Lab Safety Notes

Lab Safety Notes
PREPARING FOR LABORATORY WORK
Never perform _______________ _____________.
Know how to use the safety shower, eye wash, fire blanket and first aid kit & bucket of sand.
Do not work in the lab without your___________ in the room.
DRESS FOR LABORATORY WORK
Tie back long_________.
Do not wear ____________ ___________as they tend to get in the way.
Do not wear _____________ ______________on a lab day.
Wear lab _____________ during all laboratory sessions.
Wear safety __________ during ________ laboratory sessions
BUNSEN BURNER SAFETY:
A __________ burner flame is very hot. The center of the flame will be over 1000 degrees Celsius. Treat burner flames with respect.
Always use the main gas shut off ____________ on the desk to shut off a Bunsen burner.
The valve at the base of the burner is only used to control the flame.
If your hair or clothing catches on fire ___________, ____________ and
__________ .
If someone else's hair or clothing catches on fire, wrap them with a ________
____________ to smother the fire. Do _______ let them run.
AVOID HAZARDS
Use caution when handling _______ glassware.
When diluting acid, always add _______ slowly to _________. Never add water to acid.
Keep ______ on reagent bottles. Never switch caps.
ANIMAL SAFETY
Do not cause pain, discomfort , or injury to an animal
Wash ___________ after handling animals
SAFETY WITH CHEMICALS AND DISSECTING SPECIMENS:
Do not _________ or ______________ any chemical unless specifically instructed to do so.
_________ chemical labels more than once before using the contents - it is easy to confuse chemicals.
When working with chemicals or dissections, keep your hands away from your
________. The skin on your face is much more sensitive to irritation than your hands.
To smell something, hold it away from your nose and wave your hand over it
__________ your nose. You may pass out or inhale dangerous gases is you just stick your nose over the container and breathe in.
Flush any chemical spill on your skin with plenty of _________. The rule of thumb is
____________ _______________.
When heating anything in a test tube, point the mouth of the test tube towards a
wall, ___________ from people.
Do not lay the glass stopper from a reagent bottle on the lab _____________. Hold the handle of the stopper between two fingers while you pour from the bottle.
When mixing acids and water, pour the ______ into the __________r . Remember,
AnW (root beer)
Wash your hands when you are ____________ with the lab.
SAFETY WITH GLASSWARE & BROKEN GLASS
___________ use chipped or broken glass. If you notice chipped or cracked glassware during a lab please report it to the instructor to be replaced.
___________ glass should never be handled with your hands. Use a dustpan and broom to sweep up broken glass. Small pieces can be wiped up using a wet paper towel.
Broken glass should be placed in the proper container - either a can for broken glass or a sharps container.
Always have the instructor clean up a broken ___________ thermometer. Mercury is a poisonous substance and should not be handled. VHHS no longer has any mercury thermometers.
Broken glass that has contacted _________ must be disposed of in a sharps container.
The __________ cylinder guard on a graduated cylinder is to protect the cylinder from breaking if it should tip over. The cylinder guard is not made to slide up and down for measuring.
A graduated cylinder should be ________ ________ when it is empty - if it is laying down it can't be tipped over.
Remove glass tubing and funnels from ___________ as soon as your lab is finished.
If you do not they will become stuck in the stopper.
BLOODBOURNE PATHOGEN SAFETY
Wear ________ _________ anytime you assist someone who is or has been bleeding or vomiting.
Clean the entire area (desktop, floor, etc.) with a _________________ when you are finished caring for the person.
Remove your latex gloves by pulling them off _________ _______. Avoid contact with the outside of the gloves.
All paper towels, tissues, latex gloves and other materials used to clean up blood and other possible infectious materials should be disposed of in the proper
______________________ waste bag.
Standard Biohazardous waste bags are red with the biohazardous waste
__________ on them. If one is not available, use a regular plastic garbage bag and attach a biohazardous waste sticker to the bag.
Wash your hands thoroughly with a ____________________ when you are finished.
CLEAN UP
Consult ______________ for proper disposal of chemicals.
______________ hands thoroughly, following experiments.
IN CASE OF ACCIDENT
____________ all accidents and spills immediately.
Place broken glass in designated _____________.
Wash all chemicals from your skin immediately with plenty of running
________________.
If chemicals get in your eyes, wash them for at least _______________ in an
________________.
Laboratory Procedures Notes
Measuring Volume
The Meniscus
When water is placed in a glass or plastic container the surface takes on a
________________ shape. This curve is known as a meniscus. Volumetric glassware is calibrated such that reading the bottom of the __________________, when it is viewed at ____________________, will give accurate results. Viewing the meniscus at any other angle will give inaccurate results.
