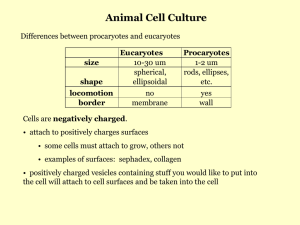
EFFECTS ON IN MICROCARRIER BIOREACTORS BY SHANE CROUGHAN
advertisement

HYDRODYNAMIC EFFECTS ON ANIMAL CELLS IN MICROCARRIER BIOREACTORS BY MATTHEW SHANE CROUGHAN B.S. Chemical Engineering University of California at Berkeley (June, 1983) in Submitted to the Department of Chemical Engineering Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June, 1988 Massachusetts Institute of Technology Signature of Author: Department of ChemicallEngineering February 23, 1988 Certified by: Daniel I.C. Wang Professor of Chemical and Biochemical Engineering Thesis Supervisor Accepted by: Robert C. Armstrong Professor of Chemical Engineering Chairman of the Committee for Graduate Students - 1 - A HYDRODYNAMIC EFFECTS ON ANIMAL CELLS IN MICROCARRIER BIOREACTORS BY MATTHEW SHANE CROUGHAN in Submitted to the Department of Chemical Engineering Massachusetts Institute of Technology on February 23, 1988 partial fulfillment of the requirements for the Degree of Doctor of Philosophy in Chemical Engineering ABSTRACT In this thesis, a fundamental approach was initiated toward the design of microcarrier bioreactors for industrial animal cell culture. Cell damage from hydrodynamic forces was considered along with the Hydrodynamic effects on the agitation requirements for mass transfer. growth and death of FS-4 cells on microcarriers were studied in small stirred-tank bioreactors. With mild agitation, growth was not affected by changes in fluid viscosity, microcarrier concentration, or level of appeared to unaffected by the hydrodynamic agitation, and thus With high agitation, a reduction in net growth was environment. observed and was found to be entirely due to cell death and removal and not growth inhibition. The mechanisms of hydrodynamic death were investigated through experiments on the effects of fluid viscosity, microcarrier concentraCell death in microcarrier bioreactors was tion, and agitation power. found to occur through microcarrier-eddy interactions, microcarrier collisions, and, in unusual circumstances, time-average flow fields. Microcarrier-eddy interactions led to cell death when the turbulence For dilute generated eddies which were smaller than the microcarriers. to the proportional be to cultures, the specific death rate was found both For regime. concentration of eddies in the viscous dissipation with increase to dilute and concentrated cultures, cell death was found agitation power and decrease with fluid viscosity. A quantitative expression was derived which related the cell microcarrier concentration, kinematic to the population kinetics the included expression The agitation power. and viscosity, hydrodynamic of function a contributions from cell growth, which was not environment, and cell death from both microcarrier-eddy interactions and microcarrier collisions. The expression was used to quantify the tradeoff between hydrodynamic cell death and oxygen transfer upon scale-up. An illustration was presented to show how bioreactor design can be approached as an optimization problem involving quantitative rate expressions. Thesis supervisor: Title: Dr. Daniel I.C. Wang Professor of Chemical and Biochemical Engineering - 2 - TABLE OF CONTENTS ACKNOWLEDGEMENTS ..................................................... LIST OF FIGURES..................................................... . LIST OF TABLES....................................................... INTRODUCTION......................................................... LITERATURE REVIEW AND THESIS FORMULATION Hydrodynamic effects on cell growth................................ The cell cycle and DNA replication................................. Assessment of cell death through DNA release measurements.......... Hydrodynamic effects on cell metabolism............................ Microscopic flow fields and cell deformation mechanics............. Effects of microcarrier and cell concentration..................... Hydrodynamic damage in microcarrier cultures....................... Cell-liquid transport in microcarrier cultures..................... Fluid-lift, airlift, and stirred-tank bioreactors.................. Cell damage from turbulence........................................ Protective polymers................................................ Cell damage from time-average flow fields.......................... MATERIALS AND METHODS Cell types......................................................... Cell culture reagents and procedures............................... Culture aeration and effect of dissolved oxygen on cell growth. Agitation power determinations..................................... Cell concentration determinations.................................. DNA assay.......................................................... - 3 - Specific fluorescence of FS-4 cellular DNA........................... 59 Effect of cell concentration in T-flasks............................. 63 Selection, use, and characterization of inert microcarriers........ 64 Viscosity and density measurements................................. 66 Selection and use of thickening agent.............................. 68 Rheological evaluation of dextran solutions......................... 73 Cell damage from direct sparging................................... 76 RESULTS AND DISCUSSION............................................... 78 CHAPTER 1. HYDRODYNAMIC EFFECTS ON FS-4 CELLS WITH MILD AGITATION 1A. Effect of fluid viscosity with mild agitation................... 79 1B. Growth at different microcarrier concentrations................. 81 1C. Growth in stagnant T-flasks.................................... 87 1D. Effect of cell concentration on growth in T-flasks.............. 89 1E. Effect of inert microcarriers with mild agitation.............. 91 1F. Cell growth with mild agitation................................ 93 CHAPTER 2. 2A. HYDRODYNAMIC EFFECTS ON FS-4 CELLS WITH HIGH AGITATION Growth reduction and cell removal under high agitation........ 95 2B. Random cell removal through normal forces......................100 2C. DNA release under high agitation...............................104 2D. Growth and death under high agitation...........................106 2E. Hydrodynamic regulation of cell growth..........................113 2F. No secondary growth after cell removal..........................114 2G. Hydrodynamic effects on glucose consumption....................117 - 4 - CHAPTER 3. HYDRODYNAMIC PHENOMENA IN y-CHO CULTURES 3A. Growth, reversible cell removal, and secondary growth..........121 3B. Hydrodynamic resistance through secondary growth...............125 3C. Selection of model cell line...................................129 CHAPTER 4. MECHANISMS OF HYDRODYNAMIC DEATH IN DILUTE CULTURES 4A. First, second, and higher order mechanisms of death........... .131 4B. Effects of power and viscosity in dilute cultures............. .132 4C. Cell death through microcarrier-eddy interactions............. .136 4D. Hydrodynamic forces in microcarrier-eddy interactions......... .143 4E. Kinetics of cell growth and death in dilute cultures.......... .146 4F. Cell damage from time-average flow fields..................... .153 4G. Effects of cavitation......................................... .155 CHAPTER 5. 5A. MECHANISMS OF HYDRODYNAMIC DEATH IN CONCENTRATED CULTURES Effect of microcarrier concentration with high agitation...... 159 5B. Cell death through microcarrier collisions..................... 161 5C. Effects of agitation power on cell death from collisions....... 167 5D. Effects of fluid viscosity on cell death from collisions....... 171 CHAPTER 6. CELL DAMAGE FROM DIRECT SPARGING 6A. Effect of antifoam addition and direct sparging on growth......178 6B. Mechanisms of cell damage from direct sparging.................181 - 5 - CHAPTER 7. BIOREACTOR DESIGN AND OPTIMIZATION 7A. Mass transfer requirements............................... .185 7B. Basic principles in bioreactor design and optimization... .192 7C. Illustration of optimization calculations................ .196 206 7D. Protection through polymer absorption and viscoelasticity .... -.. SUMMARY AND CONCLUSIONS................... .....................- 210 RECOMMENDATIONS FOR FUTURE RESEARCH....... ....... ................ 212 .... - ......... ..- ..NOMENCLATURE.............................. . ....... - .- 217 - ........... - -. -. -. REFERENCES................................ . . . -.. . . . - -. 224 APPENDIX 1: Calculation of the relative DNA release under high agitation ....... 234 APPENDIX 2: Estimation of the yield and maintenance coefficient for oxygen.....240 - 6 - ACKNOWLEDGEMENTS The author wishes to acknowledge the following organizations for financial support: National Science Foundation of the United States of America for supporting much of this research through the Engineering Research Center Initiative to the M.I.T. Biotechnology Process Engineering Center under the cooperative agreement CDR 8500003 Biogen, Inc. for supporting the initial phases of this research Exxon Corporation and E.I. Dupont Denemours Corporation for fellowship support during my coursework The author also wishes to acknowledge the following individuals and greater beings who have contributed to this thesis: God for creating the earth, the universe, and M.I.T., and for serving as the ultimate reviewer of this research Claire Malloy Croughan and Mabel Wilson Croughan for creating me, and for their continuous support of my education Kathryn Colleen Kidd, soon to be Kathryn Kidd Croughan for her joy when the experiments were successful and her support when they were unsuccessful Professors C.K. Colton, T.A. Hatton, C.L. Cooney, and A.J. Sinskey for serving as members of my thesis committee, for their review of this research, and for their insightful comments - 7 - Jean-FrancoisHamel, Elizabeth Sayre, Jeff Bigler, and Bruce Woodson for their participation in this research Don Giard and the staff for their sharing of the Cell Culture Center at M.I.T. knowledge of animal cell for culture, their services and help, and for Don's advice regarding my golf swing Professor Preetinder S. Virk for his useful and stimulating suggestions, and for his good wit John Aunins, Steve Lee, Adema, Steve Perry, Jono Goldstein, Mark Applegate, Marc Davidson, Dave Robbins, Linda Cima, Brian Kell, Mike Glacken, Enno Chris Hwang, Dawn Orton, Shiping Wang, and many others for their suggestions and help, and for the good times in the lab Professors Harvey Blanch and Frances Arnold, and Robert Lesch for spawning my interest in biotechnology at U.C. Berkeley Ruth Ayers, Diana Kenney, Marguerite Walsh, and Lynne Comeau for their services and good humor Professor Daniel I.C. Wang (last but not least) Professor Wang has been an outstanding research advisor. He exhibits a tremendous commitment to the training of his students and to the advance of scientific knowledge. For Professor Wang, who reminds me of a wise and dexterous owl, the following poem was written: An owl can see in the dark and find lost coins An owl can soar above the trees and view the entire forest An owl can build a nest and raise more owls - 8 - LIST OF FIGURES Figure 1. Growth of Vero cells at different stirring speeds (Hirtenstein and Clark, 1980)....................... 29 Figure 2. Relative growth extent of FS-4 cells versus integrated shear factor (Hu, 1983)........................... 31 Figure 3. Schematic of a sphere caught in a simple shear field..................................... 43 Figure 4. Relative growth extent of FS-4 cells versus impeller tip speed (Hu, 1983)................................ 45 Figure 5. Effect of dissolved oxygen on growth of FS-4 cells.............................................. 52 Figure 6. Increase in fluorescence versus concentration of calf-thymus DNA.............................. 58 Figure 7. Growth of FS-4 cells in stagnant T-flasks................................................. 61 Figure 8. Specific fluorescence of FS-4 cells taken from T-flasks during exponential growth....................... 62 Figure 9. Shear stress versus shear rate for various medium solutions......................................... 67 Figure 10. Chemical effects of dextran on growth of FS-4 cells with mild agitation............................. 72 Figure 11. Effect of 20 g/L dextran (78,500 MW) on growth of FS-4 cells with mild agitation......................... 80 Figure 12. Growth of FS-4 cells at various microcarrier concentrations with mild agitation..................... 83 Figure 13. Maximum surface coverage of FS-4 cells versus microcarrier concentration.................................. 84 Figure 14. Average specific growth rate of FS-4 cells versus microcarrier concentration.................................. 86 Figure 15. Growth of FS-4 cells in stagnant T-flasks................................................. 88 Figure 16. Effect of cell concentration on growth of FS-4 cells in T-flasks................................. 90 - 9 - Figure 17. Effect of inert microcarriers on growth of FS-4 cells with mild agitation......................... 92 Figure 18. Attached cell concentrations for FS-4 microcarrier cultures at different stirring speeds................. 96 Figure 19. Removal of whole FS-4 cells from microcarriers at different stirring speeds.................... 97 Figure 20. Specific rate of whole cell removal versus estimated mitotic index for FS-4 microcarrier cultures......102 Figure 21. Growth of FS-4 cells on microcarriers followed agitation at different stirring speeds after concentration.........105 Figure 22. Cumulative release of DNA into suspension for 15 g/L FS-4 microcarrier culture at 150 RPM....................107 Figure 23. Measured DNA release for 15 g/L culture at 150 RPM versus release expected under three different scenarios............112 Figure 24. Attached cell concentrations for FS-4 cultures grown at different stirring speeds.................................115 Figure 25. Glucose concentrations profiles for 15 g/L FS-4 microcarrier cultures..............................118 Figure 26. Suspended and attached cell concentrations for y-CHO microcarrier cultures at different stirring speeds...........122 Figure 27. Attachment and growth on new microcarriers for y-CHO cells taken from suspension of microcarrier culture..........126 Figure 28. Viable cells produced per microcarrier for y-CHO cultures at different stirring speeds....................128 Figure 29. Effect of 20 g/L dextran (78,500 MW) on net growth of FS-4 cells at different stirring speeds...........133 Figure 30. Effect of 50 g/L dextran (78,500 MW) on net growth of FS-4 cells at different stirring speeds...........135 Figure 31. Relative growth rate vs. Kolmogorov eddy length scale for FS-4 cultures with 0.2 g/L microcarriers.......................138 Figure 32. Relative growth rate vs. Kolmogorov eddy length scale for FS-4 cultures with 5 g/L microcarriers (data from Hu,1983).....140 Figure 33. Maximum cell concentration versus Kolmogorov eddy length for chicken embryo fibroblasts on 5 g/L microcarriers (data from Sinskey et al., 1981)...................................142 - 10 - Figure 34. Specific death rate in dilute FS-4 cultures vs. the concentration of eddies in the viscous dissipation regime......149 Figure 35. Specific death rate versus average power input per unit mass for cultures of Vero cells on microcarriers, FS-4 cells on microcarriers, and freely-suspended protozoa.........152 Figure 36. Effect of dextran (78,500 MW) on net growth of FS-4 cells in a reactor with strong time-average shear fields......154 Figure 37. Specific death rate versus eddy concentration for dilute FS-4 cultures with different levels of maximum shear stress from time-average flow fields...............................156 Figure 38. Effect of inert microcarriers on net growth of FS-4 cells with high agitation.......................160 Figure 39. Net growth rate under high agitation for FS-4 cultures with different concentrations of inert microcarriers...............165 Figure 40. Net growth at different stirring speeds for FS-4 cultures with 15 g/L inert microcarriers..................168 Figure 41. Second-order death rate constant q2 versus average power input per unit mass for FS-4 cultures................170 Figure 42. Effect of dextran (78,500 MW) on net growth of FS-4 cells at different stirring speeds with 15 g/L inert microcarriers....................................172 Figure 43. Normalized second-order death rate constant versus kinematic fluid viscosity for FS-4 cultures.................174 Figure 44. Second-order death rate constant versus the ratio of average power input per unit mass to kinematic fluid viscosity for FS-4 cultures...................................................176 Figure 45. Effect of antifoam addition and direct sparging on net growth of FS-4 cells on microcarriers.......................180 Figure 46. 100-liter reactor with 4-bladed flat-blade turbine and 4-bladed pitched-blade turbine.................................198 Figure 47. Estimated maximum concentration of FS-4 cells vs. average power input per unit mass at different fluid viscosities in 100-liter reactor................205 Figure 48. Cumulative change in Hoechst-dye fluorescence for culture fluid from 15 g/L FS-4 cultures........................235 - 11 Figure 49. Cumulative release of DNA into suspension for 15 g/L FS-4 culture at 150 RPM. Data is shown for three different methods of calculation.............................................239 LIST OF TABLES Viscosity and density measurements Table 1. for various medium solutions............................. .......... 69 Table 2. Glucose uptake by FS-4 cells in 15 g/L microcarrier cultures at different stirring speeds..........119 Geometry of 100-liter bioreactor Table 3. for optimization illustration............................ .......... - 12 - 199 INTRODUCTION Large-scale animal cell culture is currently being developed for the cells that will adhere riers appears van Wezel with high separation (Nahapetian, noted its advantages. and densities cell medium/cell simple 1986). technique and have the microcarrier have employed Many researchers First developed by technique can provide a homogeneous the microcarrier environment culture growth on the surface of microcar- to surfaces, promising for industrial operations. (1967), culture of For the industrial production of many valuable proteins. improvements Nonetheless, of the technique have and improved microcarriers, media formulations, and inoculation procedures. Although primarily been reactors limited have been no cultures, to developed thorough study the development of new and successfully used has been published for microcarrier which compares and determines the best reactor designs and agitation procedures. It widely accepted but not well documented that animal cells on is microcarriers are especially susceptible to damage from fluid-mechanical forces. wall, This susceptibility results from the lack of a protective cell the dividual translate; relatively large size of animal cells, and the lack of incell mobility. they therefore Anchored cells can not reduce can the not net experienced upon exposure to fluid-mechanical forces. - 13 - freely forces rotate and or torques In microcarrier cultures, agitation is required for cell-liquid mass growth, cell maximum For mixing. adequate liquid-phase and (oxygenation), transport mass gas-liquid transport, transfer mass be must achieved with little or no detrimental effects from hydrodynamic forces. Although condition this cultures, laboratory densities culture or it can becomes are volumes more increased. attain to difficult low-density in attained readily be as cell scale-up Successful to large-volume cultures will require a thorough understand- high-density, ing of the hydrodynamic and mass transport phenomena. Accordingly, fundamental the overall to approach microcarrier bioreactors. objective of this work is the design, operation, to formulate a scale-up of and The general approach will be to: 1) determine the effects of momentum transfer on cell growth, independent of the effects of heat and mass transfer, 2) perform experimental investigations on the mechanisms of cell damage from fluid-mechanical forces, 3) develop theoretical models to describe the mechanisms of cell damage, 4) use the verified models of hydrodynamic damage, models of mass transport, to develop along with published engineering a quantitative approach to the design of microcarrier bioreactors. The knowledge generated by this research will be useful in microcarrier bioreactors the design of and will add to the general understanding of hydrodynamic phenomena. - 14 - LITERATURE REVIEW AND THESIS FORMULATION Hydrodynamic Effects on Cell Growth of well-defined hydrodynamic forces on cell growth have The effects No investigations have been performed not been thoroughly investigated. to normal with regard forces and only a few have been performed with regard to shear stresses. Under flow conditions which do not lead to substantial cells from their growth surface, removal of cell growth appears to be unaffected by fluid shear stresses. For endothelial cells growing on glass coverslips, (1981) Dewey et al shear stresses up endothelial cells found that cell dyne/cm affected by fluid found that cell growth was not to 8 dyne/cm 2 . In more recent growing on plastic coverslips, growth was affected by not experiments Sprague et al shear stresses up with (1987) to 30 2 For moderate to high levels of shear stresses, a few quantitative studies have been performed regarding removal of cells from their growth surface. (1986) observed extensive cell removal Viggers et al surface for shear stresses of 128 dyne/cm2 . Vero, and MRC-5 cells grown on plastic or observed extensive cell removal of 30 dyne/cm2 or higher. In experiments (1985) Stathopoulos and Hellums - 15 - from the growth In experiments with BHK, glass slides, Crouch et al for shear stresses in (1985) slides, coverslips, In experiments with endothelial cells on glass the range with kidney cells on glass observed cell removal for shear stresses of 6.5 dyne/cm2 or greater. flow conditions which result in Under growth surface, their from attached 1985; is generally Stathopoulos measured in terms of the viability reported and Hellums, to be greater of trypan blue ability to reproduce and grow. which remain than 90% (Crouch et al, the However, 1985). of cells substantial removal exclusion and cells these viabilities are of the in terms not The hydrodynamic effects on cell growth are unknown for flow conditions which can cause extensive cell removal or death. When animal frequently is growth net cells are not been investigated. or death, objective inhibited, in the increase observed with an level of The biological basis behind this reduction in net growth has agitation. cell a reduction in an agitated vessel, grown in of a this The reduction could be due to growth inhibition, combination thesis unchanged, microcarrier cultures. or is of to death and growth investigate by enhanced the inhibition. whether cell hydrodynamic One growth is forces in The primary thrust will be to determine whether growth inhibition and/or cell death account for the reduced net growth in overagitated microcarrier cultures. The Cell Cycle and DNA Replication To understand the potential hydrodynamic effects on cell growth, one must first understand the growth cycle of animal cells. their DNA and pass it on to their progeny, - 16 - cells pass To replicate through a cycle with four biosynthesis General the DNA synthesis during the G2 phase, and actual For almost all animal cells growing the M phase. during during for mitosis 1983). and M (Alberts et al, during the G1 phase, occurs preparation S phase, mitosis G1 , S, G2, sequential phases: in culture, the duration of the S, G2, and M phase is roughly constant at 7 hours, 3 hours, Alberts et al, 1-2 and (Darnell respectively hours, et al, 1986; A change in the average doubling time of a cell 1983). population will occur because of a change in the duration of G, and/or because some fraction of the cell population enters or exits an out-ofcycle, non-growing state termed Go. There some debate whether a Go state actually exists or whether is all differences in doubling time are due to differences in the duration of the Gl phase the et al, At the restriction point, or at the passage between Go and G. G decision is made whether a given cell will replicate. behind decision this Independent of the will irrespective pass is not basis behind through the although understood, fully factors environmental several on depends divide Clearly, Darnell et al, 1986). 1978; a restriction point for cell growth exists at some point late however, in (Pardee (Darnell the decision, S, G2, and of the "post-decision" environment. a M cell et The basis it clearly al, 1986). stimulated phases to essentially Non-growing cells are almost always held up in the Gl or Go phase. There inhibit in are at least two ways cell growth. The which high levels of agitation may hydrodynamic - 17 - environment may affect the may affect the or it capability required for cell growth, biosynthetic These effects would be uncoupled cell's decision whether to replicate. with changes in cell metabolism which are not involved with growth. If growth, cells rather than just inhibit hydrodynamic stresses actual kill it would be interesting to know if only at certain positions in cells are killed at random or the cell cycle. When anchorage-dependent animal cells grow on a surface, cells in mitosis generally round-up and assume a less flattened morphology than the interphase cells (Alberts et mitotic cells can be selectively and For some cell lines, al, 1983). viably removed by applying mild shear growth to the stresses surface This procedure does not (Prescott, 1976; Pardee, 1978; Terasima, 1962). Cell work for many cell lines and growth surfaces (Freshney, 1983). removal is frequently not selective for mitotic cells if the agitation is excessive (Terasima, 1962) or if the cells are grown on microcarriers and have not been pretreated with colcemid (Ng et al, 1980; Mitchell and Wray, 1979). If anchored cells are killed by hydrodynamic forces, it is unclear whether death occurs before, during, or after removal of the cells from the microcarriers. result in cell It is also unclear whether lysis and disintegration. If death will removal, eventually lysis, and disintegration occur, counting whole cells in suspension will not fully Accordingly, one may wish to component which is released by the account for the number of cells killed. analyze for a stable disintegrated cells. intracellular It is then important - 18 - to know how much of this component was originally contained by each cell. Assessment of Cell Death through DNA Release Measurements quantitatively To assess one could analyze for DNA release forces, lysis and death cell from hydrodynamic into the culture fluid. For normal diploid cells, the DNA content of each cell is solely a function of the cell's position in the growth cycle and should not depend on the hydrodynamic populations environment per well-defined is se. The specific and thus DNA DNA release content can be cell of directly correlated to the number of cells killed. The amount If lysed. of DNA release will depend upon what type of cells are Go or G in cells diploid equivalent per cell. release two will be are lysed, cells in If diploid equivalents the DNA release will be one G2 per or M are lysed, cells. the DNA If cells are removed randomly throughout the cell cycle, the average DNA release will depend upon the distribution of the cell population among the phases of For a cell population growing exponentially through the growth cycle. binary fission, the distribution function, f(t), of cell ages is given by (Johnson, 1961) f(t) is where t times dt is = the time t/Td) (ln 2) 2(1 td (Eq. 1) since mitosis, Td is the doubling time, and f(t) the fraction of the cell population with an age between t and t + dt. - 19 - There average least at are two to approaches reasonable determine the For the first approach, one DNA content of a cell population. can assume that a distinct Go phase does not exist and that all cells are going the through cell All cycle. changes therefore due to changes in the duration of Gi. Sa The average DNA content then given by is Sa, per cell, rate are in growth f = (Eq. 2) f(t) S(t) dt where S(t) is the DNA content per cell in diploid equivalents at a given time t since mitosis. throughout constant If one assumes that the rate of DNA synthesis is the S period, the integral above can be solved to yield 2 (TM + TG2 + TS)/Td Sa + 2 (TM + TG2)/Td (Eq. 3) = 2 where TS, TG2, and TM are the respective durations of the S, G2 , and M phases. For a second approach to determine the average DNA content of cell populations, one can assume that a separate, out-of-cycle Go phase does exist and that the duration of the G1 phase is specific growth rate will then depend population, IGo, which resides in Go. - 20 - upon actually constant. the fraction of The the The average DNA content of the population is Sa then given by Td - Tc(l - = 0 .5 (2 (TM + TG2 + Ts)/Tc + 2 (Eq. 4) (TM + TG2)/Tc ) Td where Td is the observed doubling time and Tc is the total cycle time (TGl + TS + TG2 + TM). this In quantitatively is death cell thesis, measurements of the DNA content of culture fluids. are related the models through to cell numbers assessed through The DNA measurements presented above. For conditions of excessive agitation, the amount of cell death is compared The comparisons are used to determine growth. to the net reduction in whether cell growth is affected by hydrodynamic forces under conditions of excessive agitation. The assessed effects However, with monitoring through precursors, such the is on cell growth incorporation of different levels at thymidine, as when there excessive forces of hydrodynamic extensive cell lysis, agitation, unresolved complications radioactive the and difficulties be could also radioactive of DNA agitation. such as that which occurs precursor technique (Aherne et al, has 1977). The radioactive precursor technique is therefore not used in this thesis. Hydrodynamic Effects on Cell Metabolism Hydrodynamic forces may not only lead to cell death and lysis but could also lead to significant changes in cell metabolism. - 21 - Many of the metabolic events that occur in animal cells are not directly related to forces, or not cell growth Whether growth. cell not are which events metabolic is inhibited by hydrodynamic associated growth could be influenced by agitation. metabolism, studies have shown that cell shape, several cells, For endothelial and endocytotic activity can be strongly affected by fluid flow. The results are summarized as follows: 1) the cells align and elongate in the direction of flow over 1-2 days exposure to shear stresses above 5 dyne/cm 2 (Dewey et al, 1981; Levesque and Nerem, 1985) 2) the histadine activity decarboxylase of cells the increases linearly with shear stress for 1-2 hour exposures to shear stresses above 2.8 dyne/cm 2 (DeForrest and Hollis, 1980; Hollis and Ferrone, 1974; Rosen et al, 1974) 3) cell to permeability increases proteins for 1-hour 2 exposures to shear stresses above 7 dyne/cm (Fry, or longer 1968; DeForrest and Hollis, 1980) 4) prostacyclin production increases 6-fold upon exposure 2 and increases shear stress of 10 dyne/cm to a mean 16-fold upon a pulsed exposure at 1 Hz between 8 and 12 dyne/cm2 (Frangos et al, 1985) 5) exposure to a shear stress of 30 dyne/cm2 significantly enhances receptor-mediated binding, internalization, and degradation of low-density lipoproteins (Sprague et al, 1987) These results are due, at least in part, to the natural adaptation of endothelial cells to fluid flow in blood vessels. - 22 - For cell lines other than endothelial, there has been a very limited number direct of on how cell metabolism is studies (1986) and Hu Dodge cells, For hybridoma environment. hydrodynamic affected by the found that net cell growth was slightly reduced, but volumetric glucose consumption was unchanged, by high levels of agitation. be to lactic acid by dead or in part, to conversion of glucose due, This result may It may also indicate that the specific glucose uptake rate lysed cells. of the cells was slightly increased by agitation. epithelial For kidney glass on cells exposure shear to stress between levels of agitation on specific direct 13 dyne/cm 2 . P-interferon productivity. Besides reports in differences of Giard et al, It 1979). cell cultures metabolism between agitated and stagnant (or nearly stagnant) (Bryant, 1969; For found no are there studies, and 6.5 Schulz et al (1986) recombinant mouse-L cells on microcarriers, these and (1985) found that post-shear urokinase release was increased by Hellums effects Stathopoulos slides, is unclear, however, whether these differences are due to mass or momentum transfer. this thesis, a thorough hydrodynamic effects on cell In investigation growth, while is only made a into very the limited investigation is made into the hydrodynamic effects on cell metabolism. Before any reactor design can be fully optimized, hydrodynamic effects on both cell growth and metabolism will have to be fully understood. - 23 - Microscopic Flow Fields and Cell Deformation Mechanics When shear flow occurs over a cell anchored to a surface, the microaffected by the protusion of the cell. scopic flow field is a net torque created by the flow around its experiences The cell circumference. This torque is countered by the adhesive force between the substrate and the front of the cell. (1972a) attempted to Hyman for solve the microscopic From a literature (1972b). that his published solution was incorrect survey, the problem appears to remain unsolved. field He later admitted a cell protuding into a linear shear field. around flow The relevant Reynold's number is given by Re = the undisturbed shear rate, where Y is rical shape assumed), range (Eq. 5) Y h2/v around the For the interest in cell culture, the flow cell will be creeping with a Reynold's currently Because are of the cell height (hemisphefluid viscosity. the kinematic and v is of parameters which h is available visualization flow number below 0.5. techniques not can resolve to better than 20-30 microns, any theoretical solution to the microscopic flow field can not be experimentally verified with actual cells. Because there is no available solution to the microscopic flow field around a cell protuding into a shear field, have been correlated with the anchored cells stress. This approach is all shear 24 - on undisturbed wall shear is known taken not only because very little - effects also because but fluid mechanics, detailed the about the microscopic flow field is coupled to the cell shape and cell deformation mechanics. at least in The cell shape will be determined, field and hydrodynamic local flow field, one To determine forces. simultaneously must part, by the local flow the the cell shape and solve for both the fluid motion and the cell deformation mechanics. The deformation mechanics of red blood cells have been successfully from described Evans, 1983). purely 1972, (Hochmuth et al, models physical The deformation mechanics of nucleated cells are only In contrast currently being elucidated (Sato et al, 1987; Cheng, 1987). to a red blood cell, a nucleated cell with dynamic internal structures can not be modelled as a Newtonian will cells in Furthermore, membrane. actively 1973; solution surrounded by an elastic response to fluid shear stresses, shape by adjust their lengthening and nucleated shortening Purely physical models for nucleated cells would cytoskeleton fibers. be successful only for time scales which are shorter than the response time of the cell. It will clearly be a challenging problem to solve for the deformation mechanics of nucleated cells. the In most microcarrier cultures, cells The are exposed not only to shear forces, effects of normal forces forces effects may account for in laminar flow the but also to normal forces. on animal cells have, never been quantitatively investigated. typically fields - and 25 - The flow field is turbulent. to my knowledge, The undiscovered role of normal poor global agreement between hydrodynamic shear effects in stirred tanks, (1987). as reported by Rosenberg et al such The poor agreement may also arise due to the limited understanding of turbulence. For microcarrier cultures in turbulent bioreactors, one can only roughly estimate the magnitude and direction of the hydrodynamic forces on the cells. investigate To damage in turbulent information should be obtained both through direct experiments reactors, with the mechanisms of hydrodynamic turbulent reactors and experiments with well-defined hydrodynamic forces. mechanisms of cell damage in of translation through turbulent In forces. from this thesis, reactors investigated through experiments with turbulent reactors. attempt is results are the directly A reasonable terms of normal forces and shear made to present results in Further elucidation of the effects of well-defined forces, inc- luding the incorporation of cell deformation mechanics and the solution of microscopic flow fields, are left for future research. Effects of Microcarrier and Cell Concentration For industrial cell high culture, cell concentrations can potentially reduce production costs through four different means: 1) increased volumetric productivities and thus reduced capital and overhead costs, 2) decreased labor required per cell and thus reduced labor costs, 3) increased concentrations of the desired product and thus reduced purification costs, and 4) increased concentration other of - 26 - cell-derived products (growth factors, accelerated growth, which may result in etc..) accelerated product formation, or reduced serum requirements. For a specific cell line, the maximum number of cells per microcarAccordingly, rier is approximately constant. one wishes to if obtain It high cell densities, microcarrier concentrations must be increased. is therefore important to understand how an increase in microcarrier concentration will affect the hydrodynamic environment near the cells. concentra- of microcarrier data on the effects Currently-available tion is limited and frequently contradictory. In small spinner flasks, Hu et al (1985) found no decrease in growth rate when bare microcarriers were added to cultures, or when cultures with 5 g/l microcarriers were concentrated to 15 g/l. In contrast, however, Feder and Tolbert (1983) report that cell damage increased above decreased growth 12 g/l. rates when microcarrier occurs as et concentrations al Furthermore, Mered microcarrier concentrations (1980) were are observed increased from 1 to 5 g/l. If there is no detrimental effect of microcarrier concentration, and if similar microcarrier chemical environments concentration should lead to cell concentration. often attributed, microcarrier an increase in a proportional increase in maintained, When this proportionality without apparent proof, microcarrier collisions. of can be is not observed, it is to a detrimental effect from However, in most studies, the physical effects concentration are not - - 27 clearly separated from the of effects chemical effects detrimental The concentration. cell attributed to collisions may actually be due to nutrient limitations or other some effect from high experiments previous were Furthermore, concentrations. cell conducted with various culture The role of collisions, different levels of agitation. vessels vessel the geometry, impeller configuration, at along with any other hydrodynamic effects of microcarrier concentration, may upon the and depend level of agitation. Accordingly, the bioreactor design engineer now faces the following unanswered questions: 1) What are the physical effects associated with microcarrier concentration? 2) How do these effects vary with the level of agitation? 