 PREDICTIVITV
.6
AV SE
ER SE
ER +P
.14
65
42
79
86
93
NEAN
5% LIN
93.1
90.0
U&.2
90.9
88.5
95.1
100
Figure 4.1.15 Bootstrapped Performance Measures for the Best Maximum
Likelihood Relative-Power Detector (IBP=96%; Threshold=.60.) Histograms
of the average sensitivity to VT,VFL, and VF (aSE) (top left),
gross
sensitivity (gSE) (top right), and gross positive predictive accuracy
(gPPA) (lower left) were generated by calculating these measures over
The mean and 5 percent minimum expected
5000
(bootstrapped) databases.
performance values are indicated in the table (lower right.)
-
- 142
IOOTSTRAPPED PERFORMANCE MEASURES
: (DET
GROSS SENSITIVITY
AVERAGE SENSITIVITY
1.12-
1512.
. 5-
.51
.29-
*28
65
0) (IBP:961 (T:.2l)
i2
79
s6
ga
100
65
72
79
86
93
too
GROSS PUS PREDICTIVITY
j.q-
.
AV SE
ER SE
GR #P
.5
NEAN
5% LIN
100
100
100
69.98
100
10.0
.2955
'12
19
95
Si
100
Figure 4.1.16 Bootstrapped Performance. Measures for the Optimal
(with
respect to Maximum Sensitivity) Relative-Power Detector (IBP=96 , Threshold=.27.) Histograms of the average sensitivity to VT,VFL, and VF (aSE)
(top left),
gross sensitivity (gSE)
(top right), and gross positive
predictive accuracy (gPPA) (lower left) were generated by calculating
these measures over 5000 (bootstrapped) databases. The mean and 5 percent minimum expected performance values are indicated in the table
(lower right.)
143
-
-
IOOTSTRAPPED PERFORMANCE MEASURES
(DET 0) (IP:91 (.SS)
AVERAGE SENSITIVITY
GROSS SENSITIVITY
. -6
.1
0'
65
172
ig
86
93
lit
B1
is
-12
19
86
93
100
GROSS POS PREDICTIVITY
AV SE
6R SE
#P
e-ER
65
iE
i9
ig
93
MEAN
5% LIN
94.1
Si.i
95.2
91.9
90.1
94.1
lit
Figure 4.1.17 Bootstrapped Performance Measures for the OptimalB 2 (with
to the Maximum Sum of the Gross Sensitivity and Gross Positive
Respect
Accuracy (B2)) Relative-Power Detector (IBP=96%; ThresPredictive
hold=.58.) Histograms of the average sensitivity to VT,VFL, and VF (aSE)
(top right), and gross positive
(top left), gross sensitivity (gSE)
predictive accuracy (gPPA) (lower left) were generated by calculating
these measures over 5000 (bootstrapped) databases. The mean and 5 percent minimum expected performance values are indicated in the table
(lower right.)
-
- 144
BOOTSTRAPPED BENEFIT MEASURES
BI
:
(DET 0) (IBP=961 (T=.60)
: GSEKS
Il'
.12
.06
is
B9
91
94
97
100
189
195
BE : G5EIS + GPPA
.18
.12
.06ft
01165
111
697
183
Figure 4.1.18 Bootstrapped Benefit Measures for the Best Maximum Likelihood Relative-Power Detector (IBP=96%; Threshold=.60.) Histograms of the
Gross Sensitivity (gSE) Benefit Measure (Bi) (top) and the Sum of Gross
Sensitivity and Gross Positive Predictive Accuracy (gPPA) Benefit Measure (B2) (bottom) were generated by calculating these measures for 5000
(bootstrapped) databases.
-
- 145
VOCTSTRAPPED IENEFIT NEASURES : IDET 0) (IBP=961 (T=.E71
BI
:
G5EES
.-
0
85
88.02
9.06
91.09
32
:
S7.@B
100.1
GSENS + GPPA
1.
38-
.5
6
165 11
9
1
117193
189
9
Figure 4.1.19 Bootstrapped Benefit Measures for the OptimalB
RelativePower Detector (IBP=96%; Threshold=.27.) Histograms of the aross Sensitivity (gSE) Benefit Measure (Bi) (top) and the Sum of Gross Sensitivity
and Gross Positive Predictive Accuracy (gPPA) Benefit Measure (B2) (botfor
5000
measures
these
calculating
by
tom) were generated
(bootstrapped) databases.
-
- 146
BOOTSTRAPPED HENEFIT NEASURES : (DET 0) (IBP=96) (T=s58)
81 : GSEES
.18.12
.#65
94
91
12
:
91
100
G5EES + GPPA
.3-
.18
1{9J9
.12-
165
lii
IVJ
h8h
199
195
Figure 4.1.20 Bootstrapped Benefit Measures for the Optimal
RelativePower Detector (IBP=96%; Threshold=.58.) Histograms of theB ross Sensitivity (gSE) Benefit Measure (Bi) (top) and the Sum of Gross Sensitivity
and Gross Positive Predictive Accuracy (gPPA) Benefit Measure (B2) (botfor
5000
measures
these
by
calculating
tom) were generated
(bootstrapped) databases.
-
- 147
Table 4.1.21 Detector Benefit Values. Mean, 5 percent minimum, and 95
percent maximum expected values for the B1 and B2 benefit measures for
the optimal and ML relative-power detectors.
IBP=96%
Benefit Measure B2
Benefit Measure B12
1
Detector Type
II 5% Min
95% Max I
Mean
_____--I ____
POWR
-_~~~==- I
T
5% Min
Mean
95%
Kax
------
__
BI
B2
max
(.27)
(.58)
ML (.60)
I
100
100
100
170.0
7.
170.0
7.
170.0
7.
90.4
91.7
92.9
185.0
186.8
188.3
88.5
90.0
91.4
184.2
186.2
187.8
1 The threshold setting is parenthetically included.
2 BI: Benefit measure 1 : gross sensitivity
B2: Benefit measure 2 : sum of gross sensitivity
and positive predictivity
-
- 148
5 percent minimum,
Table 4.1.22 Detector Performance Measures. Mean,
and 95 percent maximum expected values of the performance measures for
the ML and optimal relative-power detectors.
Sensitivity
1
IBP=96%
REL.POWER
B1
(.27)
B2max (.58)
ML (.60)
Ave.toSenytvpe
1
5% 1 Mean I 95%
=------
IlPredictivityI
5% 1 Mean 1
-------
95%
100
100
100
1.91 94.1
95.7
90.4
1
94.8
88.5
i=----
100
100
93.1
Positive I
Gross Sensitivity iIGross
115%
1
Mean
100
0.01
70.0
91.7
92.9
4.1
95.2
196
90.0
91.3
5.21
96.2
1
1
95%
1
-
DetectorAve.
1 The threshold setting is parenthetically included.
70.0
97.0
-
- 149
4.2.
RESULTS FOR THE AUTOREGRESSIVE DETECTORS
150
-
-
FEATURES FOR CLASS I
t -7
.6-
I
.2-1
/
S.
a2
-1
-. 2
6
-1
-2
-1.2
-. 4
.4
a1
1.2
Figure 4.2.1 Plot of the a and a 2 coefficient pairs estimated for
four-second segment of Ventricular Tachycardia (VT).
2
each
-
151
-
FEATURES FOR CLASS 2
1T
/
/
/
.1
N
.2--
a2
-. 2-
*
**
*:-*~. :~:
*1
-2
I
I
-1.2
-. 4
I
.4
1.2
Figure 4.2.2 Plot of the a1 and a 2 coefficient pairs estimated for
four-second segment of Ventricular Flutter (VFL).
2
each
-
- 152
FEATURES FOR CLASS 3
1
1
*1
.6
2-4
or
a2
-.2r
-
-.6
S.,
-1
_r
-2
t2_
_
-1.2
I
-. 4
.4
I
1
1.2
2
2
a1
Figure 4.2.3 Plot of the a and a 2 coefficient pairs estimated for
four-second segment of Ventricular Fibrillation (VF).
each
-
- 153
FEATURES FOR CLASS 4
/
/
I1
/
.6-
.24
1~
/
CL 2
-
-. 6
_L
*1
-2
-1.2
-. 4
.4
1.2
2
a1
Figure 4.2.4 Plot of the a1 and a 2 coefficient pairs estimated for
four-second segment of electrode motion noise (NOISE).
each
Table 4.2.5 Distribution of Database Segments Among Patients
MalignantArrhythmia
No. 4-Second Data Segments
Patient
IDatabase
Tape No.(s)
Number
0
2
3
4
5
6
7
8
9
10
11
12
13
14
15
TOTAL
VT
VFL
VF
0
51
58
1
0
2
12
0
37
53
62
16
12
0
0
8
0
219
54
0
0
5
0
17
3
0
396
10
0
162
112
39
0
0
0
0
0
0
0
0
0
0
0
323
10
j
2
12251
I 4 1
I
|
20
7
482
I
II
4
||
||
1 Patient ventricular arrhythmia episodes were partitioned
into 4-second segments.
418,419
420
421,422
423
424
425,605
426
427
428,429,430
602
607
609,610
611
612
614
615
-
- 154
PATIENT 0 FEATURE DISTRIBUTION
I
I
L
VFL
VT
-I
.6
-
-
.6
I
.2-. 2-. 6
-
-
-. 6
I
-1
I
-2
-1.2
.4
-. 4
1.2
2
-2
*
-1.2
I
*
-. 4
I
.4
1.2
2
VF
1.6-
UMBER OF EVENTS
.21
VT
a
VF1 62
VF
18
-. 2-. 6-t
I
2
*
I
-1.2
I
I
-. 4
.4
1.2
2
Figure 4.2.6 Plot of the a and a2 coefficient
pairs for each class
estimated for each of tie four-second segments from patient number 0.
The x and y axes are a, and a 2 respectively.
-
- 155
PATIENT 1 FEARRE DISTRIBUTION
VT
1
VYL
-
-
I
.6-
.2-
.2-
4
-. 6
-
.2-
-1
ir
-
~1
2
-t.2
-. 4
.4
1.2
2
-2
-1.2
-. 4
.4
I.
t.2
I
2
VF
-
1
-
.6
2-
NLR1ER OF EVENTS
VT 51
VFL 16
-.2-
VF
-
-. 6
1.j
-I
-2
-1.2
-. 4
.4
1.2
2
Figure 4.2.7 Plot of the a and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 1.
The x and y axes are a1 and a 2 respectively.
-
- 156
PATIENT 2 FEATIRE DISTRIBP
i
VT
:1-
VFL
I
I
-
1
TION
/
.6-
4
-_61
-
-. 6
-1
-2
-1.2
-. 4
I
I
.4
1.2
.
-1
I
2
-2
-1.2
-. 4
.4
1.2
2
VF
.6-
JUMBER OF EVENTS
.20
-. 2i.
-. 6-
VT
58
VFL.
12
VF
162
.11.~**
-1
-2
-1.2
-. 4
.4
1.2
2
Figure 4.2.8 Plot of the a and a 2 coefficient
pairs for each class
estimated for each of te four-second segments from patient number 2.