Erlenmeyer Flasks and Beakers
Erlenmeyer flasks and beakers are used for mixing, transporting, and reacting, but ________ for accurate measurements . The volumes stamped on the sides are approximate and accurate to within about _____________.
Graduated Cylinders
Graduated cylinders are useful for measuring _____________ volumes to within about
1%. They are for ________________ purpose use, but not for quantitative analysis. If greater accuracy is needed, use a pipet or volumetric flask.
Some graduated cylinders are marked according to how much liquid they __________; others are marked according to how much liquid has been ________________ (see examples below).
amount in container amount removed
Measuring Mass
Mass is the amount of _______________ in an object. There are different kinds of balances used to measure mass. Be sure you understand how your balance works.
Some balances give a single reading. Others give two or more readings that you have to ________ together.
For example, look at the triple-beam balance below. Notice that the middle beam measures the largest amounts.
To read the mass of an object, find and record the masses shown on each of the beams.
Then add the readings. Using the example above,
200 g + 70 g + 6.5 g = 276.5 g
Measuring mass with an electronic balance:
The electronic balance has many advantages over other types of balance. The most obvious is the ease with which a measurement is obtained. All that is needed is to place an object on the balance pan and the measurement can be read on the display to hundredths of a gram. A second advantage, using the ___________ button on the front of the balance, is less recognized by beginning science students. Because one must __________ place a chemical directly on the balance pan , some container must be used. Place the container on the balance and the mass of the container will be displayed. By pressing the Zero button at this point, the balance will reset to zero and ignore the mass of the container. You may now place the substance to be weighed into the container and the balance will show only the mass of the substance. This saves calculation time
and effort. However , when the container is removed from the balance, the display will go into negative numbers until the Zero button is pressed again.
Most electronic balances also have a ________ button on the front. Pressing this button will change the units being measured. Since we have very few times when we need something other than metric units, you should not have to change the mode on the balance. Because of the Unit and Zero buttons, there are two things you must always do before placing objects onto an electric balance to be measured:
1. See that the display is reading ____________
2. See that the unit sign in the upper right of the display shows _______
When finished with the electronic balance, press the __________ button and hold it down until the display shows _________ .
Measuring Temperature
Another objective for this lesson is to use the __________ temperature scale to measure the temperature of fluids. Liquids and gases are both __________. The laboratory thermometers we have are calibrated in degrees Celsius rather than degrees
Fahrenheit. The __________ scale used to be called centigrade and is often still called that. It was called _______grade because the difference between the freezing temperature of water and the boiling temperature of water is divided into ________ degrees. 0 o C is defined as the temperature at which water freezes and melts. 100 o C is defined as the temperature at which water boils (using normal pressure). It is the scale commonly used in scientific work and in everyday use in most countries.
Scientific thermometers do ______ have to be shaken down. Quite a few laboratory thermometers have been broken by trying to shake them down next to a table. It is best
to hold a thermometer while using it instead of leaning it against the container or other object. The container it is in may or may not be able to support it. All you have to do is put the thermometer in the liquid you are measuring and watch the red alcohol level
_________. When it stops changing, read the red alcohol level. That is your measurement. You don't have to wait a prescribed length of time, just let it come to a rest.
You may have to turn the thermometer to see the numbers and line up the mercury or alcohol with the scale.
When you read thermometers, you should estimate between the lines. Try to mentally divide the sections into ______ equal parts in order to read to a ________ of a degree.
Measuring Length
In order to correctly measure the length of an object, make sure that one end of the object being measured is _________ lined up with the _______ end of your metric ruler.
Then make sure that your eye is lined up with the other end of the object you are measuring and take a measurement based on _________ where the object ________ on the scale of the metric ruler.
Using a laboratory burner:
Many chemistry experiments require something to be heated. This is done with one of several types of laboratory _________. The lab burners use natural gas delivered through the gas outlets at student lab stations.
