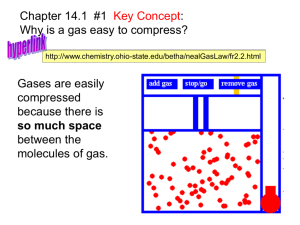
Air is a gas. Gases have various properties that can... gas pressure (p) temperature (t)
advertisement

Assignment: Gases Name: Air is a gas. Gases have various properties that can be observed including: gas pressure (p) temperature (t) volume (V) Careful scientific observation has determined that these variables are related to one another. By understanding these relationships it is possible to explain how gases behave under certain conditions. Procedure: Open the PhET simulation “Gas Properties.” http://phet.colorado.edu/en/simulation/gas-properties Click on the Run Now Button Investigate the simulation involving gas properties. Practice the following: Adding gas to the container (Note: since different gases are made of different elements, there are heavy gases and light gases, please explore the differences) Changing the size of the container (Move the little man on the side of the container) Adding and removing heat with the heat control. Opening and closing the container by sliding the lid on the top of the box. Adding Gravity Be sure to use the Reset button regularly NOTE: When you do your experiments keep extra variables constant. For example, keep the gravity and the type of the gas in the container constant (the same) for each of these first experiments. Thanks to Sarah Borenstein’s lesson on PhET Teacher’s Activity Database Experiments: Using the simulation. Explain what happens in each scenario – particle motion is important here! In your description include o immediate effects (what happens right away) o developing effects (how do things change in the system) o stabilizing effects (does the system reach a state of equilibrium with stable numbers) 1. Setting Temperature as a constant you: a. Add gas (pump the gas pump) I. Volume-Pressure graph b. Change the size of the container c. Describe (or draw) what this graph would look like 2. Setting Pressure as a constant you: a) Add heat b) Change the size of the container II. VolumeTemperature graph c) Describe (or draw) what this graph would look like 3. Setting Volume as a constant you: a. Add gas III. TemperaturePressure graph b. Add heat c. Describe (or draw) what this graph would look like 4. Setting None as a constant you will create a system and then open the lid on the top of the box. Explain what happens when you: a) Add gas b) Add heat c) Change the container size Thanks to Sarah Borenstein’s lesson on PhET Teacher’s Activity Database 5. Setting None as a constant you will create a system and then attempt to overwhelm the container to fail (the lid pops off). Explain what happens when you: a) Add gas b) Add heat c) Change the container size 6. Using your results as a guide, explain each of the following scenarios. Make sure to refer to which scenario(s) can be used as evidence for your answer. a. Explain why bicycle tires seem more flat in the winter than in summer. b. Explain why a can of soda pop explodes if left in the hot sun. c. A rigid container filled with a gas is placed in ice (ex. Nalgene bottle). What will happen to the pressure of the gas? What do you think will happen to the volume? d. An infected tooth forms an abscess (area of infected tissue) that fills with gas. The abscess puts pressure on the nerve of the tooth, causing a toothache. While waiting to see a dentist, the person with the toothache tried to relieve the pain by treating the infected area with moist heat. Will this treatment help? Why or why not? 7. For your final experiment you will use the Gravity Slider. Describe what happens when you add gravity as a factor to each of the following. a) Add gas b) Add heat c) Change the container size d) What is the chance of causing system failure with lots of gravity as opposed to low gravity? 7. Using your results from question 7 as a guide, create 2 real life scenarios that give evidence of the impact of gravity on gases. a. b. Thanks to Sarah Borenstein’s lesson on PhET Teacher’s Activity Database