The x and y axes are a, and a 2 respectively.
-
- 157
PATIENT 3 FEATURE DISTRIBUTION
VT
VFL
i-
.2-. 2
-. 6
-. 6-
-
-
-. 2
-1
-1
-2
-1.2
.4
-. 4
1.2
2
-2
-1.2
.4
-. 4
1.2
2
-F
1
.6
HUKBER OF EVENTS
.2
--Il-
-. 2-
VT
1
VFL
VF
2
112
-. 6-I
-2
-1.2
-. 4
.4
1.2
2
Figure 4.2.9 Plot of the a and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 3.
The x and y axes are a1 and a2 respectively.
-
- 158
PATIENIT 4 FEATURE DISTRIBUTION
VFL
VT
-
1
1<
-.
.2-
2d
-
-. 6
-1
-1.2
.4
-. 4
1.2
2
-2
I
-1.2
a
-. 4
.4
1.2
w
i
2
VF
10MER OF EVENTS
2
VT
B
VFL B
VF 39
.3.
9%
-1
'
a
2
-1.2
-. 4
I
.4
I
,
1.2
5
2
Figure 4.2.10 Plot of the a, and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 4.
The x and y axes are a, and a 2 respectively.
PATIENT
-
- 159
FEATURE DISTRIBUTION
I
U
V..
VT
F.
-. 6-
-I
-2
-1.2
.4
-. 4
1.2
2
-2
-1.2
-. 4
.4
1.2
2
I
VF
1.6-
WJKBER OF EVENTS
.2-
VT 2
VFL 8
F 0
-. 2-. 6-t 1I
-2
- I
i
1
.4
1.2
.I4
2
t
.2
-.
____________________________________________________a
Figure 4.2.11 Plot of the a1 and a2 coefficient pairs for each class
estimated for each of the four-second segments from patient number 5.
The x and y axes are a1 and a2 respectively.
-
- 160
PATIENT 6 FEATURE DISTRIBUTION
VT
VFL
.6-
.2-
.2
-
.2-
-
-. 2
-1
-2
-1.2
-. 4
.4
1.2
2
-2
-1.2
-. 4
.4
1.2
2
VF
.6NUMBER OF EVENTS
.2VT
VFL
W
-. 2-.
-2
-1.2
-. 4
I.
.4
1.I
2
1.2
2
12
0
8
I
Figure 4.2.12 Plot of the a, and a2 coefficient pairs for each class
estimated for each of the four-second segments from patient number 6.
The x and y axes are a, and a2 respectively.
-
- 161
PATIENT 7 FEATJRE DISTRIBUTION
VT
VFL
-
I
.6
.2
.2
-. 2-
-. 2-
-
-
-
.6-
-. 6
f:~
-
-
-. 6
-1
I
-1.2
-2
.4
-. 4
*
I
.2
*
I
2
-1
I
-2
I
-L.2
'~
~
I...
I
-. 4
I
.4
I
1.2
*
I
2
VF
-
1
.6NUKEMR OF EVENTS
.2-
VT
8
VFL 219
.2
-
VF 0
-
.6
-t
I
-2
*
I
-1.2
I
-. 4
.4
1.2
2
Figure 4.2.13 Plot of the a1 and a2 coefficient pairs for each class
estimated for each of the four-second segments from patient number 7.
The x and y axes are a1 and a 2 respectively.
-
- 162
PATIENT 8 FEATURE DISTRIBUTION
VT
VFL_
1-
1
.6-
.6
.2-
.2
-. 2
)h.
-1
-2
-1.2
-. 4
.4
1.2
2
-2
-1.2
-. 4
.4
t.2
2
VF
1.6-
UMBER OF EVENTS
.2-
VT
VFL
VF
-. 2-
37
64
0
-. 6-
-2
I '- T1
-1.2
- - -~~4
-. 4
.4
'
T
'~
I2
t.2
2
2
Figure 4.2.14 Plot of the a, and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 8.
The x and y axes are a, and a 2 respectively.
-
- 163
PATIENT 9 FEATURE DISTRIBUTION
.6
.6
.2 -
--.
-. 2-
-2
.
-. 2-
--.
6-
.
-. 6 -
-'
-1.2
2-
-. 4
.4
1.2
2
-2
-1.2
.4
-. 4
1.2
2
VF
1.6
- JKER OF EVENTS
.2-
VT
VFL
VF
-. 2
53
0
-. 6
-2
-1.2
-. 4
.4
1.2
2
Figure 4.2.15 Plot of the a1 and a 2 coefficient pairs for each class
estimated for each of the four-second segments from patient number 9.
The x and y axes are a1 and a 2 respectively.
-
- 164
PATIENT 18 FEATRE DISTRIBUTION
VFL
VT
.5
.6-
.2
.2
4
-. 2
-.2-
1't
-.6
-2
-t.2
-1-I
-. 4
.4
-.6I
1.2
2
F
-2
-1.2
-. 4
.4
1.2
2
VF
.6JKMER OF EVENTS
.2
VT
VFL
VF
18
0
8
-. 6-
-2
-1.2
-. 4
.4
1.2
2
Figure 4.2.16 Plot of the a, and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 10
The x and y axes are a, and a 2 respectively.
-
- 165
PATIENT 11 FEATURE DISTRIBUTION
VT
VFL.
.6-
.6-
.2-
.2-
-. 2--.
-2
6-
--.
-. 6 -t.2
-. 4
.4
1.2
2
.6-
-2
-L.2
-. 4
.4
1.2
2
NUMBER OF EVENTS
.2-
VT
VFL
.2-
-. 6-
2
5
VF 8
2._ T
-2 -1.2
-.4
.4
1.2
2
Figure 4.2.17 Plot of the a 1 and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 11.
The x and y axes are a and a 2 respectively.
VT
-
- 166
PATIENT 12 FEATURE DISTRIBUTION
F
VFL
1-
/
-
ro
.2-
.2-
.
5
-2
-
-I
-1.2
-. 4
.4
1.2
-1
2
I
-2
-1.2
.4
-. 4
I
1.2
2
VF
.6NUMER OF EVENTS
VT
V1I_
VF
-. 2-
225
0
0
-I
,
-. 6-1.2
-. 4
.4
1.2
I
2
1019
Figure 4.2.18 Plot of the a, and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 12.
The x and y axes are al and a 2 respectively.
-
- 167
PATIENT 13 FEATRE DISTRIBUTION
VT
VFL
.6 -.
.2
.
6-
-. 2
-. 2
-. 6 -.
-2
--.
-1.2
-. 4
.4
61.2
2
-2
-L.2
-. 4
.4
.2
2
VF
.6NUMMBER OF EVENTS
.2-
VT 4
VFL 17
-. 6
-
-. 2
-2
-1.2
-. 4
.4
1.2
2
____
_________
Figure 4.2.19 Plot of the a 1 and a 2 coefficient pairs for each class
estimated for each of the four-second segments from patient number 13.
The x and y axes are a1 and a2 respectively.
-
- 168
PATIENT 14 FEATURE DISTRIBUTION
VT
VFL
.2-
.2-
-. 6-
-. 6
-2
-1.2
-. 4
.4
1.2
2
-2
-1.2
-. 4
.4
1.2
2
VF
NUKMR OF EVENTS
2VFL 3
-. 6I
-1
-2
-1.2
-. 4
.4
1.2
2
Figure 4.2.20 Plot of the a, and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 14.
The x and y axes are a, and a 2 respectively.
-
- 169
PATIENT 1 FEATURE DISTRIBUTION
VT
VFL
I
.6
.6-
.2-
.2-
-. 2-
-. 2-
-. 6
-. 6
-
-
1
I
.4
-1.2
-. 4
a.
1.2
-l
2
-1.2
-. 4
.4
1.2
2
VF
.6
WJKBER OF EVENTS
.2
-
VT
VFL
WF
-1.2
-. 4
.4
1.2
2
7
0
0
U
Figure 4.2.21 Plot of the a 1 and a 2 coefficient
pairs for each class
estimated for each of the four-second segments from patient number 15.
The x and y axes are a1 and a2 respectively.
CUMULATIVE DISTRIBUTION:
-
- 170
MAX. DEVIATION
.2338713918
1.0-
P
R
0
0.8-
A
B
I
Y
8.2-
8.0-8
-l.S
-0.9
-. 3
0.3
.9.5
VT a
Figure 4.2.22 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a. for
Ventricular Tachycardia (VT).
The Maximum deviation between the curves
is indicated on the graph.
CUMULATIVE DISTRIBUTION:
I
T
-
- 171
tAX. DEVIATION
.2S6691784
8.20.2
8.8-
87-.
-.
.
.
-10.8
VT .ll
Figure 4.2.23 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a 2 for
Ventricular Tachycardia (VT).
The Maximum deviation between the curves
is indicated on the graph.
-
- 172
MAX. DEVIATION
CtJLATIVE DISTRIBtTION:
07521427347
1.8-
P
R
0.8-
B
A
0.6-
L
I
0.4.
T.
y
8.2-
0.0-
-1.5
-i.e
-o.S
0.0
0.5
1.0
VFL
Figure 4.2.24 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a, for
Ventricular Flutter (VFL).
The Maximum deviation between the curves is
indicated on the graph.
CX?1LATIVE DISTRIBUTION:
-
- 173
AX. DEVIATION
.89338475414
1.8-
P
R
0
8
A
B
L
I
8.8-
0.6-
8.4.
T.
y
0.2-
-1.0
-0.7
-0.4
-0.1
0.2
0.5
VFL a
2
Figure 4.2.25 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a 2 for
Ventricular Flutter (VFL).
The Maximum deviation between the curves is
indicated on the graph.
-
- 174
CMJLATIVE DISTRIBUTION:
MAX. DEVIATION: .8867986t I
1.0-
P
0.8-
R
0
B
A
B.
0.6
L
8.4.
I
T
y
8.2-
8.8
-1.1B
-0.68
-0.26
0.16
0.58
1.00
VF a
Figure 4.2.26 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a1 for
Ventricular Fibrillation (VF). The Maximum deviation between the curves
is indicated on the graph.
CLtILATIVE DISTRIBUTION:
-
- 175
MAX. DEVIATION
.05833081540
1.9-
0.8
R
0
B
A
B
I
L
I
T
y
8
0.4
0.8 -
--1.0
,
,,
'-.6
r -
-0.2
0.2
I
0.6
-
8.2-
1.0
VF a
2
Figure 4.2.27 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a 2 for
Ventricular Fibrillation (VF). The Maximum deviation between the curves
is indicated on the graph.
CMtJLATIVE DISTRIBUTION:
P
-
- 176
MAX. DEVIATION
.8364411451
8.8-
R
0
8
A
B
I
L
I
T
y
8.6
.4
8.2
8.8
8.3
8.6
1.9
1.2
1.5
Figure 4.2.28 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a1 for
Electrode Motion Noise (NOISE).
The Maximum deviation between the
curves is indicated on the graph.