3) Do collisions between microcarriers lead to cell damage? If so, at what level of agitation, and at what rate of damage? In this thesis, results are presented which address these questions. Hydrodynamic Damage in Microcarrier Cultures Hirtenstein and Clark in microcarrier cultures. there is in Their data, shown in Figure 1, indicate that an optimal stirring speed near 60 rpm for growth of Vero cells a 250-ml traditional speeds was by have studied the effects of agitation (1980) spinner vessel. The poorer growth at the slow likely due to transport limitations, probably brought about either inadequate surface aeration or incomplete suspension of the beads. The poorer growth at the high speeds was likely due to - 28 - 107 60 rpm 1- 6 10- z 0 0-J 0 O- 90 rpm U zIUiJ U 10 5 30 rpm 120 rpm 4 101 2 3 4 5 6 7 TIME (DAYS) Growth of Vero cells at different stirring speeds (Hirtenstein and Clark, 19 80) Figure the show clearly The results from excessive agitation. detrimental effects between mass trade-off transport and in Figure 1 hydrodynamic Nonetheless, these results can not be damage in microcarrier cultures. readily translated into the design of an industrial-scale reactor. Sinskey et (1981) al agitation on microcarrier by the and Hu cultures studied effects the small stirred vessels. in of As shown relative cell growth can be correlated Figure 2, data of Hu in (1983) have as given by with an integrated shear factor (ISF), 2 wrN Di ISF (Eq. 6) = (Dt - Di) where N is the impeller rotation rate, Dt is the vessel diameter, and Di is the impeller diameter. The integrated shear factor is a measure of the strength of the shear field between the impeller and vessel wall. Although the correlation of net growth with integrated shear factor is useful, it is not readily incorporated into a mechanistic model of hydrodynamic effects on cells attached to microcarriers. must be analyzed in mechanics. must be terms of the more fundamental parameters The response of the cells to the hydrodynamic clearly elucidated. The problem The effects of mass of fluid environment transport must be included in a more thorough quantitative analysis. Cell-liquid Transport in Microcarrier Cultures When investigating the effects of hydrodynamic forces, or momentum transfer, one must eliminate any other effects due to mass and heat - 30 - Impeller Diam. (cm) 100 75 Vessel Vol. (ml) 3.2 250 4.1 250 5.1 250 7.5 2000 8.5 2000 50 25 0 10 20 30 INTEGRATED SHEAR FACTOR (SEC~ 1 ) Figure 2. Relative growth extent vs. integrated shear factor (Hu, 1983) transfer. growth cell induce may it cultures, microcarrier through simply is the subsequent mixing chemical of elimination the and Dunn 1974; 1973, (Stoker, gradients shaken, a stagnant cell culture is If 1984). Ireland, For important to understand whether there are any transport limitations between the cells and medium. such as a microcarrier, When a sphere, is suspended in a turbulent vessel, mass transport between the sphere surface and bulk medium can be described by (Sano et al, 1974; Chaudhari and Ramachandran, Sh where Sh is unit mass, coefficient cultures, 2 + 0.4(F d = 4 dp is for the the the the average power dissipation per i is diameter, sphere under chemical Sherwood (Eq. 7) /Df V2 1/3 the Sherwood number, and Df by equation Sherwood number for cell-liquid mass transport, Shc. species which normalized the is diffusion For microcarrier consideration. given number 1980) represents 7 For each chemical is consumed or produced by cells on microcarriers, gradient between chemical the the concentration in the the bulk medium, Xb, and the concentration at the cell surface, Xc, is given by where if is Xb - Xc i Rc dp T Xb Df Xb Shc the surface (Eq. 8) in coverage cells per unit area, uptake rate per cell for a given chemical species. are produced by the cells, Rc will be negative. - 32 - and Rc is the For chemicals which Substituting typical values for the parameters in equations 7 and 8, one unity, on values with the cell surface are at concentrations of order the the concentrations in the bulk medium. that a conclusion parallel Furthermore, gradients. be can the The 0.1. to 0.001 less far are the normalized gradients that finds generally than chemical essentially equal therefore to A Nusselt number analysis shows made temperature to with regard and chemical temperature are gradients insignificant even if the microcarriers are suspended in a stagnant flow 2 field (Shc = 2, Nuc = ). Thus, it appears that mere suspension of microcarriers provides for adequate mass and heat transport between the supported by the results conclusion is (1983); uniform growth is levels of agitation of Sinskey et al are concentrations are and Hu (1981) observed over a wide range of nondetrimental that sufficiently are suspended, and maintained at as long suitable as the levels, the suspend to strong microcarriers and provide for adequate surface aeration. microcarriers This cells and medium. As long as the bulk the chemical effects of agitation will come solely through momentum transfer. Fluid-lift, Airlift, and Stirred-tank Bioreactors The vast majority of microcarrier cultures are conducted in Such vessels provide a relatively homogeneous culture tank bioreactors. environment almost all which can be readily assessed currently-available microcarriers such equipment. stirred- and controlled. are designed In fact, for use in Most microcarriers have a specific density in the range - 33 - Neutrally buoyant microcarriers are generally not used mild agitation. as through gravity for microcarrier cultures medium the from separated be not could they for complete suspension with relatively allows This to 1.04. of 1.03 sedimentation. Fluid-lift reactors have been employed reactors When fluid-lift 1981). (Clark and Hirtenstein, are used with microcarriers that have a specific density of 1.04 and a diameter of 185 microns, the maximum linear velocity through the bed is on the order of The cells are exposed only to very weak hydrodynamic forces 5 cm/min. (less than 1 dyne/cm2 ). The cell density and reactor height, however, are limited by the uptake of oxygen from the medium as it flows past the densities wishes one If cells. to a operate fluidized fluid forces, and high cell microcarriers with a specific density on a industrial scale, The energy dissipation more comparable to glass will have to be used. rates, bed with will then be comparable to the values in a stirred-tank reactor with microcarriers of a specific density near 1.04. reactors Airlift animal Handa et their use direct 1981; insect and cells 1987). al, for sparging Spier have been used for cultures. damage and Griffiths, to cells is freely-suspended 1984; found which document frequently on microcarriers stated that (Pharmacia, Nonetheless, no published reports 1984). were found which document such damage. - It of Boraston et al, 1986; No published reports were microcarrier causes al, (Tramper et cultures 34 - One of this purpose to is thesis and investigate experimentally document whether cell damage occurs from direct sparging of microcarrier Cell damage is known to occur from direct sparging of suspen- cultures. sion cultures that damage fact, (Tramper al, et 1987). Handa et al, 1986; likely is It In occurs from direct sparging in microcarrier cultures. given microcarrier cultures, the damage may be more extensive in that the cells are immobilized and are more susceptible to mechanical If forces. generated, foam is into the transfer of the microcarriers foam phase (Fleischaker, 1982) may cause further problems. are Independent of whether microcarriers mass transfer reactor types, high-density, airlift, there will be a trade-off between or stirred-tank reactor, fluid-lift, in a suspended three all For and hydrodynamic damage upon scale-up. the fluid-mechanical environment will be comparable this In cultures. large-scale in hydrodynamic thesis, phenomena in the stirred-tank design are investigated. Cell Damage from Turbulence agitated with a rotating impeller, When a vessel is the Reynold's number for the bulk flow is given by (Nagata, 1975) Re where vb is = N Di2 vb the kinematic viscosity of the bulk suspension, impeller rotation rate, Reynold's number exceeds turbulent (Eq. 9) (Nagata, and 1975). Di the is approximately For - impeller 1000, complete 35 - the N is diameter. flow off-bottom the If this field becomes suspension of microcarriers, virtually all stirred-tank bioreactors must be operated in the turbulent regime. In the turbulent flow field of a microcarrier culture, short-term hydrodynamic forces arise through the motion of turbulent eddies. with conjunction exists there the transfer of energy a spectrum of eddy sizes eddies, small to from large In down to the viscous dissipation For sufficiently high bulk flow Reynold's numbers, the smallest regime. eddies exist in a state of isotropic, statistical equilibrium and can be described by the Kolmogorov scales for eddy length and viscosity (Hinze, 1975). stirred-tank, the In a large energy-containing eddies nonisotropic and nonhomogeneous (Nagata, 1975). are highly Nonetheless, isotropic equilibrium at the Kolmogorov scale will exist if the eddies in the viscous dissipation regime are much smaller than the energy-containing eddies (Hinze, isotropic 1975). equilibrium exists at the viscous indicate which results several are There dissipation scale in stirred-tank microcarrier bioreactors: 1) For stirred containing tanks, eddies, Komasawa et al (1974) found or turbulent macroscales, given by one-fifth the impeller width. that the have sizes energy roughly Translating these results to most microcarrier cultures, one can estimate that the energycontaining eddies are more than an order of magnitude larger than eddies in the viscous dissipation regime. 2) For stirred tanks, Nagata (1975) found that the lateral and - 36 - This numbers. wave indicates at high nearly superimposable energy spectra became longitudinal that local isotropy at exists the viscous dissipation scale. 3) In analyzing several sets of results, including those of Sato et (1967) al spectra in for that found (1975) tanks, Wadia stirred energy the viscous dissipation regime followed the prediction of Heisenberg (1948) for isotropic turbulence in statistical equilibrium. The agitation for observation held unbaffled in an stirred tank with an impeller Reynold's number of 8400, typical of the conditions in a stirred-tank microcarrier reactor. studies of Komasawa et al (1974), 4) As shown by the tracer-particle the presence of particles the turbulent flow of a stirred tank in does not preclude the existence of isotropic equilibrium. are nearly the particles if are high, bulk-flow Reynold's numbers If the neutrally-buoyant, and if the particles are comparable in size to the Kolmogorov eddy length isotropic scale, exist in the viscous dissipation regime. equilibrium should Microcarriers are nearly neutrally-buoyant and are comparable in size to the Kolmogorov eddy length scale. In light of approaching the evidence isotropic presented, equilibrium it exists appears that the viscous in condition a dissipation regime for many microcarrier cultures. With the belief that Kolmogorov's theories of isotropic equilibrium can be applied to microcarrier cultures, - 37 - one might first consider the of role occupied by If a relatively size. eddy a microcarrier, eddy formed large microcarrier would be the in a region entrained and would rotate and translate in a manner that would reduce the net torques and forces If a relatively small eddy formed adjacent on its surface. to a microcarrier, the motion of the microcarrier would be more limited, as results of Kuboi shown by the microcarrier would have eddy. Accordingly, small intense et al (1974), to experience more cells on microcarriers eddies of of and the cells on the the full force of the will be the most damaged by a size and velocity large enough affect to individual cells, but too small to readily entrain entire microcarriers. In eddies turbulent cultures, microcarrier in viscous the dissipation regime are often intermediate in size between the cells and Because microcarriers. isotropic equilibrium, these they eddies are appear described to by exist the in a state Kolmogorov of scales (Tennekes and Lumley, 1985) where L is scale for (Eq. 10) 1/4 L = (v3 v = (ve) 1/4 (Eq. 11) 9 = (v/e)1/2 (Eq. 12) the length scale, v is the eddies. the velocity scale, are These scales functions and 0 is of only the time the power dissipation per unit mass, e, and the kinematic fluid viscosity, v. The size of the smallest eddies decreases with an increase - 38 - in power or For in kinematic viscosity. a decrease power sufficiently high inputs at a given viscosity, the turbulence should generate eddies which are smaller than microcarriers. Cell damage from turbulence should then become evident, if the proposed role of eddy length is correct. the effects of both power input and viscosity are this thesis, In to investigated determine performed are investigations the damage regard power average the to the Experimental eddies. smallest with correlates with Although the role of local power dissipation rates dissipation rates. is for scale length Kolmogorov if hydrodynamic partially investigated and discussed, more thorough investigations are left for future work. In dilute cultures, cell damage should occur and concentrated cultures. primarily through cultures, cell interactions, are performed for both dilute separate experiments thesis, this In damage may In interactions. microcarrier-eddy occur not only through concentrated microcarrier-eddy but also through microcarrier-microcarrier interactions. To provide clear and unique information with regard to each mechanism, microcarrier-eddy and microcarrier-microcarrier damage mechanisms are studied separately. Protective Polymers To eliminate or reduce cell damage from fluid-mechanical their medium. have added polymers to commonly used polymer is methylcellulose (Kuchler et al, many investigators - 39 - forces, The 1960; most Bryant, Holmstrom, 1969; 1966, 1986). 1968; Pluronic polyols have and Pirt, Birch also been used (Swim and Parker, 1960; Runyan and Geyer, 1963; Kilburn and Webb, 1968; Mizrahi, 1984), along polysucrose 1984), (Mizrahi, carboxymethylcellulose sodium with al, et Tramper 1969; (Pharmacia, 1981), dextran (Schulz et al, 1986), and hydroxyethyl starch In general, metabolic uptake (Mizrahi, 1984). insignificant (Mizrahi, 1984). value (Bryant, 1966, trace metals 1969; these polymers of is The polymers appear to have no nutritive Mizrahi, they can provide although 1984), (Thomas and Johnson, in deficient medium 1967) eliminate protein precipitation from medium with poor serum and can (Swim and Parker, 1960). It is widely believed that these polymers protect animal cells from mechanical damage. observed if In agitated systems, the medium is these protective polymers Runyan and Geyer, 1963; cell disruption is frequently not supplemented with either serum or one of (Swim and Parker, Bryant, 1966; 1960; Kuchler et al, 1960; Johnson, 1967; Thomas and Holmstrom, 1968; Kilburn and Webb, 1968; Birch and Pirt, 1969; Mizrahi, 1984). for Because the known protective polymers and serum can substitute each other with regard to mechanical protection, it appears serum contains a protective polymer, although it has not that been identified. The mechanism of the protective effect of these polymers has not been determined. The mechanism may involve a reduction of turbulent damage through an increase in viscosity. - 40 - Addition of the polymers would of size the and viscosity medium the increase likely smallest the This hypothesis eddies, and thus reduce cell damage from turbulence. this thesis. will be investigated in Cell Damage from Time-Average Flow Fields are both time-average and time- In a turbulent flow field, there flucuating and pressure over small change greatly components velocity the time-average intervals in position, If components. velocity strong hydrodynamic forces could arise and damage cells. the evaluate To fashion, thorough one profile average flow various time-average very is approach role determine might as around a microcarrier flow difficult, a in fields it circulates through this Because tank. stirred currently not if time- position-dependent the impossible, a in components velocity time-average of one might instead employ a simplified approach and ignore the dynamic effects due into down ignored, 3. Figure small small problem the This region velocity regions, in and velocity turbulent if can be simplified schematic of a sphere the flow field is the time-average If circulation. to microcarrier reactor where the to in the gradient field is approximately constant. flucuations situation a shear broken are depicted field represents in the in a time-average Such gradients will subse- quently be referred to as time-average shear rates. Lin et al (1970) developed an analytical steady-state solution to the flow profile near a neutrally-buoyant sphere, as depicted in Figure - 41 - 3. The relevant Reynold's number is given by Re Y rm2 /V = (Eq. 13) where rm is the microcarrier radius and Y is the undisturbed shear rate. For equation 13 are generally below 1, calculated from and the flow can be approximated as r, The maximum shear stress, creeping. numbers Reynold's the bioreactors, microcarrier on the sphere surface is then given by r= As shown in time-average speed, (Eq. 14) n Y the fluid viscosity. where n is maximum 3 shear rate time-average ITS, the maximum shear stress depends upon the equation 14, and is and is shear rate given, in In most fluid viscosity. proportional the reference to the frame of the vessels, the impeller tip rotating im- peller, by (Oldshue, 1983): Y = K1 ITS and where K1 maximum shear is = If a constant. rate occurs (Eq. 15) K 1 grN Di a radial radial the in flow impeller jet from is the used, the impeller. Analyzing the flow profiles developed by Nagata (1975), one can estimate that K1 takes on a value near 0.4 cm-1 for a typical paddle impeller. - 42 - Ux U00+Y(z-z V,7 0 ) z Ox Figure 3. Schematic of a sphere caught in a simple shear field - 43 - Both Hu (1983) and Sinskey et al (1981) found that cell growth does As shown by the data of Hu (1983) in not correlate with impeller tip. Figure 4, maximum growth or zero growth can be observed at the same tip As originally concluded by speed, depending on the size of the vessel. Hu, the impeller tip speed, or the maximum time-average determining whether there are role in not appear to play a fundamental shear rate, does detrimental hydrodynamic effects. In the experiments of Hu (1983), the maximum time-average shear rate was approximately 16 sec~ 1 , as calculated from equation 15. The maximum shear stress was approximately 0.4 dyne/cm2, as22calculated from equation 2 This maximum shear stress is much less than 7 dyne/cm , the minimum 14. value Hellums, shear Thus, 1985). fields cause to reported was not (Stathopoulos removal or damage cell it appears that cell damage in occurring the experiments and from time-average performed Hu by (1983). This probably accounts for the lack of correlation between cell damage and maximum time-average shear rate. To determine whether cell damage from time-average shear fields can occur, one can not only calculate and compare can also flow fields is time-average increase the investigate the stresses. viscosity turbulence. If amount occurring, an increase since this will of damage, reduce the If cell effects of viscosity. cell damage from turbulence will shear stresses, amount is in as damage from viscosity should lead to higher shear occurring, of damage, but one it in an increase will dampen the Viscosity can be used to distinguish between damage from - 44 - Symbol 1 0M0 -- I- z W 75 Impeller Diam. (cm) Vessel Vol. (m 1) 3.2 250 0 4.1 250 0 5.1 250 0 7.5 2000 8.5 2000 X 500 cc - ULU - 25- - 0- 0) 10 20 30 40 IMPELLER TIP SPEED (CM/SEC) Figure 4. Relative growth extent vs. impeller tip speed (Hu, 1983) turbulence and damage from time-average flow fields. viscosity will cause cell damage in An increase has sufficiently high time-average As was mentioned, the shear rate for most reactors occurs in maximum time-average the impeller. shear rates. a reactor only if off the jet In some reactors, the maximum time-average shear rate may occur in a region of close clearance between the rotating impeller and a stationary clearance profile in vessel between the impeller instance, For component. if and vessel wall, there the close a tangential this region may periodically assume a character the flow between concentric rotating cylinders. is flow similar to The maximum tangential shear rate may then be estimated from the solution of Lee (1966) for the flow profile between concentric rotating cylinders, and is thus given by Y = 4 r N Dt2 2(Eq. 16) 2 Dt 2 - D, where Dt is the vessel diameter, N is the impeller rotation rate, and Di is the impeller diameter. In this thesis, a vessel with a close clearance between the impeller and vessel wall is used to investigate the onset of cell damage from time-average flow fields. The effects of viscosity are investigated for shear stresses below and above 7 dyne/cm2 , as calculated from equations 14-16. Criteria are developed to predict the onset of cell damage from time-average flow fields. These criteria are used to purposefully avoid - 46 - cell damage from time-average flow fields turbulence. - 47 - during the experiments on MATERIALS AND METHODS Cell Types cells were used in Two types of representative foreskin fibroblasts engineered to produce and fully fully suspension y-interferon (7-CHO). cells were y-CHO The Jan 850-cm 2 roller School from Biogen obtained of Medicine. the FS-4 cells were propagated bottles in grow The FS-4 cells were obtained from Vilcek, New York University experimental use, to difficult are anchorage-dependent, (Perry, 1987). The FS-4 cells are diploid y-CHO cells are aneuploid and, The Research Corporation, Cambridge, MA. Dr. human (FS-4) and recombinant Chinese hamster ovary cells anchorage-dependent. not although this thesis: to doubling 14-22 in Corning, NY). No Products, (Corning Science to Prior deterioration of the cell line was detected prior to doubling 32. Cell Culture Reagents and Procedures nearly In microcarriers with cells glass petri Uppsala, (Pharmacia Inc., grown on dishes cultures experiments, roller bottles or flasks all were grown Sweden) or on in polystyrene (Corning). A few experiments were Cytodex 2 and 3 microcarriers Unless (Corning). otherwise Cytodex 1 T- conducted (Pharmacia) or in noted, the term "microcarrier" refers to Cytodex 1 microcarriers. All cultures were grown in Dulbecco's Modification of Eagle's Medium (DMEM; (Gibco), Gibco, Grand Island, NY) 50 mg/l streptomycin supplemented with 50 U/ml (Gibco), - 48 - penicillin and either 5 