Before attempting to light any lab burner, check to see that the jet hole between the base and the burner tube is free of ____________. If chemicals have covered this jet, the burner will not operate properly. After attaching the hose to the gas outlet, check the tubing for ___________ by gently stretching it and watching closely for small holes to appear. Make sure the gas control knob and the air control vent are closed, and then turn the handle on the main gas valve ____________ to the nozzle to the gas jet. The gas valve is turned off by turning
the handle 90 degrees in either direction. Open the gas control knob at the base of the laboratory burner and carefully check to see that you __________ gas escaping from the mouth of the burner tube. When you are sure that gas is escaping, bring the head of the
__________ over the burner and squeeze the striker handle. The _________ produced will ignite the gas and your burner is lit. Adjust the _______ control vent so that the flame has the proper color pictured here . A yellow flame is an indication of a lack of --
___________, meaning that the air vent needs to be opened. The hottest part of the burner flame is just at the top of the bright blue inner cone. Normal heating is done with an object at the top of the light blue outer cone, while strong heating is done with an object at the top of the bright blue inner cone. To heat a container gently , move the container back and forth through the outer cone. To __________ off the burner flame, use the main gas valve handle (NOT the gas control knob!) to quickly turn the handle 90 degrees in either direction (forward or back).
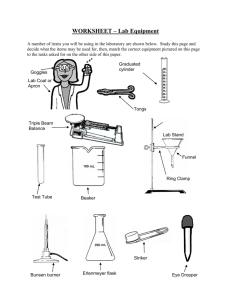
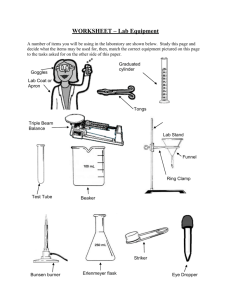
Lab Equipment Review Sheet
You should know the name of each piece and its basic use.
For exact volume measurement s of liquids.
Pipet on the left is a
___________ pipet . It has only one graduation for delivering one exact volume. Pipet on the right is a ______ pipet . It has graduations for delivering any number of exact volumes.
Pipet
Used in conjunction with a
________ connection to a water faucet to speed up filtration.
Filter flask
The _______ bottom of the
Florence flask makes it ideal for boiling liquids. It also makes this flask easy to tip over when sitting on the lab table.
Florence flask
There are several styles of fillers used to _______
_________ into a pipet.
Chemistry students use a standard pipet filler, shown at right.
Pipet filler
Never draw a liquid into a pipet with your mouth.
The Erlenmeyer flask is the most common flask in the chemistry lab. It is used to contain reaction
____________.
Erlenmeyer flask
The volumetric flask is used to make _______.
It has a precise graduation line in the neck of the flask. A solute is placed into the flask, then the
________is used to bring the total volume up to the graduation.
Volumetric flask
Beakers are the most versatile glassware in the lab and can be used for just about anything. The volume graduations on beakers should be used only for
"ballpark"
__________.
Beaker
Used to make
__________measureme nts of liquid volumes. The bumper ring on larger cylinders is to prevent breakage if tipped over.
Keep it near the top.
Graduated cylinder
This dish is used to recover dissolved solids by
__________.
While it can be heated, it should not be used for
"strong" heating.
Evaporating dish
When attached to the ring stand, this iron ring is used to
___________ glassware above the lab table.
Support ring
A watch glass can be used like an evaporating dish for very _________ amounts of liquid. It can also be used to cover beakers.
When attached to the ring stand, this clamp is used to hold a large
____________ or
Florence ________ above the lab table.
Watch glass
Utility clamp
Crucibles are used as a container when something requires
"strong"
_________.
Crucible and cover
These tongs are used for picking up crucibles and crucible covers _______ .
Crucible tongs
Wire gauze
Used as a support for
________ when placed across a support ring.
Used to _________ a lab burner. Not a toy noisemaker during lab.
Striker
Mortar and Pestle
Used to hold test tubes for _________ periods of
"gentle" heating.
Test tube holder
Used to
________ solids into powers.
Wash bottle
When lined with filter paper, used to filter suspended
_______ from a
________.
Filter funnel
Used for rinsing solids
_________ of a container when filtering.
Used to transfer
_______ from their original container to a scale for weighing.
Chemical spoon
Used to ________ hoses by pinching them together.
Hose clamps
Desiccators are used to provide a dry environment for a crucible or substance to _______ ________. This is critical in quantitative work where moisture can ________ mass
________. A common desiccant is anhydrous calcium chloride.
Colored indicator crystals are usually included to tell the user the condition of the desiccant. A gray color in the crystals usually indicates that the desiccant is dry and will absorb moisture. A pink color usually means that the desiccant is
"hydrated". When this happens, the water can be removed by heating it in an oven.