-
- 177
CQJLATIVE DISTRIBUTON:
P
MAX. DEVIATION: .8306S76489
8.84
R
0
B
A
.6
I
L
I
8.4
T
Y
0.2-
-
8.0
-0.58
-8.34
-0.18
-0.02
e.14
0.30
Figure 4.2.29 Relative Comparison of the Cumulative Gaussian Distribution Curve with the Estimated Cumulative Distribution Curve for a2 for
Electrode Motion Noise (NOISE).
The Maximum deviation between the
curves is indicated on the graph.
-
- 178
Table 4.2.30 P-values for verifing the Gaussian modeling assumption.
The p-values were calculated with the Kolmorgorov-Smirnov Test.
I
-T
a
1__2_1__2
Max.
Deviation
P-VALUE
1 .23387
0
.250671
0
aI
NOISE
VF
VFL
-
.
2
I
al
a
2
.07521
.093381
.06837
.058331
.03648
.030661
.02753
.00363
02058
.071291
.22669
.239981
I
-
- 179
DETECTOR OPERATINE CHARACTERI5TICS
DETECTOR i (VFL VS N ONLYI
GSE VS9. I-SP
GSE VS. THRESHOLD
100
-
100
< 70"'o'oo
80
at
60-
60
401
40
20
-
0
o
20
0
60
O
100
80
100
0
0
.2
84
.6
.8
1
'-U---..
G5E VS. GPPA
6PPA VS. THRESHOLD
100-
100-
80-
10-
6010
20
20I
0
a I
I
20
I
40
V
I
60
a
I
80
I
A.
I
100
V
a
t
0 2
x4
.6
of1
Figure 4.2.31 ML Detector 1 Characteristics for descriminating between
VFL and NOISE. Standard receiver operating characterisitcs (ROC) (i.e.,
the true positive rate vs. false positive rate as estimated by the gross
sensitivity
(gSE) versus 1 - gross specificity (1-gSP) (upper left).
System Operating Characteristic (SOC) ( gross sensitivity versus the
gross positive predictive accuracy
(gPPA) (lower left).
Gross Sensitivity versus threshold (upper right). Gross positive predictivity
versus threshold (lower right).
-
- 180
U
DETECTOR OPERATING CHARACTERISTICS : DETECTOR 2 (VFL VS N ONLY11
U
GSE VS. I-GSP
GSE VS, THRESHOLD
100-
8060-
20-
20-
.-,.-
0*
1
20
90
60
o0
100
1
.1
.2
0
.5
.9
1
I--
ESE VS. 6PPA
EPPA VS. THRESHOLD
100
100-
30
-
'
30
ML
60-
60
40-
20
1I
I.
-
20
0 I -0
0
0
20
40
60
80
100
a
i
I
a.E
.
I
1
26
29
I
Figure 4.2.32 ML Detector 2 Characteristics for descriminating between
VFL and NOISE.
Standard receiver operating characterisitcs (ROC) (i.e.,
the true positive rate vs. false positive rate as estimated by the gross
sensitivity (gSE) versus 1 - gross specificity (1-gSP) (upper left).
System Operating Characteristic (SOC) ( gross sensitivity versus the
gross positive predictive accuracy
(gPPA) (lower left). Gross Sensitivity versus threshold (upper right). Gross positive predictivity
versus threshold (lower right).
181
-
-
Table 4.2.33 Performance Measures for the ML Detectors 1 and 2.
Detector Te 1
I ~P
Deeco Type
DE TECTOR 1ML (1.0)
DE99.
I ML (1.0)
Predicti vity
5%OMean 15%
---
187.91 90.6
Mean
95%
--===
5%
Mean
== === ==
93.0 182.81 86.0
88.7
9I4.71
5
l8
-
5%
-
Positive
dGross
Ave. Sensitivityi Gross Sensitivity
P4.51 90.8 197.9 181.41 87.2 195.5 1 0.11
1 The threshold setting is parenthetically
included.
95%
== =
96.8
95.9
98.4
1
98.4
-
- 182
VFL
AND
WITH CAUSSIAN
NOISE DISTRIBUTIONS
VEHTPICULHP
C I SE
1-
FLUTTER
CONTOURS
PROBHBIL'ITY
.cj
'I+
-2
-1,2
-. 4
.4
-1.2-.4
2
NOISE
.4
.d
2-2
DETECTOR 1
-1
-1.2
-. 4
.4
1.
2
.,++
-1.2
-4
,4
1.2
2
2---
DETECTOR 2
-12
2
UPE S4ND IDN11UR CURV ES
FEAT
-1NTOU0UR
-2
1 .2
4
2
(VFL) and electrode motion noise
Figure 4.2.34 Ventricular Flutter
(NOISE) feature space distributions and their Gaussian model probability
contours (for the original database.) Distribution of all patient a and
a2 coefficient pairs for VFL(upper left) and NOISE (upper right). 6aussian conditional probability contours (level sets) for the probability
distributions equal to .95 (lower left). The curve encircling the horizontal axis is the noise contour. The other two contours are from the
two different estimates of the covariance matrix for VFL. The inner
ellipse is from the distribution modeled with a gross covariance matrix,
while the outer is due to the average covariance matrix. Superposition
of the three figures (lower right) illustrate why the Detector 2 with
the broader probability distribution has 1) the higher mean senstivity
to VFL , and 2) lower mean gross positive predictivity than Detector 1
(over the original database.)
:
PERFORNANCE NEASURES
-
- 183
NL DETECTOR I (VFL VS. N ONLY)
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.5-
.5-
.!-
.1-
070
0i6
82
83
94
100
70
76
32
8e
91
100
EROSS P05 PREDICTIVITY
.2-
70
6
92
88
94
NEAR
5% LIN
AV SE
90.6
87.9
ER SE
ER #P
86.0
96.8
92.8
91.7
100
Figure 4.2.35 Bootstrapped performance measures for Detector 1 (AR(2)
Gross Covariance Matrix Detector).
The histograms were generated by
calculating the performance measures for 5000 (bootstrapped)
databases.
Average sensitivity (upper left), gross sensitivity (upper right), and
gross positive predictivity (lower left). The mean and five percent
minimum expected values of the performance measures are included in the
table (lower right).
5-~
-
- 184
PERFORMANCE MEASURES : NL DETECTOR 2
(VFL VS. N ONLY)
U
GROSS SENSITIVITY
AVERAGE SENSITIVITY
.5
.s-
.1
a
.2
31
~.4d4ffILAfflhl1
0*'10
82
'15
9
as
100
'10
16
BE
88
94
100
GROSS P05 PREDICTIVITV
.5sq.-
a-
AV SE
ER SE
ER +P
.2-
MEAN
5% LIN
90.98
879.2
95.9
8.s
81.
90.1
.10
I
f0
T
16
I r
I
82
-. Aallia
F
88
I
94
:
100
____________________________________________________m
Figure 4.2.36 Bootstrapped performance measures for Detector 2 (AR(2)
Average Covariance Matrix Detector). The histograms were generated by
calculating the performance measures for 5000 (bootstrapped) databases.
Average sensitivity (upper left), gross sensitivity (upper right), and
gross positive predictivity (lower left).
The mean and five percent
minimum expected values of the performance measures are included in the
table (lower right).
-
- 185
NAXINAL BENEFIT NEASURES FOR DETECTORS I AND 2
Bi : 65E
100
95go
US
10
s1
2
fSE
82 : GPPA +
200
I I
192
188134
0
1
2
Figure 4.2.37 Optimal and ML Benefit Measures for Detectors 1 and 2.
KEY:
(+) corresponds to the maximum value of the benefit measures over
all possible detectors of types 1 and 2.
(-) corresponds to benefit
measures of the Maximum Likelihood detectors for Detector 1 and 2
schemes. The first benefit measure is gross sensitivity (gSE) to
VT,VFL, and VF as a combined classes (top). The second benefit measure
is the sum of gross sensitivity and gross positive predictivity
(gSE+gPPA) (bottom).
-
- 186
Table 4.2.38 Optimal and ML Benefit Measures for Detector Classes 1
2.
Detectors
Detector
Type
1
I
eT
.001t
2
Max. Likelihood Detector
||
-
Optimal
.021
B1a
6
T
100(69.9)
.551
100(69.9)2
.541
and
-----
B2
T
B1
B2
192.1
1.0
92.9
191.8
192.23I1.0
93.74
192.05
1 Detector threshold setting.
2 Optimal detector with respect to Benefit Measure 1.
4 Optimal detector with respect to Benefit Measure 2.
Best ML detector with respect to Benefit Measure 1.
5 Best ML detector with respect to Benefit Measure 2.
6 The Gross Positive Predictivity (gPPA) is listed parenthetically.
Detector 1 : AR(2) Gross Covariance Matrix Detector
Detector 2 : AR(2) Average Covariance Matrix Detector
-
- 187
DETECTOR OPERATING CHARACTERISTICS
:
DETECTOR I
GSE VS. THRESHOLD
GSE VS. 1-ESP
110-
"
100
t 't~
1
tML tB2Bo
s0
60-
6090
90-
20
1
0
20
90
60
80
20
0
100
.2
.9
.5
.
i
EPPA VS. THRESHOLD
GSE VS. EPPA
100
110
Boo
B2
80t ML
90
90
20
20
0
20
90
60
90
100
0
.
a2
6
as
Figure 4.2.39 Detector Characteristics
Type 1 Detector.
Standard
gross sensitivity vs. 1
receiver operating characteristics (ROC) (i.e.,
- gross specificity)
(upper left).
System operating characteristic
(SOC)
(i.e., gross sensitivity vs.
gross positive predictivity) (lower
left).
Gross sensitivity vs. threshold (upper right). Gross positive
predictivity vs. threshold (lower right).
-
- 188
DETECTOR DPERATINE CHARACTERISTICS
GSE VS. I-ESP
:
DETECTOR 2
GSE VS. THRESHOLD
100
100
80
t7-t
9
T2
60-
60-
90
0
20
20
0
20
40
60
80
0
100
GSE VS. GPPA
100 -
.6
.8
I
GPPA VS9. THRESHOLD
t
0
1
IB
60-
60
90
90
0
.9
e0
90
60
80
100
.
30I
El
.2
0
.2
s9
.A
vs8
Standard
Type 2 Detector.
Figure 4.2.40 Detector Characteristics
receiver operating characteristics (ROC) (i.e., gross sensitivity vs. 1
System operating characteristic
(upper left).
- gross specificity)
(SOC) (i.e., gross sensitivity vs. gross positive predictivity) (lower
left). Gross sensitivity vs. threshold (upper right). Gross positive
predictivity vs. threshold (lower right).
-
- 189
BENEFIT NEASURES AS A FUNCTION OF THRESHOLD
:
DETECTOR I
BI: GSE
100-
90B2
90
751
0
.6
1.E
BE:
1.8
2.
a
2.1
3
SE4EPPA
200192-A
AA
tB2
fet-
t
175168. ti
1600
.6
1.2
1.8
Figure 4.2.41 Benefit measures as a function of threshold setting for
Detector 1.
Benefit measure 1 (gross sensitivity) (top) and benefit
measure 2 (sum of the gross sensitivity and positive predictivity.)
(bottom).
The curves indicate that the benefit measures are quite
robust with respect to their threshold setting.