or 10% (v/v) fetal a single For the FS-4 cultures, serum. calf lot of fetal calf serum For (Hazelton Dutchland Inc., Denver, PA) was used for all experiments. the McLean, Laboratories, was additionally supplemented with 0.25 pM methotrexate Milwaukee, and mycoplasma contamination. for viral WI). (Flow and the medium was used for all experiments, VA) Aldrich Chemical Co., pterin, calf serum lot of dialyzed fetal a single cultures, -y-CHO (L- + ametho- All serum was screened The serum lots used in these experiments were originally tested against several other lots; they were chosen for their ability to promote superior cell growth and attachment. The microcarrier cultures were grown in 125 or 500-ml Corning SlowSpeed spinner vessels 30 NY). Corning, These are specially designed for gentle agitation and can suspend up vessels to (Corning Science Products, g/l impeller of microcarriers diameter of 5.3 35 RPM. at cm, an The 125-ml vessels have an and an impeller width of 1.9 cm, The 500-ml vessels have an impeller internal vessel diameter of 6.3 cm. diameter of 7.8 cm, an impeller width of 2.5 cm, and an internal vessel diameter of 9.6 cm. each impeller was In nearly all of the experiments, positioned at approximately half the the liquid height. the 500-ml vessels, When the 5.3 cm impeller was used in the middle of the middle of impeller was positioned at one-third of the liquid height. Prior to use, the glass components of the vessel were siliconized with Prosil 28 (SCM Specialty Chemicals, Gainesville, of the microcarriers. - 49 - Florida) to prevent adherence For each experiment, all cultures were grown at the same time from a This inoculum. single numerous of use the through achieved was (Bellco Biotechnology, stirrers all cultures vessels spinner microcarrier cultures (Corning) and magnetic the for The stirring speed for NJ). Vineland, determined with a stroboscope was (Pioneer Model DS303, Cole Parmer, Chicago, IL) and was verified, if possible, through direct The stirring speeds were found to visual observations with a stopwatch. vary less than 5% from setpoint. inoculated from roller bottles according to the (1985). They cultures were All procedures developed by Giard et al (1979) and Hu et al were grown at 37*C in a humidified incubator with 10% carbon dioxide in air. The cultures were fed by replacing 60-80% of the culture medium For each culture, with fresh medium at predetermined intervals. length between medium exchanges, IL, interval was the determined in hours from the equations 180 I = LC -- (for FS-4 cultures) (Eq. 17) (for -y-CHO cultures) (Eq. 18) m 44 I where Cm is L values - C m the microcarrier These interval and 4.5 = g/l. between concentration 7.2 and dry grams per liter. maintained glucose levels between 1.0 feeding schedules They also in limited lactic 7.45, and limitations. - 50 - acid build-up, maintained pH hopefully eliminated nutrient Culture Aeration and Effect of Dissolved Oxygen on Cell Growth Oxygen was supplied to all cultures through surface aeration. preliminary control the effect of dissolved oxygen on cell experiment, in microcarrier growth In a cultures was Two investigated. FS-4 cultures were grown at different levels of dissolved oxygen in the 125-ml vessels In at 35 RPM. between 85 culture, the dissolved oxygen level was maintained one culture, and 90% of saturation with respect to air. In the other 85% 60 and the dissolved oxygen level was maintained between saturation with respect to air. 5 shows Figure the growth of the FS-4 cells in the two cultures. Growth at 60-85% dissolved oxygen was nearly identical to growth at 8590% dissolved oxygen. essentially dissolved oxygen oxygen were it appears that growth of FS-4 cells is uniform over a dissolved oxygen range of 60-90% saturation with respect to air. this Thus, expected Accordingly, Because range. for the all FS-4 cultures were grown within similar effects y-CHO cultures, all of dissolved y-CHO cultures were also grown in this dissolved oxygen range. For nearly all cultures, 90:10 air-CO2 sidearms. provided For the the headspace gas was equilibrated with the incubator atmosphere through loosened caps on the vessel This maintained adequate oxygen levels in the headspace oxygenation through surface aeration. for sufficient culture high density cultures, however, oxygen transfer from surface aeration required elevated levels of oxygen in the headspace. the high density cultures, and Thus, for the headspace was continuously exchanged with - 51 - +*- 60-85% sat. 85-90% ---- sat. with air with air 106 CO 1- Z zZ 0 O 1Cz 10 50 150 100 200 250 TIME (HRS) Effect of dissolved Figure i. oxygen on growth of FS-4 cells 52 - 300 The flow rates a humidified mixture of air, carbon dioxide, and oxygen. of the three gases were controlled with variable-area flowmeters (Models The FMO42-07, FM042-15, and FM062-01, Aalborg Instruments, Monsey, NY). oxygen content of the headspace was adjusted so as to control the oxygen level in the culture between 60-90% of saturation with air. The carbon dioxide content of the headspace was maintained at 10% so as to control the culture pH between 7.2 and 7.45. Agitation Power Determinations For mass, e, each microcarrier the culture, average power input per unit was determined from the standard power-number correlation: = 7 N p N3 D.5/ i (Eq. 19) c where Np is the power number, N is the impeller rotation rate, Di is the impeller diameter, and Vc is the average culture volume. were determined from the flat-blade paddles in Power numbers correlations presented in Nagata unbaffled vessels. These (1975) correlations for are described by the following equations: N = p A Re Re ) B (103 + 1.2 Re +3(Eq. (10 3 + 3.2 Re 0.66 )p 20) where A, B, and p are empirical constants given by the equations: A = 14 + (w/Dt)[670(Di/Dt - 0.6)2 + 185] B = 10[1.3 - 4(w/Dt - 0.5) p = - 1.14(Di/Dt)] 1.1 + 4(w/Dt) - 2.5(Di/Dt - 53 (Eq. 21) - - 0.5)2 - 7(Di/Dt) (Eq. 22) (Eq. 23) the where w is impeller width, Dt is the impeller diameter, Di is the tank diameter, and Re is the impeller Reynold's number given by equation of a microcarier suspension was 9. The bulk kinematic viscosity, vb, estimated by the ratio of suspension viscosity (Hiemenz, 1977) to volume-average density: r;(l + 2.54 + 1042) (1- 0) + = b the fluid viscosity, where r; is the fluid density, pm is is p 4 hydrated microcarrier density, and (Eq. 24) the is the volume fraction of solids. Cell Concentration Determinations For all microcarrier cultures, supernatant and microcarrier samples Glucose concentrations were determined were taken at least once a day. through the hexokinase method (Sigma Chemical Co., concentration of cells microcarriers to attached St. Louis, MO). was measured The in duplicate with a modification of the nuclei counting technique described by Hu et al soon Samples from 1 to 15-ml were withdrawn from the spinner vessels and placed in well-mixed As (1985). as polystyrene centrifuge tubes. the microcarriers settled to the bottom of the tube, the supernatant was removed and the microcarriers were resuspended in 0.15 M citric acid with 0.5 g/l crystal violet. After incubation for 2 hours at 370 C, the samples were sheared with a Pasteur pipette. nuclei were then magnification with Complete nuclei counted phase-contrast release hemacytometer a in or always was examination of the microcarriers. - under The released 120-200 fold Hoffmann modulation microscopy. verified through microscopic For the samples with high concentra54 - of inert microcarriers, tions citric which will be susequently described, the acid concentration was increased to 0.3 M so as to overcome the dilution effect from the extra bead volume. In this thesis, results for microcarrier cultures will frequently be expressed in terms of cell concentration, or the number of cells per ml of volume, culture including solids especially volume, for counts are nuclei necessary to correct for the is it performed on a liquid volume basis, the Because solids. high with cultures of concentrations Accordingly, cell concentrations were determined from microcarriers. the equation 104 ((/E) C (Eq. 25) = 4) F (1 + F ( where C is the cell concentration in cells/ml, counted, E number of hemacytometer the is volume fraction of solids volume divided the by in final the culture, is the number of nuclei squares scored, and F is the initial To minimize volume. sample 4 the is sample any bias associated with the frequency of nuclei observation in the hemacytometer field, were samples generally in sampling, the diluted or concentrated such that the C/E was To provide an adequate volume for the range of 30 to 80. cultures with only 0.05 g/l microcarriers were grown in 500-ml vessels. Samples from all cultures were frequently microscope after being fixed with crystal violet. - 55 - observed under a The morphology of the cells qualitatively was recorded along average approximate the with These direct microscopic counts were number of cells per microcarrier. used, along with glucose and oxygen uptake data, as a redundant check on the nuclei counts. No discrepancies were found in the results presented in this thesis. For the T-flask cultures, cell concentrations were also determined by counting the number of nuclei released after treatment with crystal the cells were first release, in the In order to citric acid (0.3 M). violet and obtain complete nuclei washed twice with PBS and then incubated After the flasks were counting solution for 48 hours at 37*C. vigorously slapped and shaken, the released nuclei were counted. DNA Assay DNA The fluorometric technique complete of culture the (Brunk et al, Instruments, Scientic (Hoefer fluid, content 1979) determined employing Hoechst San Francisco, with suspended was fluid CA). with a 33258 dye Samples of culture cells but void of microcarriers, were taken before and after each interval feeding. The samples were placed in 15-ml polystyrene centrifuge were later thawed, two minutes tubes and stored at mixed for 10 seconds on a vibromixer, in a sonication bath (Model 8850, The samples -20*C. sonicated for Cole-Parmer Inst. Co., Chicago, IL) with intermitant mixing, and then centrifuged for 5 minutes at 300g. From the sample supernatant, 0.8 mls were taken and then added to a UV-fluorometric cuvette (Spectrocell Inc., 2.7 mls of phosphate buffered saline (PBS) - 56 - Oreland, PA) containing with 1 pg/ml Hoechst dye and no calcium or magnesium. The content of each cuvette was mixed and the pH was adjusted to 7.60 + 0.01 with 10 pl aliquots of dilute NaOH. Elmer, Norwalk, CT). wavelength was 458 nm, control Several and the band widths were 3 nm. were experiments concentration was determined through (Sigma Chemical Figure antibiotics. calf-thymus Co., Perkin- The excitation wavelength was 365 nm, the emission procedures listed above for the DNA assay. DNA (Model LS-5, was then measured with a fluorimeter fluorescence The St. Louis, 6 shows MO) the DNA concentration. to performed validate the The response of the assay to the use of dissolved in calf thymus DNA DMEM with 5% FCS and increase in fluorescence versus The increase in fluorescence the represents the fluorescence of the sample minus the fluorescence of a control with As shown in Figure 6, the fluorescence was found to no calf thymus DNA. plateau when the approximately 20. (1979) for ratio to of DNA dye concentration (w/w) exceeded Similar behavior has been observed for by Brunk et al the fluorescent DNA assay with DAPI dye. In Figure 6, the regressed lines through the data in the non-plateau regions have slopes of 1.04 and 0.96, respectively, a good linear correlation Brunk et al (1979). on log-log coordinates. over a wide dynamic range, This indicates as also found by For DNA concentrations below 2 pg/ml, the standard deviation of the assay was 0.06 pg DNA/ml. For a given DNA concentration in the cuvettes, the fluorescence was found to increase with pH and decrease with the relative proportion of DMEM to PBS. The pH of all samples was adjusted using small aliquots - 57 - A ,ug/mL 0.08 * 2.0 Ag/mL dye dye 1000 Slope = 0.96 100 A A A A 10 Slope 0.1 0.01 I I I I I I I I I I I I f 0.1 I I I = tI 1.04 I t I I I I I I I 10 CALF THYMUS DNA CONC. (gG/ML) Increase in fluorescence Figure 6. vs. calf thy mus DNA concentration - 58 - 100 as described of dilute NaOH above. Minor corrections then had to be made for sample dilution and the change in the proportion of DMEM. The fluorescence of a cell suspension, and subsequent to freezing thawing, was found to only be a weak function of sonication time with a The fluorescence of the calf thymus maximum at approximately 2 minutes. standards was of sonication or any of the not effected by 2 minutes other sample processing procedures. The fluorescence DNA content approximately of of medium with serum was unexpectedly high. serum was subsequently measured and found The be to the The source of this DNA was not determined; 20 pg/ml. serum was not contaminated with viable microorganisms. The DNA content, however, does correspond to a lysed cell concentration of approximately 107 cells/ml, a typical white blood cell count. Thus, the DNA in serum could have come from white blood cell lysis during blood centrifugation or processing. It could psychrophilic microbes also have come from contamination with during serum storage prior to sterilization, as observed by Shiigi and Mishell (1975). Specific Fluorescence of FS-4 Cellular DNA The specific fluorescence of the DNA from FS-4 cells was determined Thirty identical through the use of T-flask cultures. cultures were started in 75-cm 2 T-flasks containing 12.5 mls of medium and an initial 2 attached cell concentration of 2.2 x 103 cells/cm . flasks were sacrificed in duplicate - 59 At daily intervals, and the concentration of attached - The growth curve is cells was measured. Figure 7. shown in The growth curve was used, in part, to determine the growth rate of the cells taken for the DNA assay calibration. for cells obtain To the DNA assay sacrificed at 60 hours after inoculation. were flasks two calibration, The cells were in exponential 1 growth at a specific growth rate of 0.023 hr- . The culture fluid was removed through discarded and the attached cells were washed with PBS, trypsinization, centrifuged for 5 minutes at 150 x g, and resuspended in One-third of 3 mls of medium containing 5% (v/v) FCS and antibiotics. this standard cell suspension was removed and diluted 1:6.25 in the same Both medium. the normal and diluted cell samples were and stored processed as previously described for the DNA assay. Figure 8 shows fluorescence versus cell concentration for the FS-4 As found by other researchers cell samples. (Brunk et al, fluorescence increases linearly with cell concentration. is high due to the DNA from the serum supplement. 30 hours, and one can reasonably assume that TS the 1979), The intercept For these cells, Td = is TG2 is 4 7 hours, hours, and TM is 1.5 hours (Baserga, 1976; Darnell et al, 1986; Alberts et al, then An average DNA content of 1.24 diploid equivalents 1983). be calculated from equation 3. The value of 1.25 can diploid equivalents is obtained if the calculation is performed with equation 4, and if one assumes a G1 duration of 6 hours, fibroblasts (Defendi and Mason, 1963; Baserga, 1976). - 60 - typical for human diploid Macieira-Coelho et al, 1966; U U) -J -J w C) 10 10 i i 60 i 120 i i 180 240 TIME I 300 (HRS) Growth of FS-4 Figure cells in stagnant T-flasks - 61 - 360 40 38 36 34 32 30 4 2 6 LYSED CELL CONC. Figure 8. 8 10 (10 5 CELLS/ML) Specific fluorescence of FS-4 cells - 62 - There is difference between the results obtained with equa- little For the purposes of this paper, equation 3 will always be tion 3 or 4. The DNA assay calibration is thus 6.3 fluorescence units/(million used. FS-4 diploid equivalents/ml). population is Sa = The average DNA content of an FS-4 cell therefore estimated by + 0.5 ( 218.04p 27.940 ) (Eq. 26) where p is given in hr-1. Effect of Cell Concentration in T-flasks For FS-4 microcarrier cultures under mild agitation, the growth rate was found to decrease with the microcarrier and cell concentration. One could not clearly distinguish, however, whether the observed effect was due to high cell or high microcarrier concentrations concentrations. New experiments had to be designed so that the effects of microcarrier concentration and cell concentration could be studied separately. Experiments in the effects of T-flasks were performed to cell microcarrier concentration. cm 2 and 25-cm 2 T-flasks. growth surface separate concentration independently determine from the effects of Various volumes of media were added to 75- This procedure allows one to vary the ratio of to medium volume (S/V), or stagnant environment free of microcarriers. cell concentration, in a To insure that the oxygen level was at least 60% of saturation with air at the cell surface, the This depth was calculated from a maximum medium depth used was 2.0 cm. stagnant diffusion model with a specific oxygen uptake rate of 5 x 10-11 mmole/cell-hr (Fleischaker, 1981, - 1982) 63 - and a maximum surface coverage of 5 x 104 surface was cm. All cell/cm 2 (Hu et To 1985). al, that insure the culture completely wetted, the minimum medium depth used was 0.16 T-flasks 850 cells/cm 2 . contained DMEM with 5% serum and were inoculated at The cultures 250 hours. were sacrificed after Growth was measured using triplicate flasks for each surface/volume ratio. Selection, Use, and Characterization of Inert Microcarriers Cell strongly growth in microcarrier and T-flask cultures was affected by cell Thus, concentration. to found to be investigate the effects of microcarrier concentration, one would ideally want to use an "inert microcarrier". An inert microcarrier would have and density as a normal microcarrier, the same size but would be chemically inert and incapable of supporting cell growth or attachment. could be used to change the solids concentration, Inert microcarriers even during inocula- tion, without affecting the chemical environment or the number of cells inoculated per "active" microcarrier. G-50 Sephadex requirements of an beads (Pharmacia) were inert microcarrier. These beads to fulfill have the negligible Control experiments showed that they charge and ion exchange capacity. do not support cell growth or attachment. between 90-106 found The beads with dry diameters microns have a size distribution, to that of Cytodex 1 microcarriers. upon hydration, close In subsequent paragraphs, the term "inert microcarrier" will refer to this size fraction of Sephadex beads. - 64 - A reticule was microscopic Cytodex 1 to used a of have a diameter of 178 + 23 micrometers; Cytodex 1 microcarriers have size the of diameter both Hydrated in DMEM with 5% serum, and inert microcarriers. microcarriers determine 162 + Direct micrometers. 18 inert microscopic counts were used to determine the number of microcarriers per gram. 1; there There are 3.8 million microcarriers per gram of dry Cytodex are 3.3 million microcarriers per gram of dry inert microcarriers. To determine the density and specific volume of hydrated microcar- riers, microcarrier suspensions were vacuum filtered for 2 minutes remove unbound The medium. filtered The change in graduated cylinder containing medium. were used to calculate space) appears is then is 1.03 the density g/ml, added to a weight and volume the specific volume and density. Cytodex 1 microcarriers, volume is were beads to For hydrated the hydrated bead 10.2 ml/g, and the gravity-settled bed volume (including void 18 ml/g. In the Pharmacia literature (1981), the bed volume to have been accidently substituted for the bead volume, and thus the specific surface area, bead volume, and number per gram are all in error by a factor of 1.76. 