Desiccator
Scientific Method Notes
Definition : a systematic procedure for solving problems and exploring natural phenomena
A Sample Method
1. Observations : the foundation of the _____________ _____________. This observation might lead to a question regarding the event or characteristic. For example, you might drop a glass of water one day and observe it crashing to the floor near your feet. This observation might lead you to ask a question, "Why did the glass fall?"
2. Hypothesis : In attempting to answer the question, a scientist will form a
______________ (or some would say a guess) regarding the question's answer. In our example there are many possible hypotheses, but one hypothesis might be that an invisible force (gravity) pulled the glass to the floor. Characteristics of good hypotheses:
tentative explanations designed to guide experimentation
a useful hypothesis must be _______________
must be rejected or corrected when they conflict with experiment
Notice that the hypothesis postulates a relation between the independent and dependent _____________, one which produces the change, the other one in which the change is produced. The hypothesis should fit well with what we already believe about the natural order of things.
3. Experimentation : Of all the steps in the scientific method, the one that truly separates science from other disciplines is the process of _______________. In order to prove, or disprove, a hypothesis, a scientist will design an experiment to _________ the hypothesis. Over the centuries, many experiments have been designed to study the nature of gravity. In the late 16th century, it was generally believed that heavier objects would fall faster than lighter objects. The Italian scientist Galileo thought differently.
Galileo hypothesized that two objects would fall at the same rate regardless of their mass. Legend has it that in 1590, Galileo planned out an experiment. He climbed to the top of the Leaning Tower of Pisa and dropped several large objects from the top of the Leaning Tower. What happens when you drop objects from the top of the tower? The two different objects fall at the same rate (as long as we ignore wind resistance).
Data can be qualitative or quantitative. ________________ _____________ is obtained by making a measurement. _______________ __________ is descriptive data that does not involve numbers.
________________ indicates how close a measurement is to the accepted value. For example, we'd expect a balance to read 100.00 grams if we placed a standard 100.00 g weight on the balance. If it does not, then the balance is inaccurate. ___________ indicates how close together or how repeatable the results are. A precise measuring instrument will give very nearly the same result each time it is used. Data is most useful when collected under controlled conditions (experiments). Experiments must be repeatable and reproducible.
Develop a controlled experiment: Now we will take a closer look at what goes into making a "controlled" experiment.
variable - The quantities on which the outcome of an experiment depends are called _____________.
______________ variable - this will be the single observation, or the result we will be observing . In reality one would observe as many dependent variables as possible.
_________________ variable - this will be the single variable we elect to manipulate. All of the other variables must now be held constant so they don't influence our dependent variable. This way any change that occurs to the dependent variable can be attributed to our independent variable.
"____________ " - this will be the part of our experiment that does not receive the independent variable. This step is critical to the validity of a controlled experiment.
_________ - Make sure sufficient data is gathered to form a conclusion. (the more trials, the better the data
—usually!)
4. Analyze Data and Evaluate Results
Galileo's experiment proved his hypothesis correct; the acceleration of a falling object is independent of the object's mass. Why is this true? A few decades after Galileo, Sir
Isaac Newton would show that acceleration depends upon both force and mass. While there is greater force acting on a larger object, this force is canceled out by the object's greater mass. Thus two objects will fall (actually they are pulled) to the earth at exactly the same rate.
Theories
a ___________________ explanation for experimental data based on a set of hypotheses
tries to explain _______ or ________ something happens
must be discarded or refined when they can't _________ new experimental results
Natural laws
compactly summarize ___________ in a large amount of data
often apply only under ___________ conditions
tries to describe ____________ has happened
Making Measurements
Making measurements properly is somewhat more involved than it may seem. The proper __________ has to be selected for both the _________ being measured and the size . The measuring instrument must be used correctly to assure __________ of the measurement. Also, the __________ of the measurement must be noted.
Concept of Precision
____________ and ____________ are concepts that you are already familiar with. In day to day living their importance varies considerably with the situation. If you are describing a window in terms of how it relates to its room, then you might say it is four feet by three feet in size. Not very precise but it gets the message across. On the other hand, if you were giving someone the dimensions to cut a piece of glass so that you could replace a broken window, then saying 4 ft x 3 ft would not be nearly precise enough. If it is cut 1/16th of an inch too large, it won't fit in the frame. If it is cut too small, it won't fill the frame and will either fall through or not seal properly.
Note that whether we deal with general dimensions or replacing the glass, the precision necessary for an adequate description was dictated by the ____________ .
Also note that in each case, the numbers used to describe the size were rounded off.