-
- 190
BENEFIT NEASURES AS A FUNCTION OF THRESHOLD
:
DETECTOR 2
BI: USE
o
.5
1.2
mE:
1.8
2.1
3
EtEPPA
200192181
tB2
ML
196
a 21
16*
.3
,S . .. . , 'S
I5
Figure 4.2.42 Benefit measures as a function of threshold
setting for
Detector 2.
Benefit measure 1 (gross sensitivity) (top) and benefit
measure 2 (sum of the gross sensitivity and positive predictivity.)
The curves indicate that the benefit measures are quite
(bottom).
robust with respect to their threshold setting.
-
- 191
D005TRAPPED DETECTOR CHARACTERISTICS : DETECTOR I
GSE V5, THRESHOLD
GSE V5, I-G5P
94
96
88-
94-
62
9019
92 -
920I
16-
20
%o
60
o0
0
100
98
99-
96-
8e-
92-
7620
20
O
40
6
60
.8
.6
.1
I
GPPA VS8 THRESHOLD
GSE VS. GPPA
I0
0
.2
0
80
1
110
0
0
.1
.2
I
4
I
I 8
.s
-
0
-
go -
.6
I
Figure 4.2.43 Bootstrapped Detector Characteristics : Type 1 Detector.
Standard receiver operating characteristics (ROC) (i.e.,
gross sensitivity vs. 1 - gross specificity)
(upper left).
System operating
characteristic (SOC) (i.e., gross sensitivity vs.
gross positive
predictivity) (lower left).
Gross sensitivity vs. threshold (upper
right). Gross positive predictivity vs. threshold (lower right).
-
- 192
BOOSTRAPPED DETECTOR CHARACTERISTICS : DETECTOR 2
GSE VS. I-GSP
GSE VS, THRESHOLD
100
"
1 g0
BE76.
9!M
0
20
40
60
80
0
100
ESE VS. GPPA
100-
9696
sa89-
92-
76
1
21
26
010
30
1 0.1
1 .17
41
60
86
.4
.6
.8
1
GPPA VS. THRESHOLD
100
90o
.2
160
1
.2
.4
A6
.so
Figure 4.2.44 Bootstrapped Detector Characteristics : Type 2 Detector.
Standard receiver operating characteristics (ROC) (i.e., gross sensitivity vs. 1 - gross specificity)
(upper left).
System operating
gross positive
(SOC) (i.e., gross sensitivity vs.
characteristic
predictivity) (lower left).
Gross sensitivity vs. threshold (upper
right). Gross positive predictivity vs. threshold (lower right).
193
-
-
PERFORMANCE NEASURES : OPT 3I
AVERAGE SENSITIVITY
GROSS SENSITIVITY
1.u-
1.1-
1.12-
1.12-
.56
.56-
. 28
.28
65
12
19
86
93
DETECTOR I
100
65
12
19
86
93
100
GR055 P05 PREDICTIVITV
1.1-
.89
AV SE
GR SE
GR #P
.56-
NEAN
5% LIN
100
100
10.0
100
100
69.9
.28ES
12
'15
SE
93
100
Figure 4.2.45 Bootstrapped performance measures for the optimal Detector
1 with respect to B1. (Threshold = .001) Average sensitivity (upper
left),
gross sensitivity (upper right), and gross positive predictivity
(lower left).
The included table (lower right) tabulates the mean and
five percent minimum expected values of the performance measures.
-
- 194
PERFORNANCE NEASURES
:
OPT 82
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.8q
.6
.8Ig
.5-
it
i6
BE
DETECTOR I
is
9
Ito
io
I'
BE
as
94
100
GROSS FOS PREDICTIVITY
.NEAN
S LIN
.6
AV SE
IR SE
fR #P
84-
p
16
BE
is
99
932.
939.
98.0990
91.1
92.5
10
Figure 4.2.46 Bootstrapped performance measures for the optimal Detector
1 with respect to B2. (Threshold = .551) Average sensitivity (upper
left), gross sensitivity (upper right), and gross positive predictivity
The included table (lower right) tabulates the mean and
(lower left).
five percent minimum expected values of the performance measures.
195
-
-
PERFORMANCE NEASURES : NL DETECTOR I
AVERAGE SENSITIVITY
GR0SS SENSITIVITY
.8-
I0
59
'1
82
88
99
100 rig
0- 8 88
9100
0
I
63055 P05 PREDICTIVITY
.16-
0'10
AV SE
GR SE
GR fP
15
82
88
99
NEAN
5% LIN
91.8
92.9
98.8
89.3
91.
98.0
100
.
Figure 4.2.47 Bootstrapped performance measures for the ML Detector 1
(Threshold = 1.0) Average sensitivity (upper left), gross sensitivity
(upper right),
and gross positive predictivity (lower left).
The
included table (lower right) tabulates the mean and five percent minimum
expected values of the performance measures.
-
- 196
PERFORMANCE MEASURES
:
OPT 81
DETECTOR 2
GROSS SENSITIVITY
AVERAGE SENSITIVITY
1.4-19
1.1!-
1.12SSol
.569
.56-
. 28
,28-
0
- , . , - , . , .0
65
'1 19
SE 93 100
ES
'2
19
86
93
100
GROSS FOS PREDICTIVITV
.*S~AV
SE
NEAN
5% LIN
100
10.0
100
69.8
100
100
GR SE
GE fP
.56.28
65
12
'9
85
9a
100
Figure 4.2.48. Bootstrapped performance measures for the optimal Detector 2 with respect to B1. (Threshold = .021) Average sensitivity (upper
left), gross sensitivity (upper right), and gross positive predictivity
(lower left).
The included table (lower right) tabulates the mean and
five percent minimum expected values of the performance measures.
-
- 197
PERFORNANCE NEASURES : OPT 12
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.8-
8
. -
.6-
04-
.q-
10
16
82
88
91
DETECTOR 2
100
f0 76
82
8
91
100
6fOSS P05 PREDICTIVITY
.o]
.56
AV SE
GR SE
GR #P
54-
KEAN
5% LIN
931.
93.9
98.0
s1.9
92.
s.0
010
15
82
88
91
100
Figure 4.2.49 Bootstrapped performance measures for the optimal Detector
2 with respect to B2. (Threshold = .541) Average sensitivity (upper
left), gross sensitivity (upper right), and gross positive predictivity
The included table (lower right) tabulates the mean and
(lower left).
expected values of the performance measures.
minimum
five percent
-
- 198
PERFORMANCE NEASURES : NL DETECTOR 2
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.8
.8
.6-
.6
0-
10
i6
92
98
94
100
070
"6
92
18
91
100
EROSS P05 PREDICTIVITV
.5
AV SE
GR SE
GR #P
.-
10
'1
82
88
94
NEAN
5% LIN
93.5
93.7
98.e
91.8
92.3
91.3
100
.
Figure 4.2.50 Bootstrapped performance measures for the ML Detector 2
(Threshold = 1.0) Average sensitivity (upper left), gross sensitivity
(upper right), and gross positive predictivity (lower left).
The
included table (lower right) tabulates the mean and five percent minimum
expected values of the performance measures.
-
- 199
BOOTSTRAPPED BENEFIT NEASURES : OPT B1 DETECTOR I
It
:
G5EVS
.8-
12 :
ENS
GPPA
so
.6
165
171
177
183
189
195
Figure 4.2.51 Histogram distribution of the benefit measures for the
optimal detector with respect to B1 for Detector Type 1. Bi: gross sensitivity (top) and B2 : sum of the gross sensitivity and positive
The histograms were constructed by calculating
predictivity (bottom).
the benefit measures for 5000 (bootstrapped) databases.
-
- 200
OPT 82 DETECTOR I
*OUTSTRAPPED BENEFIT NEASURES
ii
G5EMS
.3-
.18
.12.@6-
v661
95
98
91
32 6 &EUS
9
9
100
189
15
+ GPPA
.18
.12-
165
171
177
193
Figure 4.2.52 Histogram distribution of the benefit measures for the
optimal detector with respect to B2 for Detector Type 1. Bi: gross sensitivity (top) and B2 : sum of the gross sensitivity and positive
predictivity (bottom).
The histograms were constructed by calculating
the benefit measures for 5000 (bootstrapped) databases.
-
- 201
BOOTSTRAPPED BENEFIT NEA5URES
81
:
:
ML DETECTOR 1
SENS
23
BiE-
.18'
.12
06
15
31
91
82 :
94
97
100
189
15
SENS + 6PPA
.18-
.0165
111
117
183
Figure 4.2.53 Histogram distribution of the benefit measures for the ML
detector for Detector Type 1. Bi: gross sensitivity (top) and B2 : sum
of the gross sensitivity and positive predictivity (bottom). The histograms were constructed by calculating the benefit measures for 5000
(bootstrapped) databases.
-
- 202
800TSTRAPPED BENEFIT NEASURES
OPT
O 9I DETECTOR 2
.9-
95
99
91
9G
97
100
119
195
12 : ESES + GPPA
.8
155
171
177
1U3
Figure 4.2.54 Histogram distribution of the benefit measures for the
optimal detector with respect to B1 for Detector Type 2. Bl: gross sensitivity (top) and B2
sum of the gross sensitivity and positive
predictivity (bottom).
The histograms were constructed by calculating
the benefit measures for 5000 (bootstrapped) databases.
-
- 203
:
BOOTSTRAPPED BENEFIT NEASURES
Bi
:
OPT 12 DETECTOR 2
ESENS
.3
.18
.1!
.06
085
88
i1
12
9497
:
1
GSENS + GPPA
a
.18.94-
165
171
177
ie
189
195
Figure 4.2.55 Histogram distribution of the benefit measures for the
optimal detector with respect to B2 for Detector Type 2. Bi: gross sensitivity (top) and B2 : sum of the gross sensitivity and positive
predictivity (bottom).
The histograms were constructed by calculating
the benefit measures for 5000 (bootstrapped) databases.
P
-
- 204
BOOTSTRAPPED BENEFIT NEASURES : NL DETECTOR 2
It : U5ENS
.Blo
.18
.12
.46
85
a8
91
94
91
100
189
195
12 : SENS * GPPA
.81
.12
.#6155
loll
17
183
Figure 4.2.56 Histogram distribution of the benefit measures for the ML
detector for Detector Type 2. Bi: gross sensitivity (top) and B2 : sum
of the gross sensitivity and positive predictivity (bottom). The histograms were constructed by calculating the benefit measures for 5000
(bootstrapped) databases.
-
- 205
Table 4.2.57 Detector Benefit Values (Mean, 5 percent minimum, and 95
percent maximum expected values for the BI and B2 benefit measures for
the optimal and ML Detector 1 and 2 detectors.)
Detector Typel
III
Benefit Measure B1 2
5% Min
Mean
|
---II
II
Benefit Measure B2 3
95% Max
5% Min
Mean
95% Max
DETECT69 I
IBI max
(.001)
100
100
100
171.1
171.1
171.1
B2
(.551)
92.5
93.9
95.0
190.3
191.9
193.1
91.4
92.9
94.1
190.1
191.7
192.9
DETCTUR 2
IB1 max
B2
max
..