3440 cm2 /g, not 6000 cm2 /g. For example, the specific surface area is For the inert microcarriers, the hydrated density is 1.03 g/ml, and the hydrated bead volume is 7.6 ml/g. Inert microcarriers were used to independently determine the effect of bead concentration in the microcarrier cultures. In one experiment, inert microcarriers were added to four identical vessels - 65 - at different All four cultures had 1.5 concentrations: 0, 8.5, 18.5, and 28.5 g/l. g/l Cytodex 1 microcarriers, agitated at 35 RPM. contained medium with 5% serum, To determine how the results of this experiment would vary with the level of agitation, was repeated at a stirring it the inert microcarriers speed of 150 RPM. At the higher speed, added to levels of 0, 5, 10, 15, 20, 25, and 30 g/l. effect and were of adding inert microcarriers were Eventually, the investigated for a number of was stirring speeds and fluid viscosities. Viscosity and Density Measurements The density of various medium solutions was measured at 37*C with a Viscosities were measured at 37*C with a balance and volumetric flasks. concentric-cylinder viscometer LVT with UL adaptor, (Model Stoughton, MA) for shear rates between 7 sec-l and 74 surface at the rate of the inner cylinder, r, Brookfield, sec- 1 . The shear was calculated from the equation (Lee, 1966) r where N cylinder is = 41r N ro2 2(Eq. r2 -r2 the rotation rate (1.258 cm), and ro 27) (sec- 1 ), ri is is the radius of the inner the inner radius of the outer cylinder (1.381 cm). Figure results rate. are Data shows 9 the plotted as is of results the viscosity measurements. The versus shear percent of maximum shear shown for water as the calibration stress standard. Data is also shown for Dulbecco's Modification of Eagle's Medium (DMEM), fetal - 66 - 30 25 _- DMEM, 5% FCS 50 g/L dextran S--Fetal 20 calf serum (FCS) DMEM, 15 5% *-DMEM 10 -+- 0 10 20 30 40 50 60 70 Water 80 SHEAR RATE (1/SEC) Figure 9. Shear stress vs. shear rate for various medium solutions FCS calf serum (FCS), DMEM with 5% (v/v) FCS, and DMEM with 5% (v/v) FCS and 50 g/L dextran of 78,500 average molecular weight. various the as solutions, determined by the The viscosities of are slopes, regressed tabulated in Table 1 along with the measured densities. All solutions exhibited a constant viscosity except fetal calf serum which (FCS), a exhibited slight very behavior. pseudo-plastic The viscosity and density of DMEM were only scarcely greater than the values Supplementation of DMEM with fetal calf for pure water. and density. slightly increased the viscosity with dextran greatly density. For Further supplementation dextran concentration, a given Methocel the raised A15LV Supplementation increase. viscosity in increased viscosity with only slight increases supplementation with a increase but a higher molecular weight allowed for a similar density greater serum only viscosity by of medium approximately with 18% 1 with g/l no significant change in density. At the beginning and end of several density of the culture fluid were measured. cultures, the viscosity and No significant changes in culture fluid viscosity or density were observed. Selection and Use of Thickening agent The mechanisms investigated, Such of cell in part, with experiments are in damage microcarrier experiments on the effects best performed with Newtonian cultures of viscosity. solutions, solutions with constant viscosity and no viscoelastic interactions. - 68 - were i.e., TABLE 1. VISCOSITY AND DENSITY MEASUREMENTS Solution Density (g/ml) Viscosity (g/cm-sec) DMEM 1.003 0.0071 Fetal Calf Serum 1.015 0.0088 5% (v/v) FCS 1.004 0.0074 DMEM, 25% (v/v) FCS 1.006 0.0078 20 g/L Dextran, 78,500 MW 1.008 0.0104 35 g/L Dextran, 78,500 MW 1.013 0.0135 50 g/L Dextran, 78,500 MW 1.018 0.0185 60 g/L Dextran, 78,500 MW 1.022 0.0227 80 g/L Dextran, 39,000 MW 1.030 0.0275 1.069 0.0275 DMEM, DMEM, 5% (v/v) FCS, with 180 g/L Dextran, 9,000 MW - 69 - Viscous effects independent then be uniquely determined, can the of For Newtonian solutions, the viscosity is a effects of viscoelasticity. single physical constant that can be easily measured with a viscometer. medium, culture could and significantly or no toxic little a Newtonian fashion with in the viscosity increase Several thickening agents with very low molecular effect on the cells. weights cell in soluble completely agent which was conducted for a thickening A search was therefore were tested, including sucrose, and 1,3-butanediol, glycerol, These substances are known to form Newtonian solutions 1,7-heptanediol. At concentrations sufficiently high to give a when dissolved in water. 2-fold or higher increase in viscosity, all of these substances either killed the cells or severely inhibited growth. (A15-LV, Methylcellulose carboxymethylcellulose Sigma Chemical (LV grade, were also investigated as thickening agents. to be nontoxic, essentially completely clear solution. the but did Small, of 10-100 microns, would order Midland, Dow Chemical Co., not Co., MI) and sodium St. Louis, MO) Methylcellulose was found dissolve readily clear particles, gradually form into with diameters in the a on methylcel- lulose-medium solutions even after filtering through 0.2 micron filters. Sodium carboxymethylcellulose gave similar problems with solubility, and was found to be toxic when positive growth surfaces were used, such as Cytodex 1, 2, and boxymethycellulose Nakamura, 1973, 3 microcarriers. Both methylcellulose are known to be viscoelastic 1974) (Hoyt, and car- 1985; Amari and and frequently give solutions with pseudoplastic - 70 - (Nicodemo rheology relatively al, et 1974; 1985), Dow, although viscosities independent of shear rate can probably be obtained with the lowest molecular weight methylcellulose (Dow, 1985). The search finding a for aqueous solutions. promising candidates. nontoxic. nonionic low-molecular-weight, Newtonian rheology in as For eventually directed toward a thickening agent was aqueous of which exhibited Dextrans were soon identified are generally low-molecular-weight dextrans, Dextran polymers solutions polymer are nonionic Newtonian rheology can be expected, as will be subsequently shown. (Sigma Chemical Co., Low-molecular-weight dextrans St. Louis, MO) were tested for their effects on cell growth with mild agitation. The dextran polymers were dissolved directly in DMEM to give entirely clear solutions. filter were Three 39,400, sterilized different average and 78,500 weights were the DMEM-dextran solutions Prior to use in cell cultures, and molecular daltons tested in supplemented with weights were (clinical grade). order to determine 5% FCS and antibiotics. investigated: 9,000, The different molecular the lowest molecular weight which was suitable as a thickening agent. Figure 10 shows the effect of various dextran supplements on growth Specific in FS-4 microcarrier cultures at 35 RPM in the 125-ml vessels. growth rate, relative to a control culture with no dextran, against dextran concentration. is plotted Data is included for three different - 71 - A 78,500 A 39,000 * MW MW 9,000 MW 1.20 0.90 F- 0.60 0 0.30 0</) 0.00 I..J Lu -0.30 -0.60 40 DEXTRAN 80 120 CONCENTRATION 160 (G/L) Chemical effects Figure 10. of dextran on cell growth 72 - 200 molecular weights: and 9,000 daltons. 39,000, 78,500, was collected under conditions of mild agitation. in dynamic damage All of the data There was no hydro- as will be shown in any of the cultures, the Results and Discussion section. The data in Figure 10 illustrates the chemical effects of dextran on For an average molecular weight of 78,500 daltons, dextran cell growth. g/l have below 25 concentrations effect on no concentration of 60 g/l reduces the growth rate by 12%. to a If one wishes 3-fold, use the viscosity by approximately increase while cell growth, of 78,500 MW dextran will lead to a 12% reduction in growth rate, 39,000 MW will lead to a 25% reduction, 78,500 was MW and 9,000 MW will lead to cell death. moderate within nontoxic acceptably Dextran of increases in This substance was therefore suitable as a thickening agent viscosity. as long as it exhibited Newtonian rheology. Rheological Evaluation of Dextran Solutions In the measurements with covered shear the concentric-cylinder rates between 7 and 74 sec-1, molecular-weight dextrans to found were viscometer, which aqueous solutions of low- have constant viscosities. Typical results were shown in Figure 9 for medium with 50 g/L dextran of to 176,800, solutions and for for molecular weights up general, In 78,500 average molecular weight. up concentrations to 280 of aqueous dextran are known to have constant viscosities for shear rates up to an over 1000 sec- 1 (Margaritas and Pace, 1985). range g/l, shear rates observed - agitated in 73 - This is well beyond the microcarrier cultures. Newtonian rheology can thus be expected as long as the dextran polymers do not exhibit viscoelastic interactions with the turbulence. Through macromolecular extension, many polymers exhibit viscoelastic interactions with turbulent the bursting viscoelastic A process. interaction occurs when a polymer molecule has a relaxation time which is near the duration time of a turbulent burst (Virk, 1975; Denn, 1980). The relaxation time, A, of a polymer molecule can be estimated from the results of Zimm (1956): A = (Eq. 28) 0.42 Mw [rq] rs/ R T the molecular weight of the polymer, where Mw is [r;] the intrinsic is viscosity, ns is the solvent viscosity, R is the ideal gas constant, the T is the temperature. can be estimated The minimum duration of a turbulent burst, from the time Kolmogorov for scale the 9 mini highest frequency eddies in the viscous dissipation regime: 6 min = (v/E 0.01 and 0.001 seconds. be no typical values of cell culture vessels, In animal which is (Eq. 29) )1/2 If a polymer molecule has 0 min range between a relaxation orders of magnitude less than these values of 0min, viscoelastic interaction between the polymer time there will molecules and turbulence. To determine whether such viscoelastic dextran solutions, the characteristic - 74 - interactions relaxation time could occur in of 72,000 MW water at dextran in The 1979). al, (Wolf et calculated from published viscosity data 20*C was 1982) through standard methods (Rodriquez, nsp = viscosity, intrinsic [q], calculated was from the equation: [1] + k'[1]2 x (Eq. 30) x where x 3 The intrinsic viscosity was thus determined to be 24 cm /g. viscosity. This corresponds over seconds, to three relaxation characteristic a orders magnitude of should interactions not between occur than smaller the x 3 10-7 minimum the Thus, viscoelastic turbulence Other of molecular weight near 78,500 daltons. have noted that low-molecular-weight of time turbulent burst durations in microcarrier reactors. polymers the specific g/cm3 and 77sp is in the polymer concentration is dextran and researchers dextrans generally do not exhibit viscoelastic behavior in most turbulent flows (Hoyt, 1985). For a typical commercial mixture of dextran polymers with an average molecular weight near 78,500, such as Dextran T70 (Pharmacia), the ratio of weight-average Mw to number-average Mw is approximately 1.6. This degree of polydispersity should not lead to non-Newtonian behavior. In 78,500 of dextran polymers summary, exhibit all of the desired properties solubility, polymers relative were therefore used in the viscosity. - of a thickening agent: and nontoxicity, 75 - molecular weight average Newtonian experiments rheology. on the complete These effects of Cell Damage from Direct Sparging of effect The sparging Two experimental microcarrier cultures. was growth cell on examined FS-4 for cultures were grown in 500-ml Bellco spinner flasks (Model 1965-00500, Bellco Biotechnology, Vineland, cell damage from time-average To diameter of 10.2 cm. flasks have an internal The NJ). flow fields, eliminate in the Teflon impeller each flask was cut to a diameter of 5.4 cm and a width of 2.7 cm. A filter placed through a stick was Vineland, NJ) (Ace Glass, porosity C of The filter sticks were rubber cork stuck in the sidearm of each flask. modified such that the solid tubular sections ran down the side of each flask then curved radially inward near and each the bottom of on layed almost horizontally regions the bottom. The fritted this flask; allowed for free bubble rise without interference from the filter stick. experimental One culture was sparged at a superficial Foaming was essentially cm/sec with a 90:10 air-CO2 mixture. of 0.01 gas velocity eliminated through the daily addition of 20-40 ppm Medical Emulsion AF antifoam (v/v) solution and was ultraviolet radiation. tifoam in sparging. filter were through sterilized 6 hours of exposure to A second experimental culture was grown with an- an identical vessel, complete with filter stick, but with no A third experimental culture was grown with no antifoam, stick, impeller. FCS, The antifoam was added as a 1% (Dow Corning, Midland, MI). no a 500-ml Corning vessel with a 7.8 cm and no sparging in All cultures contained 2.0 g/l microcarriers in DMEM with 5% identically inoculated from the same inoculum, and replenished with nutrients on the normal interval feeding basis. - 76 - were Due to the low cell concentrations, dissolved oxygen levels were essentially at saturation with 90:10 air-CO 2 gas mixtures. The stirring speeds were set at the minimum levels to provide microcarrier suspension: 50 RPM in the Bellco flasks and 35 RPM in changes in the Corning flask. Net cell growth, were used to assess the effects viable cell concentrations, of sparging, antifoam addition, and filter stick presence. - 77 or - RESULTS AND DISCUSSION As mentioned in the Introduction, the objective of this thesis is to formulate a fundamental approach to the design, operation, and scale-up of microcarrier bioreactors. This objective was fulfilled by a series of experiments on the following topics: 1) hydrodynamic effects on growth of FS-4 cells with mild agitation, 2) hydrodynamic effects on growth of FS-4 cells with high agitation, 3) hydrodynamic phenomena in y-CHO cultures, 4) mechanisms of hydrodynamic death in dilute cultures, 5) mechanisms of hydrodynamic death in concentrated cultures, 6) cell damage from direct sparging The topics listed above constitute the first six chapters of the Results and Discussion section. the seventh chapter The results from these chapters are used in a quantitative approach to bioreactor to develop design and optimization. - 78 - CHAPTER 1. lA. HYDRODYNAMIC EFFECTS ON FS-4 CELLS WITH MILD AGITATION Effect of Fluid Viscosity with Mild Agitation The hydrodynamic investigated under effects on growth of FS-4 cells conditions of mild agitation. were first This established a baseline for the subsequent analysis for conditions of high agitation. Hydrodynamic effects on growth with mild agitation were investigated with experiments on the effect of fluid viscosity. growth with and without a 20 g/l mild agitation in Figure 11 shows cell dextran supplement the 500-ml vessels. (78,500 MW) under The cultures were agitated with 5.3 cm impellers at 60 RPM, slightly above the minimum speed of 45 RPM for complete microcarrier g/l microcarriers, dilute. suspension. or 0.002 volume fraction solids, the microcarriers concentrations dextran. and were thus very The cells exhibited the normal growth observed in FS-4 microca- rrier cultures with mild agitation. to The cultures contained only 0.2 were and Over 95% of the cells were attached excluded essentially Supplementation with trypan blue. with identical 20 g/l The or attached without dextran did not cell 20 lead to g/l toxic chemical effects or mass transfer limitations. At 60 RPM, input of approximately 10 cm2 /sec3, or an average power there appeared to be no cell death from time-average shear fields microcarrier-eddy interactions was If cell death from microcarrier-eddy interactions. occurring, or an increase in viscosity should have increased the minimum eddy size, reduced the death rate, and increased - 79 - + 20 g/L Dextran No Dextran 106 0 10 10 30 60 90 120 150 180 TIME (HRS) Effect of 20 g/L dextran Figure 11. (78,500 MW) on growth with mild agitation - 80 - If cell death from time-average cell concentrations. the viable flow fields was occurring, an increase in viscosity should have increased the maximum shear stress, increased the death rate, cell concentrations. Because an increase no on the viable effect significant cell cell in concentrations, death from hydrodynamic and reduced the viable viscosity had essentially there forces appeared to be no in these dilute cul- tures. Growth at Different Microcarrier Concentrations 1B. With mild agitation, microcarrier-eddy interactions and time-average for the dilute data lead to fields apparently did not shear significant cell death. cultures, however, did not The indicate whether cell death can occur through hydrodynamic interactions between microcarriers. even though the be referred to as collisions, will be subsequently interactions These interactions primarily may involve fluid the flow between microcarriers which come in close but not actual contact. To determine whether death cell occurs through microcarrier collisions with mild agitation, the hydrodynamic effects of microcarrier Cultures of FS-4 cells were grown over concentration were investigated. a range of microcarrier concentrations between 0.05 and 15 g/l. The cultures were fed according to the interval feeding schedules and were inoculated with 17 minimum inoculation to microcarrier, per cells requirement identified by Hu et al nutrients, 23 6 of cells per clearly above microcarrier, the as Through provision of excess defined (1985). and through prevention of the build-up of toxic metabolites, - 81 - it was that hoped could be the physical effects of microcarrier concentration investigated without being strongly influenced by differences The experiments were first performed with environments. in chemical by medium supplemented To (v/v) serum. 5% if determine there were nutrient limitations associated with the 5% serum level, the experiments were repeated with a serum level of 10%. Figure 12 shows the growth of FS-4 cells at different microcarrier was microcarriers grown a in 35 at 5% serum concentrations with RPM. 500-mi g/l culture with 0.05 The to vessel Corning an provide All other cultures were grown in 125-ml adequate volume for sampling. Corning vessels. At all microcarrier concentrations, at least a four-fold increase in was concentration cell for microcarrier performance This observed. good 1985). (Hu et al, of FS-4 cells cultures relatively represents Nonetheless, the cultures with high cell or microcarrier concentrations showed very long multiplication concentration). lag ratios phases, decreased (final cell 13 the cultures. and decreased initial cell The cells in the high density cultures also appeared to shows maximum the low density cultures. the maximum surface coverage microcarrier concentration. that over concentration be much more "spread out" than the cells in Figure rates, growth surface These normalized coverage was (cells/cm 2 ) at each results lower for clearly indicate the high density However, the detrimental effect of increased microcarrier or - 82 - 10 5% FCS . 15 g/I -&-A~ 5 g/ 6 10 -Ar + .----- - +--O 10 -- 1.5 g/I 0.5 g/l 5 0.1 5 g/I 0- 0.05 g/1 10 10 4 3 0 50 150 100 200 250 300 TIME, (HRS) Growth at various microcarrier Figure 12. concentrations with mild agitation - 83 - 350 6 10 10% FCS 0 Avg. slope = -0.16 0 wU 10 5% FCS w0 0 w 10 4 I I I I IIIII I -2 10 11111111 i i j ! ! ! ! 1 i ! ! ! t 1 111 -1 10 10 MICROCARRIER CONCENTRATION (G/L) Maximum surface coverage Figure 1 3. vs. microcarrier concentration - 84 - 2 cell concentrations dependency was very on microcarrier weak and had an average exponential of only -0.16. A concentration two-fold increase in microcarrier concentration resulted in only a 10% reduction in maximum surface coverage. present with approximately both 5 and Nonetheless, 10% serum. the weak effect was clearly Furthermore, the same at both serum levels. the slope is Even though the cells in 10% FCS generally grew to 50% higher concentrations than the cells in 5% FCS, the additional did serum not eliminate or even reduce the detrimental effect of higher microcarrier or cell concentrations. Figure 14 microcarrier maximum at shows the average concentration. approximately specific growth rate With 5% FCS, 0.2 g/l a at maximum growth microcarriers, concentration of 1.5 x 104 cells/ml. reaches the approximately With 10% 0.15 concentration of 1.1 x 104 cells/ml. g/l, as a function of or FCS, or rate reaches an the an initial a cell growth rate initial cell The existence of these maxima indicates that the growth rate was influenced by at least two competing factors. As the microcarrier or cell concentrations were increased, at least one factor exerted a positive influence on the growth rate while at least one other factor exerted a negative influence. It is well-documented promoting factors on many animal cells secrete growth- As microcarrier or cell concentra- (Butler, 1986). tions are increased, influence that cell-derived growth factors could exert a positive the growth It rate. is also well-documented that many animal cells secrete growth-inhibiting factors (Stoker and Piggott, - 85 - 10 1LU 0 10% FCS 0 -2 10 0 5% FCS 0 C,, w 10 -3 II I I I I I I I I I IIIII till,, I I I -1 I I I 10 I I. I 1 MICROCARRIER CONCENTRATION (G/L) Average specific growth Figure 14. rate vs. microcarrier concentration - 86 - 2 1974; McMahon et al, 1982; Bohmer et al, 1985; Hsu and Wang, 1986). or microcarrier cell concentrations could exert a negative inhibitors increased, are cell-derived on the growth influence As rate. The Figure 14 could result from competition between cell-derived maxima in growth promotors and inhibitors. Cell damage from microcarrier collisions could also exert a negative influence on the observed growth rates. or net observed growth which represent rates, growth and death. damage concentrated microcarrier in As will be shown in 14 actually presents Figure the the difference section cultures, a between on hydrodynamic collision mechanism should decrease the observed growth rate in direct proportion to the however, For the high-density cultures, microcarrier concentration. a two-fold increase in microcarrier concentration resulted in less than a 25% reduction clearly did in not concentration. observed decrease This is growth rate. direct in the first The observed proportion indication to the growth rates microcarrier that the slower growth in the high density cultures was not due to microcarrier collisions. 