Any measured value (except for counting) cannot be described exactly. __________ values are rounded off in a way that depends on how they are measured .
Where the number is rounded off is its _____________ .
Whether it is measured correctly is its ________________ .
In science classes there are rules that determine what is expected of you when you make and record measurements. ___________ ___________ measured values to the same degree of precision with which you measure them.
In general, it is expected that you will make and record your measurements to a precision that involves estimating between the closest lines on the scale.
Measurement Estimation
All instruments intended for measurement are marked according to a scale on their surfaces. You are expected to estimate _________ the lines that make up the scale of an instrument to be most precise when you use any instrument. For examples, see below.
Example 2 - parts a, b, c.
On this scale the numbers mark 1, 2 and 3 cm. Each numbered division is divided into 10 parts, so each marked division is 0.1 cm. You are expected to measurements to 0.01 cm. This is not hard but it is important. read between the lines, so you need to report
Look at line a. It extends past the 1.8 cm mark on the scale. By one estimate, it goes to
1.84 cm so that is what would be written down. You might see it the same way or estimate it at 1.83 cm or 1.85 cm. Either of those would be a reasonable measurement.
Line b presents a bit of a problem. It extends to the 1.5 cm mark on the scale and no further. It could be written down as 1.5 cm, but you are supposed to estimate between the lines. Since Line b seems to line up _____________with the mark you should write
1.50 cm. That gives the measurement to the nearest 0.01 cm and says that the measurement is right on the line as far as you can tell. In this measurement, the 0 that is after the 5 is a measured value and should be included in the measurement.
Line c is somewhat similar to line b. Here the length of the line matches a numbered mark. so you need to put in two 0's in order to show that the measurement was measured to 0.01 unit. You should record your measurement as 2.00 cm.
Also note that you shouldn’t extend any of these numbers by using any extra unmeasured digits. When dealing with precision, 1.50 cm does not mean the same thing as 1.500 cm. When writing down a measurement, put down all measured numbers including the estimate between the lines, or zero if on the line, but no more.
Example 2 - Parts d and e.
On this scale, the numbers mark 10, 20 and 30 cm.
Again each numbered division is divided into 10 parts, so each mark is 1 cm. Since we read between the lines, measurements should be made to 0.1 cm. Thus line d is 14.3 cm rather than 14 cm or 14.30 cm. Occasionally in cases like this, people will forget that each mark is 1 cm instead of 0.1 cm and read the length as 10.43 cm. Try to avoid that mistake if you can.
Line e measures at more than 20 cm and less than 21 cm and is estimated to be 20.8 cm. Be careful to note that the value is 20.8 cm and not 28 cm.
General Information on Metric Measurements
The sciences use a lot of _______________. Measurements have two parts: a
____________and a _________. The unit is just as __________ as the number.
Writing a number without its unit is nonsense
—it has no _____________. Some measurements can be measured directly, such as those below.
1. Length
2. Mass
3. Time
4. Temperature
Other measurements need to be calculated, such as those below.
1. Speed
2. Acceleration
3. Force
The units that we will be using are international standards called Le Systeme
International d'Unites , or ______Units for short.
Below is a table of the SI Base Units for some of the quantities mentioned above. Please know which unit is used to measure which quantity. NOTE: the actual SI base unit for mass is the kilogram —however, since we rarely use quantities in the science lab that are large enough to measure in kilograms, we will use a smaller, more convenient unit — grams.
QUANTITY
Length
Mass
Time
Temperature
Amount of Substance
SI UNIT meter gram second
Kelvin mole
SI UNIT SYMBOL m g s
K (this is a capital K) mol
In science, we can measure quantities that are very large or very small. To avoid having to use very large or very small numbers we change the base units by adding prefixes to them. For the purposes of this class, we'll be using only three prefixes: kilo-, centi-, and milli-.
Kilo- means 1,000 times the base unit.
1 kilo = 1,000 base unit OR 1/1,000 kilo = 1 base unit
Centi- means 1/100 times the base unit.
1 centi = 1/100 base unit OR 100 centi = 1 base unit
Milli- means 1/1000 times the base unit.
1 milli = 1/1,000 base unit OR 1,000 milli = 1 base unit
If you notice in the lists above, there is no quantity that measures ____________ . We do measure a lot of volumes in science. Therefore, we need to use a ____________ . We can derive a unit for measuring volume by using _________________ . In previous math classes you learned that the volume of a solid is calculated using the formula
______________ = length x width x height
If we measured a solid using meters then the derived unit would be cubic meters (m 3 ) .