.=
.j..
..
.. .
.
ML (1.0)
(.021)
100
100
100
170.1
170.1
170.1
(.54)
92.4
93.9
95.0
190.3
191.9
193.1
92.3
93.7
94.8
190.3
191.9
193.1
ML (1.0)
1 The threshold setting
is parenthetically included.
2 Bi: Benefit measure
1 : gross sensitivity
B2: Benefit measure 2 : sum of gross sensitivity
and positive predictivity
-
- 206
Table 4.2.58 Mean, 5 percent minimum, and 95 percent maximum expected
values of the performance measures for the ML and optimal Type 1 and 2
detectors.
Positive
vi . Gross
ctivity
ItPredi
95%
5% Mean
5%
Mean 1 95%
Mean I
5%
===b=
DE TE CTOR1I------=======-=----
Detector Type
B1
max
B2
1001
1 100 111
100 1
9391
100
1 100
1~
9.9
II"1001 I
(.68)
91.
93.2
94.7 192.
93.9
95.0
"89.
91.8
93.71191.
92.9
93.9 I8.0
DETECTOR21
(.02)
max
B2
Gross S
(.01)
ML (1 0)
B1
Ave. Sensitivity
(.94)
ML (1.0)
97.
70.0
70.0
98.0
98.5
98.8
99.3
70.0
70.0
-==
100
'.11
1.9 93.7
"100
1.8
I
100
95.2
93.5 195.1
1100t
I1
100
1
100
1L
92.41 93.9
1192.31
1
93.7
9.91
----
1
95.0
7.0
98.0
98.8
94.8
7.31
98.2
98.9
1 The threshold setting is parenthetically included.
PERFORMANCE MEASURES
-
- 207
: ML
DETECTOR i (BS BY EVENTSI
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.8-
.6
82-
62-
i
W
BE
so
94
too
70
16
82
88
91
too
EROS5 POS PREDICTIVITV
.8AV SE
ER SE
#P
.qR
10
i6
92
38
91
NEAN
5% LIN
sits
ga.
98.8
89.3
91.4
9810
100
Figure 4.2.59 Performance measures for the Type
1 ML
detector
bootstrapped over events.
The histograms were generated by calculating
the performance measures over 5000 double bootstrap iterations.
Average
sensitivity
(upper left),
gross sensitivity (upper right), and gross
positive predictivity (lower left). The included table (lower right)
tabulates the mean and five percent minimum expected values of the performance measures.
i
208
PERFORMANCE MEASURES
:
-
-
ML DETECTOR 2 IBS BY EVENTS)
AVERAGE SENSITIVITY
GROSS SENSITIVITI
1-
1-
.8-
.8-
.5-
.6-
0
70
76
82
88
I
94
0
100
70
Jr
bI~.
-
76
i2
98
94
100
ER055 POS PREDICTIVITY
as.5.9.
U
1 T9 T W I
t a 75
B2
as
I I
91
'1
J
AV SE
GR SE
ER #P
MEAN
5% LIN
935
93.7
98.2
91.8
92.3
97.3
100
a
detector
2 ML
Figure 4.2.60 Performance measures for the Type
The histograms were generated by calculating
bootstrapped over events.
Average
the performance measures over 5000 double bootstrap iterations.
gross sensitivity (upper right), and gross
sensitivity
(upper left),
positive predictivity (lower left). The included table (lower right)
tabulates the mean and five percent minimum expected values of the performance measures.
-
- 209
PERFORNANCE NEASURES : NL DETECTOR 1 18S BY PATIENTS)
AVERAGE SENSITIVITY
GROSS SENSITIVITY
.9
io
.8-
16
iE
88
94
100
10
16
82
8
94
100
GROSS P05 PREDICTIVITY
.8
s6
AV SE
ER SE
. +P
10
16
82
83
;9
NEAN
5% LIN
92.2
93.0
98.5
88.2
95.1
ga.
log
detector
1 ML
the Type
measures for
Figure 4.2.61 Performance
calculatwere
generated
by
The
histograms
bootstrapped over patients.
ing the performance measures over 5000 double bootstrap iterations.
Average sensitivity (upper left), gross sensitivity (upper right), and
The included table (lower
gross positive predictivity (lower left).
right) tabulates the mean and five percent minimum expected values of
the performance measures.
The Libraries
Massachusetts Institute of Technology
Cambridge, Massachusetts 02139
Institute Archives and Special Collections
Room 14N-118
(617) 253-5688
This is the most complete text of the
thesis available. The following page(s)
were not included in the copy of the
thesis deposited in the Institute Archives
by the author:
-
- 211
PERFORNANCE NEASURES
:
NL DETECTOR 2 IBS BY PATIENTS)
GROSS SENSITIVITY
AVERAGE SENSITIVITY
.8-
.8-
.6-
.6
.14
.2
10
.2
16
82
88
94
100
10
76
82
88
9t
100
GROSS P05 PREDICTIVITY
.8
.
AV SE
GR SE
ER +P
.4-
710
76
92
88
9
NEAN
5% LIN
93.14
93.6
97.
9.1
88.1
94.3
100
Figure 4.2.62 Performance measures for the Type
2 ML
detector
bootstrapped over patients.
The histograms were generated by calculating the performance measures over 5000 double bootstrap iterations.
Average sensitivity (upper left), gross sensitivity (upper right), and
gross positive predictivity (lower left).
The included table (lower
right) tabulates the mean and five percent minimum expected values of
the performance measures.
-
- 212
Chapter 5
5.
DISCUSSION
Chapter 3 discussed the design theory of three detection schemes to
noise
discriminate
also
detailed
bootstrapping
respect
to
discusses
Chapter 3
and artifact from malignant arrhythmias.
the
analysis
tools
(e.g.,
ROC
and
SOC
curves
and
techniques) to be used to select an optimal detector with
some
criteria
(i.e.,
benefit
measures.)
This
chapter
the results of the design and analysis phases in the develop-
ment of these three detector genera.
To facilitate the discussion,
the following terms
are
defined
or
restated from earlier chapters.
1) The following is the notation for the four data classes:
VT, Class 2 = VFL, Class 3 = VF, and Class 4 = Noise = N.
Class
1
=
Class V = the
combined classes of VT,VFL, and VF.
2) The "peak frequency(F)" of a spectrum is the frequency at
which
the
describes how the width of
the
spectrum is maximal.
3) The "inner-bandwidth percentage"(IBP)
inner
band of the relative-power detector is selected.
For example, an
inner-bandwidth percentage of 90% means that the inner band is
about
the
centered
spectral peak (at peak frequency F) and extends from .55F to
1.45F.
4) A "genus of detectors" is a group of detectors with the
strategy.
In
this
study,
there
same
design
are three genera of detectors, each
-
- 213
denoted by the shorthand notation below
a) Detector 0 :
tor")
This
genus
detector
was
"reference
or
(also called the "relative-power detector"
in this study as a
implemented
of
reference for comparison with the results
detec-
following
the
two
novel autoregressive detectors.
b) Detector 1 :
(also called the "AR(2)
Gross Covariance Matrix Detector" or "Type
1 Detector")
c) Detector 2
(also called the "AR(2)
Average
Covariance
Matrix
Detector"
or
"Type 2 Detector")
5) A "detector class" is a subset of a detector genus.
only
the
relative-power
of
curve is
this
study,
detector genus has a set of detector classes.
Each class is specified by a different
class
In
inner-bandwidth
percentage.
A
Every point on the ROC
detectors defines a unique ROC curve.
a "specific" detector within that class and is uniquely
speci-
fied by decision costs and a-priori probabilities or by some fixed value
of a benefit function.
(Refer to section 3.4.1.)
6) A detector is "optimal" in the sense that it
to a specific benefit measure.
is the best with respect
Since there are two benefit measures (Bi
= gSE, and B2 = gSE+gPPA ), it is likely that there
will
be
two
dif-
ferent optimal detectors, each best with respect to one cost function.
-
- 214
7) The notation optimalBI and optimalB 2 means a
detector
optimal
with
respect to benefit measures BI and B2 respectively.
The analysis of
The results are presented in the following scheme.
Detector
0 is presented first. Subsequently, the analysis of Detector 1
and 2 are presented in parallel because of their similiar design nature.
A
of
comparison
all
three
detectors is discussed in chapter 6.
The
analysis strategy was to first find the best detector within each of the
three
detector
and then to find the overall optimal detectors
classes
among all detector classes.
5.1.
DISCUSSION OF THE REFERENCE DETECTOR RESULTS
Figure 4.1.1 shows the distribution of the frequency
spectral
peak
for
each
of
of
the
main
the four classes over the entire database
bimo-
(i.e, peak frequency distribution.) Classes VT,VFL, and VF show a
dal
distribution.
The
peaks below 1.3 Hz in each of these classes is
due to the widely changing baseline in some four-second
upper
mode
corresponds
to
the
peak
frequency
arrhythmia in the remainder of the segments.
The
segments.
distribution
distribution
The
of the
of
the
peak frequency for noise is generally below 2 Hz.
The feature of interest in the relative power detector is the ratio
(R)
of
power
in a small bandwidth centered about the spectral peak to
the power in an larger
outer
bandwidth.(See
section
3.2.1)
experiments, the outer bandwidth was fixed from 1.5 to 24 Hz.
bandwidth was a percentage of the peak frequency
observation.
for
each
For
all
The inner
four-second
Thus the center and absolute width of the inner bandwidth
varied with each observation; however, the inner bandwidth
was
a
con-
stant
-
- 215
the sense that it was the same relative width (percentage) of
in
the peak frequency (i.e., a fixed IBP.)
As described above, any fixed IBP defines
The
the
IBP
a
class
of
from 40% to 150% of the peak frequency.
varied
following
inner
bandwidth
percentages
Specifically,
were
tested
40,50,60,70,80,90,92,94,96,98,100,110,120,130,140,150.
Thus
classes of the relative power genus detector were studied.
next
three
figures
(figures
4.1.2-4)
detectors.
sixteen
Each of
the
shows the distributions of the
feature R for VT,VFL,VF, and Noise (for different inner
bandwidth
per-
centages.)
Comparing these figures shows that as the inner
tage increases, the mean of each
bandwidth
distribution for each class increases.
Note however that the shape of the noise distribution is
stant
in
comparison
with
of
segment.
the
outer
relatively con-
the changes in histogram shape of the other
classes. The peak frequency of the spectrum is
range
percen-
estimated
only
in
the
bandwidth and not the entire spectrum of the data
Since the outer bandwidth is from 1.5 Hz to 24
Hz,
and
most
noise segments have their spectral peak below 1.5 Hz (see figure 4.1.1),
the spectrum of noise in the region for estimating R is relatively flat.
Thus,
increasing the IBP should increase R linearly for each noise data
segment.
This causes the mean of the distribution of
R
for
noise
to
increase while maintaining the same distribution shape.
Figure 4.1.2 shows that even for a small
tage,
inner
the R distribution for VFL is large near R=1.
bandwidth
This is reasonable
since many VFL segments have narrowly peaked spectrum and thus
the
power
would
be
concentrated
tightly
about
percen-
most
of
the peak frequency.