1C. Growth in Stagnant T-flasks To further investigate whether cell damage from collisions occurred in the microcarrier cultures at cultures was compared to growth in the growth curve RPM, growth 35 stagnant T-flasks. grown in for FS-4 cells in the microcarrier Figure 15 shows stagnant T-flasks. The data show the concentration of attached cells and was previously presented in Figure 7. The cells exhibited a period of rapid exponential growth - 87 - 10 U Cl) -J -J ul C) 10 10 3 I 60 120 I 180 I I 240 I I 300 TIME (HRS) Figure 15. Growth of FS-4 cells in stagnant T-flasks - 88 - I 360 followed by a period of constantly-decreasing growth rate. In comparison to the microcarrier cultures, the T-flask cultures showed initial growth rate but lower subsequent growth rate. the T-flasks may have been accelerated by the derived growth promotors at the cell surface. a higher Initial growth in local build-up of cell- Subsequent growth may have been inhibited by the build-up of growth inhibitors. the T-flask cultures had an average Over the entire growth period, growth rate of 0.0085 hr-1. For an FS-4 microcarrier culture at 35 RPM with the same ratio of growth surface area to medium volume (6.0 cm-1), one would expect an average growth rate of 0.009 hr- 1 . in mildly-agitated average, rate in mixing, as cells cultures grow just as microcarrier Although the in stagnant T-flasks. Thus, FS-4 cells on the quickly, average growth T-flasks may have been somewhat affected by the lack of the it appears that or no hydrodynamic little reduction of cell growth occurred through microcarrier collisions at 35 RPM. ID. Effect of Cell Concentration on Growth in T-flasks If cultures decreased the was not due growth to rate of collisions, the but high-density was rather microcarrier due to growth inhibitors or other chemical effects, a similar trend should be observed for cultures grown in T-flasks. The growth rate in T-flasks should decrease with increased surface/volume ratios, or cell concentrations. Figure 16 shows the effect of surface/volume ratio, or cell concentration, on the average growth rate in stagnant T-flask cultures. - 89 - 0.020 wr H 0 Avg. slope = -0.40 0.015 0 0 a wf T-Flasks, 5% FCS 0.010 i I 1 0 1 GROWTH SURFACE AREA/MEDIA VOLUME, (CMl\) Effect of cell density Figure 16. on growth in stagnant T-flasks - 90 - 10 As fact, the average same as that surface/volume cell lower a at occurs ratio approximately is in decrease the cultures, T-flasks for However, ratio. growth In the cultures. microcarrier high-density the for observed in surface/volume dependency of -0.40 exponential average the cultures, microcarrier increase decreases with an rate growth the with observed previously rate than concentration with for This difference may be due to inhibitor build-up microcarrier cultures. at the cell surface in stagnant T-flasks. Although no conclusive evidence has been shown, it appears that the decreased performance of the high density microcarrier cultures may have experiments microcarrier chemical However, the results from the cell damage from microcarrier collisions. inert or other There was no indication of significant of cell concentration. effects inhibitors growth to in part, least at due, been further will indicate whether microcarrier collisions had any effect. lE. Effect of Inert Microcarriers with Mild Agitation To investigate from the effects at various cultures proximately of cell concentration, levels were the effects of microcarrier concentration separate microcarrier to agitated 7 cm2 /sec 3 at , and 35 inert microcarriers cultures or RPM, contained in average 1.5 125-ml power were added vessels. inputs g/l microcarriers of All ap- with 5% (v/v) serum. Figure 17 shows the attached, or viable, cell concentrations for the - 91 - 106 Symbol C. (g/l) A 0 A 8.5 o 18.5 * 28.5 5% FCS 1.5 g/I Cytodex 1 35 rpm 100 50 150 TIME, (HRS) Effect of inert microcarriers Figure 17. on growth with mild agitation - 92 - 200 cultures with growth was nearly identical in all of the cultures. Cell Ci. inert microcarrier concentrations, different If cell death from microcarrier collisions was occurring, an increase in inert microcarrier concentration should have the death rate, increased increased the collision frequency, cell concentrations. and reduced the viable However, the viable cell concentrations were clearly not effected from increases in even up to 28 g/l. the inert microcarrier concentration microcarrier from cultures at 35 RPM. therefore was collisions For the not Cell death significant the in cultures grown with high microcarrier concentrations at 35 RPM, the decreases in surface coverage (Figure 13) and observed growth rate were (Figure 14) due to the effects of cell concentration and not microcarrier concentration. Cell Growth with Mild Agitation lF. With mild agitation, increase also not in viscosity or inert microcarrier concentration. affected by small to culture volume, the in increases previously observed by Hu (1983). area cells was not affected by an growth of FS-4 the Growth was level of agitation, as For a given ratio of growth surface in cells mildly agitated microcarrier cultures grew just as quickly, on the average, as cells in stagnant Tflasks. All of this evidence indicates that little or no hydrodynamic death occurs in FS-4 microcarrier cultures with mild agitation. In general, if cell growth or death was influenced from any type of hydrodynamic mechanism, in net cell growth should be affected by a change a fundamental hydrodynanic variable, - 93 - such as agitation power, fluid viscosity, or microcarrier concentration. Because changes in these three variables had no effect on net cell growth with mild agitation, it appears that cell growth and death were not significantly influenced by hydrodynamic forces with mild agitation. This conclusion is in line with the published results which show that growth of attached cells is not affected by mild shear stresses (Dewey et al, 1981; Sprague et al, 1987). This conclusion is also in line with the results of Dodge and Hu (1986), which show that growth of freely-suspended the agitation. affected by moderate increases in - 94 - animal cells is not CHAPTER 2. 2A. HYDRODYNAMIC EFFECTS ON FS-4 CELLS WITH HIGH AGITATION Growth Reduction and Cell Removal under High Agitation microcarrier cultures. hydrodynamic forces. or no hydrodynamic damage in there was little With mild agitation, Cell These growth was not apparently conclusions provide a affected by baseline for the analysis of cell growth with high agitation. Numerous were performed experiments to high agitation on cell growth and death. cell concentrations for FS-4 cultures at microcarrriers The data was determined at An actual at 60 RPM. 35 RPM and was slightly decreased reduction in indicates on grown Net growth of attached cells was each speed from duplicate cultures. at of Figure 18 shows the attached different stirring speeds in 125-ml vessels. highest the effects investigate attached cell concentration was observed at 150 RPM; this from the there microcarriers. was hydrodynamic In general, as of removal the cells increased, the the stirring speed was cells were either removed from the microcarriers and/or inhibited from growing at their maximum rate. Because FS-4 cells are completely anchorage-dependent, any cells in suspension must have come either through incomplete attachment during inoculation, to subsequent or through cell removal from the microcarriers attachment. Figure 19 the removal shows microcarriers at different stirring speeds. from measured concentrations of whole cells The results were calculated of whole cells in suspension. No correc- tion was made for incomplete attachment of the inoculum; such a - 95 - from the + 35 rpm 60 -A- rpm --- A--- 150 rpm 10 10 6 10 40 120 80 160 200 240 TIME (HRS) Attached cell concentrations Figure 18. at different stirring speeds - 96 - A 35 rpm .. 60 -- +-- rpm 150 rpm 12 -e0 ,---------0' 10 ,0' / 40 120 80 160 200 TIME (HRS) Removal of whole Figure 19. cells from microcarriers - 97 - 240 Cell fragments were observed in the correction would have been minor. However, these fragments from the cultures at 60 and 150 RPM. samples could not be accurately counted and were not included in the whole-cell counts. Figure 19 shows that removal of whole cells from the microcarriers at all occurred stirring speeds, and throughout the duration of all At 35 RPM, the whole cells removed generally represented less cultures. than 5% of the total observable cells (whole cells on microcarriers and in suspension). cells At 60 RPM, cell removal increased slightly; the whole observable removed generally represented about 9% of the total cells. 150 RPM, At greatly increased; cell removal cells the whole removed eventually represented nearly 60% of the total observable cells. The cells whole disintegrate suspension were counts below of the cells removed. cell fragments fragments suspension, 1-2 over a period between suspension cell all in were days. to generally lyse and This lysis reduced the the values which would fully account for The lysis also resulted in the release of was unclear, It into suspension. generated found solely through lysis however, of whether the whole cells in or whether the fragments were also generated through lysis of attached cells. Whole animal cells shape, due in suspension will generally assume a spherical to the high surface energy of the cell membrane - 98 - in aqueous To maintain a flattened morphology with a high solutions (Evans, 1983). membrane surface area, cells must generally exert mechanical forces with Upon removal from the growth surface, most cells their cytoskeleton. will develop mechanical cells exposed Animal become spherical. stiffness and may have to shear, however, can a non-spherical shape for several hours after removal from the growth surface (Sato et al, 1987). of absence the in Nonetheless, fixing animal dead agents, cells generally do not remain attached and flattened on a growth surface in an agitated environment. At all stirring speeds, cells attached the to the microcarriers exhibited flattened morphologies and did not uptake trypan blue. in Thus, terms of the ability to exclude trypan blue and remain attached with a flattened morphology, the cells on the microcarriers always appeared to be viable. In contrast, the cells suspension in always appeared to be nonviable. Generally over 90% took up observable amounts of trypan blue stain. Most suspension of cells them over lysed had extensive a surface period trypan blue, In terms the cells in that the cells in 1-2 days. The irregularities and roughness. They did not attach and grow upon inoculation T-flask cultures. between into new microcarrier or the ability to reproduce and exclude of suspension were always nonviable. It appears suspension were essentially killed upon removal from the microcarriers. - 99 - Random Cell Removal Through Normal Forces 2B. The removal of cells from the microcarriers increased with the level of agitation and been have of action the to due this removal was selective for mitotic cells, If hydrodynamic forces. to appeared thus which tend to be more rounded, the specific rates of removal should have increased with mitotic index. To investigate whether cell removal was selective for mitotic cells, the rates of whole cell removal presented in Figure 19 were analyzed on an interval basis. For the culture concentrations cell presented in insignificant hydrodynamic death. interval time to t1 t2 can The attached the agitation was mild. 35 RPM, at 18 Figure The mitotic thus be cell growth with reflect index, determined IM, from during the each equation presented in Johnson (1961): IM= TM y 1n 2 where TM is the ti and and C1 t2 , (Eq. 31) TM ln(C 2 /C 1 ) (t2 - ti) ln 2 the duration of mitosis, interval, times - and C2 are yu is the specific growth rate in the attached cell concentrations at can be The respectively. duration of mitosis reasonably estimated as 1.5 hours (Alberts et al, 1983). In the culture at 35 RPM, there was a small but measurable removal For each time interval, of cells from the microcarriers. - 100 - the specific can be estimated from the equation: rate of whole cell removal, Jw Jw W2 = (t2 - - 32) (Eq. W1 ti) (C2 + Cl)/2 where Wi and W 2 are the suspended whole cell concentrations at times ti and t2 , respectively, and Cl and C2 are the attached cell concentrations at times ti and t2 , respectively. For culture at the 35 RPM, Figure 20 the shows whole cell removal versus the estimated mitotic index. was selective for mitotic cells, the increase linearly with mitotic index. scatter with a linear appears cells. culture that cell removal was rate should the data exhibits random However, random If cell removal removal specific correlation coefficient specific rate of of only 0.16. and not Thus, it for mitotic selective The same conclusion can be drawn from a similar analysis for the at 60 RPM, introduces although the minor hydrodynamic damage some error in the calculation of the mitotic index from equation 31. to indicate These results are not the first hydrodynamic forces in microcarrier cultures. on DEAE-dextran microcarriers, random cell removal by When CHO cells are grown cell removal from excessive agitation is apparently random unless the cells are pretreated with colcemid al, 1980). These results are surprising if one believes (Ng et cell removal occurs primarily through shear stresses. In response to a shear field at the growth surface, distracting torque a rounded mitotic cell will experience than a flattened interphase cell. a higher Furthermore, rounded mitotic cell will probably have fewer attachment sites and a - 101 - a 2.00 I 1.50 0 12 1.00 Lu Hr 0.50 0.00 -0.50 -1.00 - 0.00 0.02 0.01 0.03 MITOTIC INDEX ESTIMATED Specific rate of whole cell removal vs. estimated mitotic index Figure 20. - 102 - 0.04 weaker attachment cell. If cell to growth the removal occurs surface through the in mitotis should be more rounded cells interphase than a flattened action of shear susceptible to stresses, removal than flattened cells in interphase. However, also from normal forces. In microcarrier cultures, in the generated by pressure fluctuations normal forces can be In turbulent flow fields. response to a pressure fluctuation near a microrcarrier surface, may be but cell removal can occur not only from shear stresses, subjected to a distractive normal force. a cell Because the pressure fluctuation will occur on the length scale of a turbulent eddy, which is much larger than a cell, the magnitude of the distractive force will be roughly proportional to cross-sectional surface. area of the cell on the growth If the cell has a constant number of attachment sites per also be area, cross-sectional the total attachment proportional to the cross-sectional area. force will Overall, both the attachment and distractive forces will be proportional to cross-sectional area of the cell. The cross-sectional area, or shape, cancel out as a factor. rounded up removal cell than a If in mitosis flattened of the cell will then cell removal occurs through normal forces, should roughly be no more cell in interphase. For susceptible a to microcarrier cultures, the observed lack of selectivity for removal of mitotic cells may indicate that cell removal occurs primarily through normal forces. - 103 - 2C. DNA Release Under High Agitation When FS-4 cultures are exposed the microcarriers. cell the to of cells from growth along with random and lethal removal reduction in part, to high agitation, there is a net in The net reductions forces. from hydrodynamic death and removal at least in growth are due, The question remains, however, whether this was the sole effect of agitation on net cell growth, or whether growth inhibition occurred along with lethal cell removal. account of the To answer this question, one needs a very accurate number of cells killed and removed by hydrodynamic This was achieved by monitoring the DNA forces, both whole and lysed. release into suspension from a high-density microcarrier culture. To investigate the release of DNA into the culture fluid under high agitation, The cultures were operated at 45 RPM to vessels with 7.8 cm impellers. eliminate microcarrier agglomeration. When nearly microcarriers were pooled, concentrated to 15 g/l, two identical 125-ml vessels. two 500-mi in FS-4 cells were grown on 5 g/l microcarriers the confluent, and transferred to One of these 15 g/l cultures was agitated at 35 RPM while the other was agitated at 150 RPM. Figure 21 shows the growth of the cells in the cultures with 5 g/l microcarriers. The data show the concentration of cells attached to the microcarriers; it conditions of represents typical Figure mild agitation. growth 21 for also FS-4 shows cells the under subsequent performance of the nearly-confluent cultures with 15 g/l microcarriers. The nearly-confluent culture at 35 RPM showed minor growth with a 25% - 104 - - 5 g/L 45 rpm 15 35 A g/L --rpm - 15 g/L 150 rpm 10 A A \A 10 0~ 10 60 180 120 240 300 360 TIME (HRS) Growth followed by agitation Figure 21. at different speeds after concentration - 105 - at The nearly-confluent culture attached cell concentration. in increase a 15% 150 RPM exhibited decrease in attached cell concentration. Cell death and removal clearly occurred in the nearly-confluent culture at 150 RPM. into suspension from Figure 22 shows the cumulative release of DNA The data are plotted in terms culture agitated at 150 RPM. the 15 g/l of FS-4 diploid equivalents and were calculated relative to the control culture at 35 RPM. The error bars represent the combined uncertainty in both the DNA measurements and in the correction for the control culture, as more thoroughly described in Appendix 1. there was extensive In the culture at suspension. from both the lysed and whole cells in 150 RPM, The data include the DNA release of DNA into the culture fluid. This provides further evidence of significant cell death and removal. 2D. Growth and Death Under High Agitation In overagitated an occurring if increasing. rate q, culture, microcarrier the total number of cells, cell growth must both attached and removed, be is If cell death and removal are occurring at a specific death the DNA concentration, D, in the culture fluid should increase according to the relation dD dD = Sr q C (Eq. 33) dt where C is the cell concentration and Sr is the cells removed. The DNA concentration, - 106 - the average DNA content of D, includes the DNA for both 1.80 1.50 1.20 0.90 0.60 0.30 0.00 40 20 TIME SINCE TRANSFER 60 (HRS) Cumulative release of DNA into Figure 22. suspension for 15 g/L culture at 150 RPM - 107 - 80 the lysed and whole cells in there the is random cell removal, average DNA average DNA content is content content given the as measured by our assay. If such as for FS-4 cells on microcarriers, of the of by suspension, cells cell population, equation 26 as Sr, removed, Sa. presented is equal to the average DNA Materials and This in the Methods section. In FS-4 cultures with high agitation, and removal. there is extensive cell death The specific growth rate will then be strongly dependent Secondary growth, as referred to on whether secondary growth can occur. in this thesis, represents cell growth over areas from which other cells previously were removed through hydrodynamic forces. In a manner analogous to the stimulation of growth through direct mechanical removal of cells, as in Alberts described might culture contact-inhibited through hydrodynamic removal. at high agitation may be et al (1983), stimulated if cell areas growth are in a opened up If secondary growth can occur, a culture actually grow faster than a culture at low agitation which is more confluent and contact-inhibited. To investigate the nature of cell growth in the 15 g/l cultures at 35 and 150 RPM, the DNA release data were compared to the predictions of three mechanistic models. both cultures were growing at the same rate at any given time cells in since inoculation. agitation, model, For the first model, it was assumed that the nor Cell stimulated by which represents was growth cell neither removal. inhibited According by excessive to the growth and death with no secondary growth, - 108 - first the specific growth rate, p, in each time interval was calculated from the equation: ln(C2, 3 5 /G1 ,3 5 ) (Eq. 34) (t2 - ti) where C 1 ,3 5 and C 2 ,3 5 are the attached cell concentrations at 35 RPM for The apparent specific death rate, q, at times ti and t2 , respectively. 