This doesn't help us much if we want to measure the volume of a liquid. It just so happens that one ________ centimeter (cm 3 ) equals one __________ (mL) . We will also use the unit, _________ (L) , to measure the volume of liquids.
Data Analysis Notes
An experiment was performed in which plants were grown under the exposure of different colors of light
. The hypothesis being investigated is that plants will grow best in green light. After four weeks this is what our data chart looks like.
So now we have lots of ___________. What do we do next?
We want to be able to use the data in the _______ to draw a __________ about what happened in our experiment. But it can sometimes be difficult to do this when the data are organized in a chart. Let’s take our information and ________ it. A graph really helps show _________. We can use a graph to ___________the heights of our plants more easily. Two types of graphs that are often used in science are ________ graphs and
_______ graphs. Line graphs are often used to show how one variable in an experiment changes over _______. Bar graphs are used to display data in ____________ categories.
For our experiment we could use a line graph to show how the heights of the plants changed over time, or we could use a bar graph to show the heights of our four plants at the end of the experiment. Both would be good ways to show the data in our chart. Bar graphs are good to show comparisons of change between items.
How do I set up a graph?
There are a few rules about what goes where on a graph. In general, the
_______________ (or manipulated) variable is plotted on the ___________ axis ( x ), and the ______________ (or responding) variable is plotted on the ____________ axis ( y ).
The independent variable is the variable that you ________ during the experiment. In our experiment the independent variable was the color of light the plants received. The dependent variable is the one that _____________ as a result of the independent variable.
The dependent variable is the one that you ___________ or observe during the experiment. In our experiment the dependent variable was the heights of the plants.
How would the data look if we used a bar graph?
Here is a bar graph of some of the data from our experiment. This graph shows the average heights of the plants _______four weeks (at the end of 4 weeks).
How would our data look if we used a line graph?
Let’s use a line graph to show how the plants’ heights changed ___________the last two weeks of the experiment. In this case time is the independent variable, and average plant height is the dependent variable. We’ll use a different line for each light color.
So we’ve made graphs from our data. What are we supposed to do with the graphs?
Now that the data are in a graph form, we can __________the data and make ____________________ . If you look at the graphs, you should be able to see some patterns. For instance, we can see that the plants grew to different heights, so we can conclude that the color of light that the plants received may have had an effect on their growth. In order to be certain we would have to repeat this experiment many times. We can also see that the tallest plants grew in blue light, the second tallest in red light, the third-tallest in white light, and the shortest in green light. The tomato plants grown in red and blue light grew more than our control, and the plants grown in green light grew less than our control.
Wow! And we thought the green light would make the plants grow best. I guess our experiment was a waste of time, huh?
Of course it wasn’t a waste of time! It’s true that our data don’t support our hypothesis, but that’s O.K. A _____________ is an educated guess; it doesn’t have to be
_____________. Though we weren’t able to support our hypothesis, we discovered that the tomato plants that grew in the blue and red light were taller. This is important information that can lead to a new hypothesis to test.
If the plants that received green light had grown taller than the others, could we have said that our hypothesis is true?
No. We performed only a single experiment, and we used only eight plants. If we repeated our experiment, we might get slightly different results. In addition, we tested only tomato plants. If we tested other types of plants, we might find that different types of plants grow best in different colors of light.
Is a hypothesis kind of like a theory?
A ___________ is a general explanation of a set of observations about the natural world.
A theory helps explain how things happen the way they do in nature. Unlike a hypothesis, a theory is supported by _________ of data collected from
_________different experiments and observations. Theories can ____________over time as scientists gather more evidence. Hundreds of years ago scientists proposed a theory called spontaneous generation. This theory stated that some living things could develop from nonliving materials. For instance, it was thought that maggots could form from rotting meat. However, as scientists made more observations about the world around them, the theory of spontaneous generation was eventually replaced with the theory of biogenesis. The theory of biogenesis states that living things can come only from other living things. For example, it is now known that maggots hatch from tiny eggs that flies lay on rotting meat.
S C I E N C E
S C I E N C E
Can our data tell me why the tomato plants grew taller in red and blue light than in green light?
Unfortunately, our data do not answer that question. We would need to do much more research to find out how light affects plant growth. We would need to learn about the properties of red, blue, and green light. We would also need to understand how light is reflected and absorbed by plants and how plants use the light that they absorb. Maybe you could come up with another investigation!