-
- 216
percentage
Figures 4.1.3 and 4.1.4 show that as the inner bandwidth
increased,
the histograms are exponentially increasing near R=1.
the spectra of VF and VT are not as concentrated as that
for
is
Since
VFL,
the
histograms of VF and VT are not as concentrated near R=1 as for VFL.
The ML detectors for each class of relative power detectors
(i.e.,
for each inner percentage bandwidth) were found by determining where the
conditional probability of R given that the observation
was
Noise
was
equal to the conditional probability of R given that the observation was
VT,VFL,or VFL (i.e.,not Noise).
That is,
p(RIN) = p(RIV).
Figures 4.1.5-7 display the conditional
of
distributions
estimated by the histograms of the distribution of R.
as
R
probability
4.1.5 shows the conditional distributions for R of
percentage
an
inner
The distributions are equal at R=.51.
of 40%.
bandwidth
Figure 4.1.6
percen-
shows the conditional distributions of R for an inner bandwidth
tage
of
96%.
A
threshold
Figure 4.1.7 show the
hold of .71.
of .6 gives the ML detector for this IBP.
conditional
bandwidth percentage of 150%.
Figure
distributions
of
R
for
an
inner
The ML detector is specified for a thres-
The benefit values for these detectors are given in
Table
4.1.9.
One could imagine sliding a verti.cal bar horizontally across a plot
of
the
4.1.5).
specifing
conditional
distributions
of
R from 0 to 1 (e.g., for figure
A fixed position of the bar along the axis would correspond
a
particular
detector.
observation was not noise if
The
detector
to
would decide that an
the estimate of R for that observation fell
-
- 217
to the right of the bar and noise if it fell to the left.
performance
evaluated
measures,
and
hence
the
benefit
from
functions,
for every fixed postion of the threshold.
of the benefit functions were recorded as the
0
to 1.
In addition,
values
were
measures
figure 4.1.8.
be
The maximum values
threshold
recorded
Thus, for each
was
detector
increased
class,
BImax, B 2 max, B1ML, and B 2 ML .
values and their thresholds are shown in Table 4.1.9.
benefit
could
the values of the benefit functions for the
ML detectors were also recorded.
benefit
Likewise, the
Plots
four
These
of
these
as a function of inner bandwidth percent are shown in
(Maximum benefit values are denoted
by
a
(+).
Maximum
Likelihood benefit values are denoted by a (-).)
Figure 4.1.8 shows a plot of the maximum and
for
each inner bandwidth'percent.
ML
benefit
As the top graph indicates, for each
inner bandwidth percent, there is at least one detector which
fect
sensitivity
has
with
per-
(gSE=100%) to VT,VFL, and VF. The best detector among
all the detectors which could achieve perfect gross sensitivity
one
measures
is
the
the fewest false alarms (i.e., the highest gPPA.) All classes
of detectors achieved the same highest gPPA (gPPA=70.0%) and
perfect gross sensitivity.
maintained
Thus all IBPs produce detectors optimal with
respect to the first benefit measure.
The same figure indicates that the gross
detectors
sensitivity
varied as a function of inner bandwidth percent.
for
the
The maximum
sensitivity for a ML detector was 90.0% and found for detectors with
inner
bandwidth
percentage of 96 or 98%.
ML
an
As Table 4.1.9 indicates (as
expected), the gPPA for the ML detectors were significantly higher
than
those detectors which were specified to be maximim sensitivity detectors
-
- 218
(i.e., optimalB1 detectors.)
The bottom of figure 4.1.8 displays the second benefit values
for
both ML optimalB2 detectors.
This graph shows that the optimal and
ML benefit values are nearly equal for most
indistinguishable
150%.
for
inner
(B2)
bandwidth
detector
classes
and
are
percentages of 40,70, 120, and
The optimalB2 detector is specified by an IBP of 96 or 98% and
a
Likewise, the best ML detector is the ML detector for
threshold of .58.
an IBP of 96 or 98% (and specified by the threshold of .60.)
Table 4.1.9 tabulates the values plotted in figure 4.1.8 as well as
the
threshold
relative-power detectors.
with
an
inner
percent
optimal detectors.
the ML and best detectors for each class of
for
values
This table indicates that detectors
designed
width of 96 or 98% can produce the best ML and
The table implies two other facts.
First, for
each
IBP, the threshold for the optimal detector with respect to B1 is significantly less than thresholds of
optimal
is
with
respect to B2.
the
ML
detectors
or
the
detectors
Second, the threshold for the ML detector
greater than or equal to the threshold for the optimal detector
respect
with
to B2 for 40 < IBP <98. Otherwise the ML threshold is less than
or equal to the other threshold.
The next three figures describe the
detector
characteristics
for
detectors with inner bandwidth percentages of 40,96, and 150%.
The ROC and SOC curves were calculated for all inner bandwidth percentages.
The
"knees" of the ROC curves increased for inner bandwidth
percentages over the range of 40% to 96% to a maximium at
and
then
decreased
96%
and
98%
as the inner bandwidth percentage was increased to
-
- 219
Comparing figures 4.1.10-12 shows the full range of deviation
150%.
the
of
ROC and SOC curves from the worst cases (figures 4.1.10 and 4.1.12)
to the best case (figure 4.1.11.) The points on the curves indicate
the
ML and optimal detectors for each particular IBP.
As shown in Table 4.1.9, the
bandwidth
of
class
showed
detectors
with
an
inner
96% can produce the best ML detector as well as detectors
optimal with respect to both benefit
4.1.11
of
the
measures.
As
discussed,
Figure
ML and optimal detector settings along the detector
characteristic curves.
It is instructive to see how the benefit measures change
threshold
setting.
in
the
gradient
may
detector
coming
from
particular class types.
robust the detector scheme is to
changes
selecting the detector threshold setting.
each threshold setting along the ROC curve
ratio
of
set-
traditional
cost
measures
and
functions
of
oberva-
Thus these curves show how
the
in
parameters
used
in
(Note, however, that although
MAY
be
described
by
some
a priori probabilities, the
optimal detectors selected in this study were determined
benefit
the
be considered to be a function of the relative costs assigned
to detector decisions as well as the a priori probabilities
tions
of
the benefit measure as a function of detector setting (for a
particular database.) As described in section 3.4.1, the
ting
the
Figure 4.1.13 shows the benefit measures as a func-
tion of threshold setting. These curves indicate
change
with
by
maximizing
which were NOT based on the traditional cost assign-
ment rules mentioned in section 3.4.1.
The cost functions used in
this
study were selected to maximize the utility of the detector to a patient
for
-
- 220
1) a detector where every alarm was immediately
answered
(Bi:
Maximum
the
fastest
sensitivity), and
2) a detector where the staff/machine system
would
yield
alarm response (B2 : Maximum(sensitivity + positive predictivity).)
Figure 4.1.13 shows the benefit measures as a function of threshold
The
setting.
curves.
the
and
ML
detector
These curves show that the
after
a
optimal
optimal
threshold of .7.
settings are indicated on the
benefit
measures
upper
graph
are
decline
More importantly, this figure shows that the
threshold on the lower graph and the
B2
steeply
relatively
insensitive
optimalB1
B
threshold
in
to small changes in the
threshold setting.
The detector characteristics displayed in
benefit
curves
of
figure
4.1.11
and
the
figure 4.1.13 describe the results for the original
database. The following 7 figures answer some
questions
regarding
the
distributions of performance measures and maximum benefit values for the
optimal and ML
curves
then
detectors
over
many
(bootstrapped)
databases.
These
suggest an estimate of the variance of how well a detector
would work on another database which was selected in the same manner
as
the original database.
Figure 4.1.14 shows the detector characteristics (superimposed) for
ten
detector
classes
all with an IBP of 96%.
and SOC curves.) Each curve describes the
one
database.
(i.e., bootstrapped ROC
detector
positive
for
Comparison of the ROC curves (upper left) and the gross
sensitivity curves (upper right) with the SOC curves
gross
characteristic
(lower
left)
and
predictivity curves (lower right) shows that the varia-
-
- 221
bility of gross sensitivity with different databases is greater than the
variability of gross positive predictivity.
The variablity of the performance measures (aSE,gSE, and gPPA)
different
detectors
is
shown
in
detector
The performance
figures 4.1.15-17.
measures for the ML detector are shown in figure
4.1.15,
th
optimalBI
in figure 4.1.16, and the optimalB2 detector in figure 4.1.17.
The histograms of the performace measures were generated by
the
for
performance
measures
for 5000 (bootstrapped)
and 5 percent minimum expected value
of
each
calculating
databases.
performance
The mean
measure
is
shown in the lower right table on these graphs.
Figures 4.1.18-20 illustrate the bootstrapped benefit measures
the
best
ML
detector
(figure
4.1.18),
optimalB1
detector
for
(figure
4.1.19), and the optimalB2 detector (figure 4.1.20.) These curves
indi-
cate how robust the expected benefit measures are with respect different
(bootstrapped) databases.
Tables 4.1.21 and 4.1.22 tabulate the mean, 5 percent minimum,
95
percent
maximum
and
expected detector performance and benefit measures
repectively.
5.2.
DISCUSSION OF THE RESULTS OF THE AUTOREGRESSIVE MODEL DETECTORS
In the previous section,
tors
were
discussed.
the results of the
relative-power
detec-
This section focuses on the detection results of
two autoregressive detection schemes.
To aid in the discussion,
the definitions defined in section 4.1.
1) Detector 1 (Type 1) : AR(2) Gross Covariance Matrix Detector
recall
-
- 222
2) Detector 2 (Type 2) : AR(2) Average Covariance Matrix Detector
The autoregressive detector results are presented in the
order.
First,
the
feature
space
description
presented as a tool in interpretating
detector
of
the
following
database
results.
Second,
is
the
modeling assumption that the aI and a2 features are Gaussian distributed
is tested by comparing the Gaussian cumulative probability
distribution
curve against the empirical cumulative distribution curves for a
1 and a2
for each of the classes.
the
Third, the difference in
performance
between
Type 1 and Type 2 detection schemes is presented in an illustrative
example in which the detectors discrimiate between NOISE and
Fourth,
the
results
of
both
VFL
only.
detection strategies for distinguishing
between noise and the combined classes of VT, VFL, and VF are presented.
This
fourth
section
the major conclusions of this research.
presents
Last, the results of bootstrapping by patients is compared with those of
bootstrapping by events.
Feature Space Description of the Database
Recall that the ventricular arrhythmia and noise episodes were sectioned
into
4-second
segments.
Figures
4.2.1-4.2.4
illustrate the
feature space description of those four-second segments for each of
the
four classes (all patients combined.)
Table 4.2.5 lists
patient
in
the
the number of segments of
Malignant Arrhythmia Database.
second VT, VFL, and VF segments were 482,
There
were
artifact.
a
total
of
519
396,
each
class
for
each
The total number of 4and
323
respectively.
four-second segments of electrode motion
Since noise was not considered patient specific,
segments
of
-
- 223
noise were not assigned to specific patients.
Figures 4.2.6-21 display the feature distributions for
and
for each patient individually.
each
class
These figures indicate the intrapa-
tient and interpatient variability of features
among
four-second
data
segments.