150 RPM was then calculated from the equation: ln(C 2 ,1 50 /C1 ,150) (t where p is cell - 2 for the 35) t1 ) and C1 , 1 5 0 and C2 , 1 5 0 are the attached given by equation 34, concentrations (Eq. RPM 150 culture at times t1 and t2 , Assuming there was only cell death and removal with no respectively. growth inhibition or secondary growth at 150 RPM, one can calculate the expected change in DNA concentration from the equations: D 2 - Di = - Di = D2 Sa q p - q - C for C1 + C 2 (Eq. 36) - C2 (Eq. 37) for C1 Sa q C 1 ,1 5 0 (t2 -ti) where y and q are given by equations 34 and 35, respectively, Sa is the average DNA content per cell and is given by equation 26, and Dl and D2 represent the DNA for both the lysed and whole cells in suspension at 150 RPM, as measured by our assay, for times t1 and t2 , respectively. For the second model, it was assumed that secondary growth occurred, and that growth in the overagitated cultures was fully corrected for the - 109 - reduced contact inhibition due to cell removal. had been removed, For areas where cells secondary growth was assumed to occur at the same rate as the normal initial growth, with all growth regulated through contact inhibition. effect The of contact inhibition on growth incorporated with the following empirical equations which fit was the data presented in Hu et al (1985) for FS-4 cells: where Z P = ymax (100 - Z)/60 for Z > 40 (Eq. 38) y = ymax for Z < 40 (Eq. 39) is the percent confluence. For the culture with 15 g/l microcarriers at 35 RPM, the best fit to equation 38 is given with pmax 0.024 hr-l and full confluence at 4.0 x 106 cells/ml. For the secondary second model, represents growth growth, the specific growth rate for the agitation was calculated average which percent in each time interval death confluence was used along with the and full confluence at 4 x 106 cells/ml. rate, expected change with culture with high from equation 38. 0.024 hr-1 and and The estimated pmax of The specific death in DNA concentration, were calculated from equations 35-37. For the third model, it was assumed the reduced net growth at high agitation arose solely through growth inhibition and not cell death and removal. clearly not realistic in Although this model is light of the data presented in this thesis, the predictions of the model are included for the sake of comparison and completeness. - 110 - For the third model, the DNA in the culture fluid should not increase at 150 RPM relative to the control at 35 RPM. Figure 23 shows the predictions of the three mechanistic models along with the measured cumulative release of DNA. The predictions of the two growth and death models scatter because the data was analyzed on an interval basis and because the which are somewhat imprecise predictions (±10%). rely on nuclei counts The measured DNA release clearly does not match the expected release for growth inhibition without cell death and removal, measured or for growth and death with secondary growth. data does, release however, match the expected release The for growth and death without secondary growth. To the degree that cell removal was random, cells on microcarriers, cells in the data in Figure 23 as indicated for FS-4 demonstrates that the the 150 RPM culture were essentially growing at the same rate as the cells different in the 35 RPM culture. at 35 RPM, the than the growth rate would not have matched If the growth rate at 150 RPM was the expectation for amount of DNA release growth and death without secondary growth. Thus, the data strongly indicate that there is a situation of growth and death with no secondary growth. The reduction in net growth at 150 RPM appears to have been due entirely to cell death and removal, and not growth inhibition. Even though there was extensive cell removal and death, growth appears to have been unaffected by hydrodynamic forces. - 111 - 3.50 - -90 3.00 2.50 + 2.00 ----- Measured G & D with 2nd growth 1.50 -- G & D without 2nd growth 1.00 -- -- A - A - Growth inhib. with no death 0.50 0.001 -0.50 I 20 TIME SINCE 40 I 60 80 TRANSFER (HRS) Figure 23. Measured DNA release vs. release expected under three different scenarios At both 35 and 150 RPM, the attached cells exhibited the normal ability These findings to reproduce, and thus appear to have been truly viable. are the first published results which response of cells under high agitation. document a growth and death A growth and death response was originally proposed in 1984 as published in Croughan et al (1987). 2E. Hydrodynamic Regulation of Cell Growth For FS-4 cultures exposed to mild agitation, growth is unaffected by hydrodynamic forces. shear forces unaffected by hydrodynamic growth is Sprague For endothelial cells exposed to mild fluid flow, et al, 1987). cells under high For FS-4 et al, (Dewey 1981; agitation, growth appears to also be unaffected by hydrodynamic forces. The apparent not surprising. lack of growth regulation from hydrodynamic The decision to replicate depends to environmental genetically-programmed response on factors. forces the is cell's For normal cells, such as FS-4, this genetic programming has come through years of It primarily depends upon the cell's role in evolution and adaptation. the original animal donor. exposed to response fluid flow in If a particular cell type was not normally vivo, it to hydrodynamic forces. will have no genetically-programmed Unless fluid flow can influence the normal growth regulation processes, the decision to replicate should not depend on the hydrodynamic environment. for most animal cells, the For FS-4 cells, decision to replicate does depend on the hydrodynamic environment, to influence normal growth regulation. - 113 - and probably not appear to and fluid flow does not appear Lack of Secondary Growth After Cell Removal 2F. The data in Figure 23 indicates that there is no secondary growth in To further investigate this FS-4 cultures exposed to high agitation. observation, FS-4 cultures were grown at different stirring speeds from the point of inoculation. stirring speeds The cultures were held at their respective as the culture under mild agitation reached confluence and stopped growing. If there was secondary growth in the overagitated The cultures, a clear and very strong trend should have been observed. observed the growth rate at high agitation observed growth rate confluent agitation became at low agitation stopped and should have increased relative the as growing. culture The have been constant throughout the duration of all cultures, been greatly culture reduced as the shows the results under low in difference or the observed specific death rate, observed growth rates, low at should not but should agitation became confluent. Figure 24 of experiment. Attached concentrations are shown throughout the duration of the experiment. cell The cultures contained 3 g/l microcarriers and were grown in 100-ml spinner vessels as with 3.8 cm impellers, described in Groughan et al, 1987. The straight lines through the data represent which assumes that all the best fit to a model cultures were growing at the same rate at any inoculation, and that the specific death rates were constant in each culture throughout all time periods. The data follows given time since these assumptions, which represent growth and death with no secondary growth. Furthermore, the data clearly indicates that the specific death - 114 - 10 6 10 10 4 0 100 50 TIME (HRS) Figure 24. Attached cell concentrations for cultures grown at different stirring speeds - 115 - 150 2G. Hydrodynamic Effects on Glucose Consumption Growth of FS-4 cells appears to be neither inhibited nor enhanced by hydrodynamic forces. therefore not metabolic events The metabolic significantly which are events required altered by agitation. not associated growth for growth are Nonetheless, all could be strongly influenced by agitation. For the cultures with 15 g/l microcarriers glucose concentration profiles are taken just before and just after data in each time interval ti shown in given in Figure 25. each medium exchange. to t2 , one Figure 21, the Samples were Analyzing the can calculate the specific glucose uptake rate, Rg, from the equation (G2 - Gl) ln(C 2 /C1 ) (t2 - ti)(C 2 - Ci) Rg (Eq. 40) where Gl and G 2 represent the glucose concentrations at times ti and t , 2 respectively, and C1 and C2 represent the attached, or viable, cell concentration at times ti and t2 , respectively. For each time interval, rates. RPM. It Table 2 gives the specific glucose uptake also gives the specific growth rates for the culture at 35 There is glucose uptake no correlation between specific growth rate and specific rate (correlation coefficient = 0.18). In all of our experiments with FS-4 cells, no correlation has ever been found between specific growth rate and specific glucose uptake rate. glucose uptake is not growth associated for FS-4 cells. - 117 - It appears that rates did not significantly decrease when the culture at low agitation This is further indication that there is no secondary became confluent. growth in FS-4 microcarrier cultures exposed to high agitation. lack of secondary growth may be due to the nature of The apparent cell removal by hydrodynamic forces. adherent, Cell hydrodynamic remnants may secondary growth. For FS-4 cells, which are strongly forces may not generally leave might remain which remove complete the area cells. unsuitable for The previous attachment and removal of a cell from a given position may preclude future growth over that position. There is secondary growth and lack of growth a clear analogy between lack of through contact inhibition, wherein cells can grow over areas already occupied by other cells. If cells can not grow over areas previously occupied by other cells, growth would not be stimulated as areas were opened up by hydrodynamic cell removal. The cultures with signficant cell removal would exhibit growth regulation as contained a if they greater number of cells, as if they were more contact inhibited. in part, for the similarity This effect may account, growth rates between cultures in attached agitation and more confluent cultures at low agitation. at high The similarity may also arise through an unidentified mechanism of growth regulation. Cell growth is controlled not only by contact many other poorly-understood mechanisms - 116 - inhibition, (Darnell et al, but also by 1986). + 35 ---+- 35--150 rpm rpm _1 z 0 F- z Z z O 0 0 LU 0 D IJ 09 40 20 TIME SINCE TRANSFER 60 (HRS) Figure 25. Glucose concentration profiles for the 15 g/L cultures - 118 - 80 TABLE 2. GLUCOSE UPTAKE BY FS-4 CELLS IN MICROCARRIER CULTURES Specific glucose uptake rate (1/hr) (10-12 gm/cell-hr) Interval 35 RPM Specific growth rate 35 RPM 150 RPM 46 0.0106 48 -0.0069 3 52 0.0073 4 40 0.0013 5 44 0.0018 Avg. 46 0.0028 - 119 - The data Within a 99% gm/cell-hr, or only 76% of the value 1 0-12 on the average At 150 RPM, the average uptake uptake rate was 46 x 10-12 gm/cell-hr. 35 x 35 RPM, For the culture at the hydrodynamic environment. rate was dependent 2 show that glucose consumption is Table in at 35 RPM. confidence limit, the difference in average uptake rates significant. was statistically one accounted for glucose uptake by If the whole or lysed cells in suspension, the difference in average rates would even greater. be agitation reduces that high It appears the glucose uptake rate of FS-4 cells. For FS-4 cells, glucose uptake is not growth associated and is significantly For hybridoma cells, reduced by high levels of agitation. Dodge and Hu (1986) found that net cell growth was slightly reduced, but volumetric glucose agitation. This glucose result may have been due, in specific glucose uptake rate of by part, to lactic acid by dead or lysed cells. the that unchanged, was consumption the high levels of to conversion of It may also hybridoma indicate cells was slightly increased by agitation. this In have been Review thesis, the effects of hydrodynamic extensively section, there investigated. are limited As but hydrodynamic effects on cell metabolism. be fully optimized, hydrodynamic discussed interesting effects 120 - in on cell growth the results Literature regarding Before any reactor design can metabolism will have to be fully understood. - forces on both cell growth and HYDRODYNAMIC PHENOMENA IN CHAPTER 3. 3A. y-CHO CULTURES Growth, Reversible Cell Removal, and Secondary Growth Nearly all of the results for this thesis were derived from experi- experiments were performed with a few for the purposes of comparison, However, ments with FS-4 cells. y-CHO cells. y- Figure 26 presents limited results on the effects of agitation in CHO microcarrier concentrations shown are for and 5% dialyzed FCS microcarriers and attached Both cultures. grown two cultures in the 125-ml cell suspended with 1.0 Corning vessels. g/l One culture was agitated at 35 RPM; the other at 100 RPM. In contrast with the FS-4 cells, the monolayer. surface However, coverage. cell growth y-CHO cells grew to more than a still appeared to Growth plateaued after the be regulated cells were 2-3 by layers thick on the microcarriers. In parallel with FS-4 cells on microcarriers, the y-CHO cells on the microcarriers of agitation. excluded trypan blue and appeared healthy at both levels However, in somewhat of a contrast with the results from the FS-4 cultures, the y-CHO culture at 100 RPM exhibited essentially identical attached cell concentrations as the For an FS-4 culture, the same increase in reduction in average observed growth rate. -CHO culture at 35 RPM. agitation would bring a 50% Microcarrier cultures of y- CHO cells are less sensitive to agitation than microcarrier cultures of - 121 - w R 10 10 Attached 35 rpm o 5'4\ 10 Attached 100 rpm - "-Suspended 100 rpm A- Suspended 35 rpm 10 10 I 0 40 80 I I 120 I 160 I 200 I I 240 TIME (HRS) Figure 26. Suspended and attached cell concentrations for -y-CHO cultures I 280 FS-4 cells. In time each occurred a 26, Figure drop vertical was medium spent rapid very 0.04 hr- 1 or greater. pre-exchange their values. on the The order of 1 These rates are much higher than 0.004 hr- , the growth specific medium. suspended cell concentrations rates associated with these recoveries were specific maximum to recoveries concentration fresh with exchanged Subsequent to each medium exchange, the exhibited cell suspended in y-CHO of rate parable medium (Perry, 1987). Thus, cells suspension with com- in the rapid increases in suspended cell concentrations were not due to cell growth, but were rather due to cell removal from the microcarriers. Prior each medium exchange, to the cell concentrations suspended were essentially independent of the time since the last medium exchange. The suspended cell concentrations were therefore not steadily increasing in each feeding interval, between the data points concentrations were as improperly indicated by the straight lines in Figure 26. Instead, re-established apparently constant soon after each medium exchange, as if suspended the and held cell relatively a sort of equilibrium existed between the cells on the microcarriers and those in suspension. In Figure 26, the suspended cell concentrations represent the whole cells higher in suspension, both viable and dead. level concentrations of agitation than the The y-CHO culture at (100 RPM) exhibited higher culture - at the 123 - lower level the suspended cell of agitation (35 Thus, RPM). y-CHO cells, removal of cell from the for both FS-4 and increased with the level of agitation, microcarriers and thus appeared to come about, at least in part, through hydrodynamic forces. The culture at 100 RPM exhibited much more extensive cell removal and yet had essentially the same attached cell concentrations Even though significant cell removal occurred after culture at 35 RPM. medium each exchange, cell concentrations the attached recovered their pre-exchange values or greater by the next medium exchange. indicates that the as to This y-CHO cells exhibited secondary growth over areas the where cells had been previously removed through hydrodynamic forces. The cells y-CHO cells suspension in in suspension. In the appeared much y-CHO cultures, different than FS-4 70-90% of the suspended cells appeared healthy and excluded trypan blue. In the FS-4 cultures, none of the suspended cells appeared healthy and less than 10% excluded trypan blue. To truly measure viability, however, one must determine the ability of the cells For reproduce. to the FS-4 cultures, the cells in suspension did not viably attach and multiply on fresh growth surfaces. To investigate the reproductive capability of a new microcarrier the culture was inoculated y-CHO culture at 100 RPM. y-CHO cells in suspension, from the suspended cells in The cells were removed during the medium exchange at 237 hours, centrifuged for 5 minutes at 150 x g, resuspended in fresh medium, and then used to inoculate a new culture with 1.0 g/l - 124 - microcarriers at 35 RPM in a 125-ml vessel. In terms of trypan blue exclusion, the cells had a viability of 80%. The concentration of suspended cells which excluded trypan blue was used to determine concentration. "TPB viable" microcarrier the trypan blue viable, or "TPB viable", inoculum Figure 27 shows the attached cell concentrations and the inoculum concentration. cultures, it Given the appears that most, normal if not lag phase all, suspended cells which excluded trypan blue were actually viable. 29 hours, essentially all of "TPB viable" attached the Within to the The culture subsequently grew at microcarriers and started to multiply. virtually the same rate as the cultures shown in Figure 26. cells of of inoculated from roller bottles Thus, in striking contrast to FS-4 cells, many y- CHO cells in suspension are viable. 3B. Hydrodynamic Resistance through Secondary Growth In FS-4 microcarrier cultures, cell removal from excessive agitation is irreversible and lethal. Secondary growth does not occur over areas where removal has occured. The cultures under high agitation exhibit growth regulation as if they were just will permanently and inhibited as more An FS-4 culture exposed to high confluent cultures under low agitation. agitation as contact irreversibly deviate from a control culture with low agitition, as shown by Figure 24 in Chapter 2. In y-CHO cultures, removal cell 125 excessive agitation is Secondary growth will occur over frequently reversible and not lethal. - from - + "fTPB viable" Attached cells -+- inoculum 106 Z - 0 Z Z z F- 10 I IIIII 60 40 20 I I 80 TIME (HRS) Attachment and growth Figure 27. 7-CHO cells taken from suspension - 126 - I 100 Although the cells grow to more than areas where removal has occurred. a monolayer, coverage. Thus, to be appears still growth regulated by maximum surface cell growth should be stimulated if areas are opened up When cultures are at or near the through hydrodynamic cell removal. maximum surface coverage, the cultures with a high degree of removal should exhibit higher total growth rates than cultures with a low degree of removal. Figure 28 shows the viable cells produced per microcarrier over the time course of the per microcarrier cells subsequent y-CHO cultures at 35 and 100 RPM. cumulative increase represents the to in the number of Data are presented for both attached inoculation. and total viable cells. The cells produced The number of total viable cells was calculated including those as the sum of the attached and viable suspended cells, As the suspension cell concentrations removed through medium exchange. prior to 117 hours were very low and were not measured, they were not included in the calculations. The number of attached cells produced per microcarrier was nearly identical at 35 and 100 RPM. However, the total number of viable cells produced per microcarrier was 30% higher at 100 RPM. Total cell growth was clearly higher at 100 RPM. Cell removal was also higher at 100 RPM. As discussed, the cells in suspension did not primarily arise through growth in suspension, but rather through cell removal from the microcarriers. At 100 RPM, - 127 - the vw 400 350 300 A A Total 100 rpm 250 0 .- Total 35 200 ----- rpm Attached 35 rpm 150 A- Attached 100 rpm 100 50 I I 40 I I i I I 80 I I S 120 I I I 160 I II 200 I II I 240 TIME (HRS) Figure 28. Viable cells produced per microcarrier for y-CHO cultures II 280 produced cells suspension viable of number microcarrier, per which roughly represents the number of viable cells removed per microcarrier, was 3-fold higher than at 35 RPM. Thus, as went removal one total increased hand-in-hand with increased cell secondary growth, expect with might Growth growth. was Even stimulated as areas were left void through hydrodynamic removal. though cell removal was much more extensive at 100 RPM, the culture was able to maintain comparable attached cell concentrations to the culture at RPM. 35 hydrodynamic growth Secondary damage. fast was growth Secondary can to enough overcome lead clearly to any reduced hydrodynamic sensitivity. It is likely that secondary growth accounts for the difference in hydrodynamic It also is because the y-CHO microcarrier cultures. growth can occur likely that secondary cells are frequently reversible removal may leave growth. FS-4 and sensitivity between removed whole in and y-CHO cultures viable. few remnants to hinder future Such reversible removal may come about because secondary y-CHO cells are aneuploid and do not exhibit the same attachment properties cells, such as FS-4 cells. Such as diploid Future research should be performed to more thoroughly elucidate the nature of cell removal and secondary growth. 