Examination of the Gaussian Modeling Assumptions
the
Figures 4.2.22-29 show the relative differences between
sian
cumulative distribution curve and the empirical cumulative distri-
bution curves for a1 and a2 for each of the four classes.
cumulative
The a1 and a2
The max-
were estimated by their histograms.
distributions
imum deviation from the Gaussian cumulative distribution is
on
Gaus-
also
The Kolmogorov-Smirnov Test[15] was applied to determine
the graph.
how closely the feature distributions were modeled by the Gaussian
tribution.
given
The
table
of
dis-
p-values (the probability that the data was
Gaussian distributed) for each of the feature distributions is given
in
Table 4.2.30.
Table 4.2.30 shows that the noise features are well
Gaussian
process
(p>.20).
VF.
results
and only fairly well
The Gaussian assumption was applied because of its
as a tool in developing a
show
that
the
by
a
However, the assumption fails miserably for
the distribution of the VT segments (p=O)
and
modeled
first
detector
order
autoregressive
worked
well
for
VFL
simplicity
detector.
The
under this assumption.
Future work may investigate different data transformations to scale
autoregressive coefficients into Gaussian-distributed features.
the
-
- 224
Demonstration of the Difference between the Two Autoregressive Detection
Strategies
example
This section presents an illustrative
between
the gross covariance matrix detection strategy
average covariance matrix detection
shows
the
results
of
the
ML
strategy
detectors
descriminate between VFL and NOISE only.
characteristics for Detector 1.
tor
of
(Type
the
difference
(Type 1) and the
2.)
example
This
for both strategies as they
Figure 4.2.31 shows the detec-
Similarly, figure 4.2.32 shows the
detector characteristics for Detector 2.
The ML detector thresholds are
indicated on the curves in these figures.
The ROC and SOC curves were calculated over the original
A
of
comparison
the
curves
between
database.
two figures shows that the
the
"knees" of the ROC and SOC curves are (slightly) higher for the
strategy than for the Type 2 strategy
1
(over the original database.) Thus
we would expect that we can always choose some
perform better than any Detector 2.
Type
Detector
1
that
would
The detectors selected in this case
were the ML detectors for the original database.
We see that Detector 2
performed better than Detector 1 over the original database.
Table 4.2.33 shows the performance measures for Type 1 and 2 detectors
over
the
original database.
It shows that the average and gross
sensitivity measures are higher in Type 2 than Type 1.
that
the
superior to
gross
the
It
also
shows
positive predictive accuracy of the Type 1 detector is
Type
2
detector.
This
difference
is
graphically
explained in figure 4.2.34.
Figure 4.2.34 shows the distribution of the features for VFL (upper
left)
and
-
- 225
for NOISE (upper right) for the original database.
The dis-
tributions of a1 and a2 of VFL and NOISE are assumed to be Gaussian distributed as discussed above.
Detector 1 estimates the covariance matrix
of the conditional distribution for VFL by estimating
ments over all VFL segments.
data
matrix
ele-
Detector 2 estimates the covariance matrix
by averaging covariance matrices calculated for each
patient's
the
segments alone.
patient
from
the
All three of the two-dimensional Gaus-
sian conditional distribution functions have elliptical projections onto
the
feature
space.
graph of figure
through
the
These projections are indicated in the lower left
4.2.34.
(The
contours
describe
the
cross
distributions when all the conditional distributions equal
.95) The contour encircling the horizontal axis is from the
tribution.
section
The
quasi-concentric
contours
noise
dis-
are from the VFL distribu-
tions: the inner one from Detector 1 and the outer one from Detector 2.
The lower right graph in figure 4.2.34 displays
of
the
other three graphs in the figure.
conditional
Detector
more
1.
Since
distribution of VFL with the average covariance matrix
is broader than that for the gross covariance matrix,
call
superposition
This figure explains why the
sensitivity of Detector 2 is greater than that for
the
the
Detector
2
will
"borderline" observations VFL than will Detector 1. For this
same reason, the false alarm rate of Detector 2 is greater than Detector
1.
This is reflected in Dectector 2's lower mean gross positive predic-
tivity.
The variability of the performance measures are illustrated by Figures
4.2.35 and 4.2.36 which show the bootstrapped performance measures
for Detector 1 and 2 respectively.
The tables in each of these
figures
-
- 226
the mean and minimum five percent expected values for the per-
indicate
formace measures.
one
1.
By comparing the means of the
performance
measures,
could prematurely conclude that Detector 2 was superior to Detector
However,
ures
for
by noting that the distributions of the
Detectors 1 and 2 overlap,
difference
between
bootstrapping
detectors
demonstrates
is
influence
not
other
significant.
Specifically,
that the database is insufficient to deterBootstrapping also shows
that
of a single, dominant patient (such as patient Number 7)
may be excessive.
the
meas-
one concludes that the performance
mine which detection scheme is superior.
the
performance
Here we see that the aSE distribution is bimodal
distributions
are
broader than those for Detector 1.
and
This
occured because the detector was not tuned to the great number of events
from
patient 7, but weighted the influence of that patient equally with
all others.
Discriminating between Noise and VT,VFL, and VF
The previous section described
inate
bewteen
Noise
and
VFL.
ML detectors designed
to
This section describes autoregressive
detection schemes designed to descriminate between noise and
of malignant arrhythmias.
schemes,
of
both
descrim-
all
types
To illustrate the difference between detection
the benefit measures were maximized over all possible detectors
Type
1
and Type 2 types.
detectors are plotted in
figure
The results for the optimal and ML
4.2.37
and
are
tabulated
in
Table
4.2.38.
As with the relative-power detectors,
the
can be tuned to be maximally sensitive (100%).
autoregressive
detector
Figure 4.2.37 shows that
the optimalBI Types 1 and 2 detectors have identical statisitics
(i.e.,
they
can
achieve
maximal
-
- 227
senstivity with a gPPA = 69.9%.) Thus, both
B1
autoregressive schemes are equally good with respect to the
benefit
measure.
Table 4.2.38 also shows that the maximal performance of both Detectors
1
and
2
are
quite
similar
with
to
respect
B2.
Detector 2
(B2max = 192.2) is not statistically significantly superior to
1
(B2max = 192.1.)
on the original database.
Figure 4.2.37 shows that
the ML detector (-) benefit measures are similar to
detectors
Detector
the
optimalB 2
(+)
but are significantly less than the optimalBl detectors.
The
optimalB2 and ML detectors are similar since
minimize the number of false positives.
both
effectively
try
to
(Refer to section 4.1.)
Figures 4.2.39 and 4.2.40 show the detector characteristics for the
Type
1
and Type 2 detectors respectively.
settings are indicated on the curves.
curves
for
both
detectors
shows
The optimal and ML detector
Superimposing
the
ROC
and
SOC
that there is negligible difference
between the detection schemes for the original database.
(This was con-
firmed by the benefit measures plot described above.)
Figures 4.2.41 and 4.2.42 show the benefit measures as
of
threshold
values of
(Recall
setting.
both
the
These
detectors
similar
are
curves
with
a
function
describe how robust the benefit
respect
to
threshold
settings.
discussion in section 4.1 describing the case for
the relative-power detector.) Since the benefit measures do not increase
for thresholds greater than 1 as shown in figures 4.2.41 and 4.2.42, the
detector characteristic curves are shown for thresholds varying
to 1 (e.g., figures 4.2.39-40.)
from
0
-
- 228
Figures 4.2.41 and 4.2.42 point out
a
significant
feature.
The
of a benefit measure of a detector optimized with respect to that
level
measure is quite stable (flat) with respect to
Thus,
any
threshold
the
threshold
setting.
near the optimalB1 (optimalB 2 ) setting will yield
favorable benefit values with respect to B1 (B2).
Figures 4.2.43 and 4.2.44 show the
variability
of
the
detection
schemes over different (bootstrapped) databases for Type 1 and 2 schemes
respectively.
As discussed in section 4.1, the bootstrapped ROC and SOC
curves indicate how robust the detector characteristics are with respect
to the design and testing databases.
Figures 4.2.45-47 show the bootstrapped
the ML and optimal Type 1 detectors.
performance
measures
for
Figure 4.2.45 shows that the vari-
ability of the average and gross sensitivity measures is smaller for the
detector
designed to optimize sensitivity than for the other two detec-
Comparing figures 4.2.46 and 4.2.47 shows that mean of the sensi-
tors.
tivity
measures
detector.
is
higher
for the optimalB 2 detector than for the ML
In contrast, the mean gross positive
predictive
measure
is
lower for the optimalB 2 detector.
Figures 4.2.48-50 show the bootstrapped
the
optimal
and
ML
performance
detectors of Type 2 strategy.
strategy, the variability of the sensitivity measures
the
optimalB1 detector.
measures
for
As with the Type 1
is
smallest
for
The performance of the ML and optimal detector
with respect to B2 are nearly identical.
Figures 4.2.51-53 show the bootstrapped benefit
optimal and ML Type 1
detectors.
measures
for
the
The mean, minimum 5 percent, and max-
-
- 229
imum 95 percent expected values of the benefit mesasures
in
4.2.57.
table
are
tabulated
The benefit histograms were generated by calculating
the measures for 5000 (double bootstrap) iterations.
As expected the standard deviation
larger
than
measures.
of
B2
for
all
detectors
is
that for B1 since it is a sum of two (not one) performance
Nevertheless, the deviations of
the
benefit
functions
are
less than 2% of the mean estimate (see table 4.2.57.) Hence, the benefit
functions are robust with respect to different databases.
from
the
performance measure distributions
As
expected
(figures 4.2.45-50),
the ML
and optimalB2 detectors have nearly identical benefit distributions.
Figures 4.2.54-56 show the bootstrapped distributions of the
fit
measures
for
the ML and optimal Type 2 detectors.
percent minimum, and 95 percent maximum values
4.2.57.
These curves indicate,
are
The mean, five
included
in
Table
as with the Type 1 class detectors,
the B2 deviations from the mean are larger than those
detectors.
bene-
for
B1
for
that
all
Likewise, all deviations from the mean for both performance
measures are less than 2%.
Table 4.2.58 tablulates the mean, 5 percent minimum, and 95 percent
maximum expected values for the performance measures
(aSe,gSE,
and gPPA)
for the ML and optimal Type 1 and 2 detectors.
Comparison of Bootstrapping .y Patients Versus Bootstrapping by Events
The previous discussion describes the results for bootstrapping
creating
new
from a pool.
databases
by
databases by randomly selecting
This discussion focuses on
the
by
(with replacement) events
effect
of
creating
new
randomly selecting with replacement patients from a pool.
-
- 230
The events from each selected patient then make the final data sample.
on
Figures 4.2.59-4.2.62 show the difference between bootstrapping
patients
and
bootstrapping
on events for detector Types 1 and 2.
histograms in all cases were generated by
calculating
measures for 5000 double bootstrap iterations.
the
The
performance
Comparing figures 4.2.59
with 4.2.61 and 4.2.60 with 4.2.62 shows that the variance of
the
dis-
tributions
of all performance measures is broader when bootstrapping by
patients.
This occurs because many patients do not have events from all
classes.
(Refer
to
table 4.2.5 for the distribution of the number of
events of VT,VFL, and VF among patients.)
-
- 231
Chapter 6
6.
CONCLUSIONS
This chapter presents conclusions based on
over
all three detection schemes.
the
comparing
results
For convenience, table 6.1.1 summar-
izes the benefit measures for the optimal and ML detectors of the
three
detection schemes.
Table 6.1.1 Benefit Measures of the Optimal and ML detectors for Detectors 0, 1, and 2.
2
Benefit Measure B2
1
Benefit Measure B1
Detector Type
5% Min
Mean
95% Max
5% Min
Mean
95% Max
B1
100
100
100
170.0
170.0
170.0
B2 max
90.4
91.7
92.9
|| 185.0
186.8
188.3
186.2
187.8
(IBP=96%)
II
ML
II
I
88.5
I
I
90.0
III
91.4
|| 184.2
DE TE
J...~
B1max
100
100
100
171.1
171.1
171.1
B2max
92.5
93.9
95.0
190.3
191.9
193.1
ML
91.4
92.9
94.1
190.1
191.7
- ---
192.9
170.1
170.1
170.1
190.3
191.9
193.1
190.3
191.9
193.1
2----1
DETE T4I
B1 m
100
100
100
B2 ma
92.4
93.9
95.0
ML
92.3
93.7
94.8
II
II
II
II
U
1 Bi: Benefit measure
1 : gross sensitivity
2 B2: Benefit measure
2 : sum of gross sensitivity
and positive predictivity
I
-
- 232
Best Optimal Detector With Respect to B1
The results show that all
achieve
maximal
three
detector
schemes
sensitivity with the same gPPA (69.9%).
were
able
The detectors
achieved the maximal sensitivity by assigning all observations to
V.
to
class
Since the total number of events in class V was 1201 and there were
519 noise events, the worse case gPPA was
1201
_= 69.9%
519 + 1201
Comparing the gPPA among results published earlier would
this
since
measure
is
highly
useful
for
comparing
a
mistake
dependent upon the different number of
events in separate classes for different databases.
is
be
detectors
However,
the
gPPA
tested on databases with the same
number of episodes in corresponding classes.
(i.e., there is no problem
bootstrapping new databases of the same size as the original data-
with
base.)
Best Optimal Detector With Respect to B2
Table 6.1.1 shows that based on the
tuned
to
optimize
1
sum
of
gross
estimates
of
detectors
sensitivity and gross positive
the detectors are ranked as : Detector 2 (191.9) =
predictivity,
tor
the
mean
(191.9) > Detector 0 (186.8).
Detec-
Detector 0 is clearly inferior to
Detectors 1 and 2 since the 95% confidence interval for B2 for
Detector
0 (188.3) is less than the 5% confidence interval for B2 for Detectors 1
and 2 (190.3).
and
2
are
Because the confidence intervals for B2 for Detectors
identical
(190.3,
1
193.1), either Dectector 1 or 2 is con-
sidered the best detector with respect to B2.
-
- 233
Comparision Between ML Detectors 0, 1, and 2
The ML detectors of each scheme were
results
of
different
schemes
implemented
to
compare
which used identical thresholds.
the
Table
6.1.1 shows that for the same threshold setting, all three schemes
formed statistically significantly differently.
A Two-sample T-test was
applied to test the null hypothesis that the the means
distributions were the same.
of
the
benefit
Thus, with respect to benefit measure B1, Detector 2 (93.7) >
Detector 1 (92.9) > Detector 0 (90.0).
Detector
benefit
The hypothesis was rejected (with a signi-
ficance level >.01) for all pairs of mean estimates of the same
measure.
per-
2
(191.9)
>
In addition, with respect to B2,
Detector 1 (191.7) > Detector 0 (186.2).
Thus,
Detector 2 is the best ML detector with respect to both cost functions.
Comparsion Between Optimal and ML Detectors
Table 6.1.1 shows that the results for both benefit
similar
for
optimalB 2 and ML detectors.
with
a
are
We initially would not expect
the ML and optimalB2 detectors to be similar since the
designed
functions
ML
detector
is
fixed threshold based on assigning costs to decisions
and estimating the a priori probabilities for each class.
On the
other
hand, the threshold for the optimalB2 is selected by finding that detector which optimizes the sum of two performance
measures.
However,
as
described below, both the ML and optimalB 2 detectors minimize the number
of false positives.
Recall the expression for the expected cost of a detector based
E(Cost) = C 00 P(H0 ) +C
a priori
0 1 P(HI)
+
the cost assignments and estimates of the
on
probabilties:
(6.1)
-
- 234
(C10
00)P(H O)PF
-
(C0 1 - Cll)P(Hl)PD
-
where the hypothesis H0 corresponds to noise and H 1 corresponds to
tricular arrhythmia.
ven-
C.. is the cost of deciding class i given that the
observation came from class j.
P(H ) is the
a priori probabiltiy of an
observation from class i.
The ML decision criteria minimizes the expected cost given that C
=
0,
C0 1 = C1 0 , and P(Ho) = P(H1 ).
Substituting these conditions into
equation 6.1 gives
E(Cost)ML = MINU1-PD) + PF}
(6.2)
The condition in equation 6.2 is equivalent to
E(Cost)ML = MAX{(1-PF) + PD}
(6.3)
Recall that our estimate of 1-PF is the specificity (SP) and that for
PD
was the sensitivity (SE).
Thus,
E(Cost)
MAX{SE + SP}
=
ML
(6.4)
Comparing Eqn. 6.4 with the definition of the benefit measure B2
E(Cost)
B2
MAX{SE + PPA}
=
shows that both the ML and optimalB 2 minimize the number of false
By definition,
B2
=
SE + PPA
=
TP
TP + FN
+
tives.
TP + FP'
We see that B2 weights the FN error equally with the FP
C10
=
posi-
C01
as with the ML detector.)
It
error.
(i.e.,
is therefore reasonable that the
thresholds for the ML
and
optimalB2
-
- 235
detectors
are
similar.
It
is
interesting to note that we could have selected a third benefit function
which maximixed the sum of the sensitivity and specificity of the detector
for class V events (i.e., B3 = MAX( SE + SP ).)
optimal detector with respect to this third benefit
The solution of the
function
would
be
the ML detector.
Future Directions
Following this initial groundwork a number of short
term
projects
could be investigated.
1) Exploring the detector's response to other arrhythmias and artifact
This thesis investigated a means of discriminating VTVFL, VF, from
electrode
motion
artifact.
It
would be instructive to determine the
response of all the detectors
to
other
superventricular
atrial flutter and fibrillation, and seg-
tachycardia,
ments of noisy ECG.
The MIT/BIH database
rhythm
is
an
disturbances
excellent
such
source
as
of
examples.
2) Adaptive Decision Making
One could modify the decision scheme reported
the
detector
sions.
For
to
Clearly,
example,
above
by
make a decision based on the history of it
requiring
past deci-
there are numerous ways to design an adaptive detector.
the detector designer may decide to wait longer than four
seconds before sounding an alarm,
or to wait until at least two consecu-
tive 4-second segments are declared class V before alerting the staff.
-
- 236
3) Noise Stress Test
A critical project is to determine the signal to noise ratio
when
noisy
normal sinus rhythm causes false positives.
(SNR)
A model of the
testing situation is shown in figure 6.1.2.
s[n] J-
DETECTOR
DECISION
G
w[nI
Figure 6.1.2 Noise Stress Test. The signal produced by the heart (s[n])
is corrupted by different levels of noise (w[n]) by altering the gain,
G, of the noise channel.
To model a noisy electrocardiogram, a noise-free ECG
corrupterd
by
signal
(s[n])
is
additive noise (w[n]) at different SNR levels (gain, G).
The performance of the detector is monitored for each gain
benefit measures are evaluated as a function of G.
level.
The
The performance will
decrease as G increases below some (presently unspecified) threshold
of
unexceptable
response.
-
-237
With this information, one could make a state-
ment describing how robust different detection schemes are with
to the SNR.
respect
238
-
-
References
1.
J. N. Herbschleb, R. M. Heethaar, I. van der Tweel, A. N.
E.
Zim-
merman, and F. L. Meijer, "Signal analysis of ventricular fibrillation," Computers in Cardiology Conference, pp.49-54 (1979).
2.
J. N. Herbschleb, R. M. Heethaar, I.
Meijer,
van
der
Tweel,
and
"Frequency analysis of the ECG before and duing
lar fibrillation," Computers in Cardiology
Conference,
F.
L.
ventricupp.365-368
(1980).
3.
A. E. Aubert, B. G. Denys, H. Ector, and H. De Geest, "Fibrillation
recognition
M
4.
using autocorrelation analysis," Computers in Cardiol-
Conference (1982).
M. E. Nygards and J. Hulting, "Recognition of ventricular fibrillation
utilizing
the
power spectrum of the ECG," Computers in Car-
diology Conference, pp.393-397 (1977).
5.
F. M. Nolle, K. L. Ryschon,
analysis
of
ventricular
and
A.
fibrillation
Computers in Cardiology Conference,
6.
E.
Zencka,
"Power
spectrum
and imitative artifacts
pp.209-212
(1980).
F. K. Forster and W. D. Weaver, "Recognition of ventricular,
rhythms,
,"
other
and noise in patients developing the sudden cardiac death
syndrome," Computers in Cardiology Conference (1982).
-
- 239
7.
S. Kuo and R. Dillman, "Computer detection of ventricular fibrillation," Computers in Cardiology Conference, pp.347-348 (1978).
8.
F. E. M. Brekelmans, J. S. Duisterhout, and R. A. A.
"Detection
trough
of
series
life-threatening
analysis,"
by
arrhythmias
Computers
in
F.
Dam,
van
successive peak-
Cardiology
Conference,
pp.361-364 (1980).
9.
A. Langer, M. S. Heilman, M. M. Mower, and M. Mirowski, "Considerations
in
the
development of an automatic implantable defibrilla-
tor," Medical Instrumentation Vol. 10(3), pp.163-167 (1976).
10.
A. E. Zencka, J. D. Lynch, S. M. Mohiuddin, M. H.
Sketch,
and
V.
Runco, "Clinical assessment of interactive computer arrhythmia monitoring," Computers in Cardiology, pp.209-212 (1977).
11.
R. B. Kerr, Electrical Network Science, Prentice Hall, Inc., Englewood Cliffs, N.J. (1977).
12.
William McC. Siebert,
Circuits,
Signals,
and
Systems,
The
MIT
Press, Cambridge, MA (1986).
13.
George B. Moody, Warren K. Muldrow, and Roger
stress
test
for
arrhythmia
detectors,"
G.
Mark,
"A
noise
Computers in Cardiology
Conference (1984).
14.
B. Efron,
"Bootstrap Methods
: Another Look at the Jackknife,"
The
Annals of Statistics Vol. 7, pp.1-26 (1979).
15.
Bernard W. Lindgren, Statistical Theory, MacMillian Publishing Co.,
Inc., New York, NY (1976).