3C. Selection of Model Cell Line The viability of study of hydrodynamic y-CHO cells in suspension greatly complicates the phenomena in - these 129 - cultures. Because the cells can reattach after they have been removed from the microcarriers, rate of removal is not easily measured and does not simply correlate A form of equilibrium exists between the cells with the rate of death. in suspension and the and removal the those reverse The the microcarriers. on rate reattachment of forward must rate of measured be simultaneously. In an FS-4 culture, all of the viable cells are on the microcarriers and all of the dead cells are in The rate of hydrodynamic suspension. Hydrodynamic cell correlates directly with the rate of death. removal removal is not reversible; there is no equilbrium. The forward rate of death and removal can be measured and modelled directly with no required correction for the reverse rate of reattachment. cultures were chosen as the simpler extend the cells, system, model experiments were performed with FS-4 cells. For this reason, FS-4 and nearly Future work will hopefully analysis based on irreversible removal, suitable to an analysis based on reversible removal, - 130 - all suitable for for FS-4 y-CHO cells. MECHANISMS OF HYDRODYNAMIC DEATH IN DILUTE CULTURES CHAPTER 4. First, Second, and Higher Order Mechanisms of Hydrodynamic Death 4A. Cell death and removal have been identified as the primary response It of FS-4 cells to excessive agitation. type hydrodynamic of this cause forces is For removal. and death what however, not clear, microcarrier cultures with high agitation, cell death may arise through turbulent microcarriers, no Papoutsakis, and the 1986b). fields, collisions and microcarriers between solid the From the data currently available, it appears arises damage significant microcarriers between collisions or components of the vessel. that flow time-average eddies, solid through of components Because microcarriers the collisions vessel between (Cherry and are very small and almost neutrally buoyant, they should not rapidly penetrate the boundary layers surrounding the solid vessel components. In general, the mechanisms of hydrodynamic death can be grouped into The first order mechanisms first, second, and higher order mechanisms. represent the interaction of a single microcarrier with the surrounding field. flow The second mechanisms the represent simultaneous interaction of two microcarriers with the surrounding flow field. higher order mechanisms represent the simulateneous interaction The of multiple microcarriers with the surrounding flow field. The first The second order mechanisms should be predominant in order mechanisms become should - 131 - more dilute cultures. significant as the microcarrier The higher order mechanisms concentration is increased. only in should be predominant very concentrated cultures. The higher order mechanisms will not be investigated in this thesis. To provide clear and unique information regarding the first mechanism, experiments were first performed with dilute cultures. order These experiments elucidated the nature of the first-order mechanisms without interference from the second-order mechanisms. were performed with concentrated cultures. Subsequent experiments The higher rates of damage in the concentrated cultures were analyzed to investigate the nature of the second order mechanisms. 4B. Effects of Power and Viscosity in Dilute Cultures For dilute microcarrier cultures, the mechanisms of cell death from turbulence were investigated through experiments on the effects of power and viscosity. through Cell damage from time-average flow fields was avoided average shear rate and fluid limitations on the maximum time viscosity. The maximum shear stress from time-average flow fields was than 5 dyne/cm2. less riers, The cultures contained or 0.002 volume fraction solids, only 0.2 g/l microcar- and were therefore not subject to significant damage from microcarrier collisions. Figure 29 shows the effect of 20 g/L dextran supplement (78,500 MW) on cell growth in The cell the dilute cultures at different levels of agitation. concentrations the represent viable, or attached, cell To eliminate cell damage from time- concentrations for the cultures. - 132 - 106 -N 60 rpm No dextran 10 0 --- A 77----a e rpm 20 g/L dextran 220 rpm 20 g/L dextran ..... .. .. ... ......... 10 60 220 rpm No dextran 10 3 30 60 90 I I I I 120 150 I 180 TIME (HRS) Figure 29. Effect of 20 g/L dextran (78,500 MW) on net growth at different stirring speeds the cultures were grown in the 500-ml vessels with average flow fields, 5.3 power and The data for the control cm impellers. or a at 60 RPM, cultures 2 input of approximately 10 dyne/cm , has already been illustrated discussed in Figure 11 1. of Chapter It for repeated here is reference. At 60 RPM, there was no significant hydrodynamic cell in viscosity and death and removal. At 230 RPM, there was no effect from an increase death and removal from hydrodynamic forces were strongly evident. For the cultures with and without dextran at 230 RPM, concentrations were however, indicate, lower than that in the controls at 60 RPM. cell hydrodynamic the viable cell death and The data removal can be At reduced with a viscosity increase through dextran supplementation. the 230 RPM, culture with 20 g/L dextran exhibited much higher viable cell concentrations than the culture with no dextran. Figure 30 shows the effect of a 50 g/L dextran supplement (78500 MW) on cell growth in the dilute cultures at different levels of agitation. Again, to eliminate cell damage from time-average shear fields, cultures were grown in the 500-mL vessels with 5.3 cm impellers. RPM, supplementation with 50 g/L dextan cell concentrations. dextran led to an increase in due to viscous At 60 led to a reduction in viable This reduction was due to the mild toxic effect of 50 g/L dextran on cell growth. was the At 185 RPM, supplementation with 50 g/l viable cell concentration. This increase reduction of cell death and removal. With 50 g/L dextran, the viable cell concentrations in the culture at 185 RPM were - 134 - 106 -- - - 10 60 rpm No dextran 60 rpm 50 g/L dextran ,.0 185 ---- rpm 50 g/L dextran 10 - 185 rpm No dextran 10 30 60 90 120 150 180 TIME (HRS) Effect of 50 g/L dextran (78,500 MW) Figure 30. on net growth at different stirring speeds Supplemen- nearly equal to the concentrations in the culture at 60 RPM. completely g/L dextran almost 50 tation with eliminated hydrodynamic death and removal at 185 RPM. Numerous other experiments, similar to the one described above, were the on performed dextran employed experiments The fields. flow collisions from microcarrier to cell damage were not subject at different dextran supplements All cultures contained 0.2 g/l microcarriers levels of agitation. average various effect of and time- or supplements between 20 and 50 g/L (78,500 MW) and were performed over a wide range of of levels results The agitation. the followed trends shown in Figures 29 and 30 and are included in the subsequent data analysis. 4C. Cell Death Through Microcarrier-Eddy Interactions As discussed section, the riers. death from turbulence hydrodynamic turbulence and Thesis should become evident when isotropic regime, the equilbrium appears of size average to exist in the viscous the estimated by the average Kolmogorov length scale, 3 - 1/4 The size in power or can be L: (Eq. 41) the average power input per unit mass and v is fluid viscosity. increase eddies smallest L = (v / i) where F is Formulation generates eddies which are smaller than the microcar- Because dissipation Review Literature the in the smallest eddies of a decrease the kinematic decreases with an in kinematic viscosity. For suffi- ciently high power inputs at a given viscosity, the turbulence generate eddies which are smaller than microcarriers. - 136 - should Cell damage from turbulence should then become evident. To investigate whether the proposed role of eddy length is correct, For each the experimental data for the dilute cultures was analyzed. culture, the relative observed growth rate, pr, was calculated from the equation (Eq. 42) Ar -= Pobs//d where pobs was the average observed growth rate of the culture, was the mild average observed growth rate of a control agitation the with culture grown under average The concentration. dextran same and pd observed growth rates, which represent the difference between the actual growth rates and death rates, were calculated through linear regressions on the attached cell growth rates use The concentrations. relative of observed corrects for the mild effects of dextran toxicity which were observed with dextran supplements greater than 25 g/l. Figure average 31 eddy Kolmogorov viscosities. decrease shows the plot of relative observed growth rate versus length number a for different of Cell death through hydrodynamic forces, as indicated by a growth rate, becomes apparent when the in relative specific average Kolmogorov length scale falls below about 130 microns, 2/3 of the fluid microcarrier diameter of 185 microns. As or about expected, hydrodynamic death occurs when the turbulence generates eddies which are smaller than the For microcarriers. a number of different fluid viscosities, hydrodynamic death in dilute cultures correlates well with Kolmogorov length scale. The correlation describes the effect of both - 137 - * 0.74 cp 1.04 cp A 1.10 1.35 cp + cp * 1.79 A 1.85 cp cp 1.20 0.90 0.60 0.30 0.00 -0.30 -0.60 I I 50 I 100 KOLMOGOROV , I , 150 , ,, I 200 I , , 250 LENGTH SCALE (MICRONS) Figure 31. Relative growth rate vs. Kolmogorov eddy length scale for FS-4 cultures with 0.2 g/L microcarriers i 300 viscosity and power The (v3/f)1/4. isotropic that as combined in the unique length correlation provides existence of this equilibrium exists at scale more evidence Kolmogorov the group, scale in overagitated microcarrier cultures. The results in Figure 31 are not the only data which illustrates the correlation length scale. of hydrodynamic death with Kolmogorov Prior to the experimental work in this thesis, the data presented in Hu (1983) and Sinskey effects et al (1981) were analyzed. Both researchers studied the of agitation on net growth of cells cultures. microcarrier in Experiments were performed on the effect of vessel geometry but not on the effect of fluid viscosity. Figure 32 shows cells. the correlation of the data of Hu for FS-4 (1983) Relative growth extent, or relative net positive growth after inoculation, is compared to average Kolmogorov eddy length scale. death is the relative growth extent, indicated by a decrease in proportional Cell which is to relative observed growth rate for positive net growth. Figure 31. The data show a similar trend to the results in Cell death from turbulence becomes evident when the average Kolmogorov length scale Further decreases in falls below about 130 microns. scale lead to a sharper decline than observed in be expected with the This would and the The plateau at very small eddy lengths corresponding collision damage. indicates no net positive Figure 31. concentrations microcarrier higher Kolmogorov length growth occurred. negative growth might have occured. - 139 - It is unclear whether net 10080- 60- Symbol 40- Impeller Diam. (cm) A 20- 0- 50 100 150 KOLMOGOROV EDDY LENGTH SCALE Vessel Vol. (ml) 3.2 250 4.1 250 5.1 250 7.5 2000 8.5 2000 200 (MICRONS) Figure 3 2. Relative growth rate vs. Kolmogorov eddy length scale for FS-4 cultures with 5 g/L microcarriers (data from Hu, 1983) In well Figure for 32, a single correlation describes the a number that indication of different vessel cell regime. dissipation death Such arises geometries. eddies through eddies exist in a data reasonably This in state is further the of viscous isotropic equilibrium which depends only on the local power dissipation rate and kinematic viscosity, and not on the vessel geometry. eddies, in contrast, Energy containing are highly dependent on reactor geometry. If cell death arose through the action of the energy-containing eddies, a strong effect of geometry should be observed. Figure 33 shows the correlation of the data of Sinskey et al (1981) Maximum cell concentration is for chicken embryo fibroblasts. compared As observed in Figure 32, a single to average Kolmogorov length scale. correlation fits the data for a number of different vessel geometries. Hydrodynamic death, concentration, is as indicated by evidenced when decrease a the length in scale the maximum falls cell below 100 The microcarrier diameter was not reported and may have been microns. Cell growth drops very sharply for slightly smaller than 185 microns. eddies smaller than 100 microns; this may indicate a difference between FS-4 cells and chicken embryo fibroblasts. The plateau at very small eddy lengths does not indicate whether net negative growth occurred. For a number of different microcarrier scale. cultures data sets, correlates well hydrodynamic death in with average Kolmogorov length As expected, cell death occurs when the turbulence eddies that are smaller than the microcarriers. - 141 - dilute generates In parallel research 6A w U 5- (0 0 4- 0 A A U Symbol z 0 3.3 4.4 0 7.5 A U 2 - Impeller Diam. (cm) Vessel Vol. (ml) 250 250 1000 -J ------- 0o-QA 100 KOLMOGOROV 200 300 EDDY LENGTH SCALE (MICRONS) Figure 3 3. Maximum cell concentration vs. Kolmogorov eddy length scale for chicken embryo fibroblasts on 5 g/L microcarriers (data taken from Sinskey et al., 1981) - 142 - with cultures observed a of freely-suspended animal cells, similar correlation of cell McQueen et al death with Kolmogorov length eddies smaller Cell death occurs when the turbulence generates scale. than about 3.5 microns, (1987) or about 1/3 to 1/2 the cell diameter. Hydrodynamic Forces in Microcarrier-Eddy Interactions 4D. To determine whether the correlation of cell death with eddy length has a fundamental basis, produce hydrodynamic Currently, however, hydrodynamics one forces there is near a particle eddies should determine whether the sufficiently no in thorough strong to can damage cells. the complex description of a turbulent flow field. Accordingly, only very rough estimates of the forces can be made. If Matsuo and Unno forces predominate, viscous (1981) suggest that the shear stress on the surface of a sphere in a turbulent flow field is given by r where r; is = the 2rq(2/15)1/2 (Eq. 43) 1/2 fluid viscosity. If Reynold's stresses are important, Matsuo and Unno (1981) suggest that r = 0. 3 7p f(e/v)d (Eq. 44) where pf is the fluid density and dp is the microcarrier diameter. For the conditions under which cell death was observed, the average power input per unit mass was first used to estimate the shear stresses from equations 43 and 44. From equation 43, shear stress estimates in - 143 - the range of 1-3 dyne/cm 2 were obtained. These values are somewhat less than 7 dyne/cm2 , the minimum shear stress for which significant cell damage has been equation 44, obtained. reported (Stathopoulos shear stress estimates and Hellums, 1985). From the range of 2-16 dyne/cm2 were in The upper range of these values are in the range known to cause cell damage. The shear stress estimates can alternatively be performed with local instead power dissipation of average In a stirred tank, the rates. power dissipation rates in the impeller discharge stream are much higher than the average dissipation rate. (1981) and Placek dissipation rates in and For Rushton turbines, Okamoto et al (1985) Tavlarides that report the power the impeller discharge stream are approximately fold higher than the average dissipation rates. 6 This ratio was used to estimate the maximum local power dissipation rate and the corresponding maximum shear From equation 43, the maximum are in the range of 2-6 dyne/cm2 , again still known to cause estimates values stress. From equation damage. 44, stress estimates were in the range of 10-100 dyne/cm2. shear stress less than the the maximum shear These values are all within the range known to cause cell death and removal (Crouch et al, 1985; Stathopoulos and Hellums, 1985). As cells already mentioned with regard to selectivity of cell removal, could be damaged or killed not only by shear on microcarriers stresses, but also by normal forces. Normal forces will be generated by velocity and pressure fluctuations in the turbulent flow field of a - 144 - microcarrier culture. In terms of cell death, the critical fluctuations occur on a scale which those which will be in the viscous dissipation regime. eddies unit area on the microcarrier magnitude in size intermediate These flucuations will involve and microcarriers. between cells is Thus, the normal the force per Fn, might be estimated from the surface, fluctuations which occur on the scale of the of the pressure viscous dissipation regime: F P - n (Eq. 45) vdr where P'vdr represents root mean square turbulent pressure or pressure intensity, due to the eddies in the fluctuation, viscous dissipation regime. in Turbulence isotropic viscous dissipation regime is essentially and has a characteristic Reynold's number near unity (Hinze, The pressure fluctuations due to the viscous dissipation eddies 1975). might the thus be estimated Hinze (1975) P' where u' from an extension of the result presented in for isotropic turbulence of low Reynold's number: = (Eq. 46) Pf u2 and P' represent the velocity and pressure intensity, respec- tively. In terms fluctuations The effective of are cell relevant the death, pressure and velocity dissipation scale. those which occur on the viscous velocity intensity might thus be estimated by Kolmogorov velocity scale for the viscous dissipation eddies. - 145 - The normal force per unit area on the microcarrier surface, Fn, is then given by F pf (EV)1/ 2 = (Eq. 47) where pf is the fluid density. Using estimate power average normal forces rates dissipation equation on the order of 1-4 dyne/cm 2 which exhibited hydrodynamic rates in If the death. increased to 2-10 dyne/cm2 . 47, one can for the cultures local power dissipation the normal forces estimates are the impeller stream are used, strength can damage in It is unknown whether normal forces of this or remove cells from a growth surface. In fact, a literature review indicates no published data with regard to the effects of normal forces on animal cells. performed in 4E. Future research will hopefully be this area. Kinetics of Cell Growth and Death in Dilute Cultures Although the investigated more thoroughly, a near hydrodynamics surface must be the comparison of experimental data with simplified models can help to elucidate excessive agitation. microcarrier the mechanisms of death from The data in Figures 31-33 indicate that cell death occurs when the turbulence generates eddies which are smaller than the microcarriers. length scale, Based upon the correlation of cell death with Kolmogorov a model was developed to describe hydrodynamic damage in dilute microcarrier cultures. the kinetics This "eddy-length" model is based upon the following assumptions: 1) hydrodynamic damage occurs through microcarrier-eddy encounters - 146 - of 2) damage will occur only if a microcarrier encounters an eddy smaller than a critical length, Lc, assumption is based upon the correlations This 130 microns. of approximately Figures shown in 31 and 32. growth inhibition, are the only death and removal, and not 3) cell forms This assumption is based upon the damage. of hydrodynamic experimental results previously presented and discussed. 4) a constant fraction of the culture volume is appearing eddies regime. Kolmogorov the in filled with randomly This assumption is implicit in the derivation of the Kolmogorov scales from an energy balance. Under the fourth assumption, the effective eddy concentration in the proportional to the inverse of the eddy volume, Kolmogorov regime is or (E/V3)3/4. Because cell death occurs through cell-eddy encounters, the total rate of cell death is proportional concentration times the eddy concentration. proportional therefore to the Expressed in mathematical form, dC -- eddy dt q where p is - pC = Ke(/v3)3/4 of the cell The specific death rate is concentration, or (i/v3)3/4. the kinetic "eddy-length" model becomes =PC = to the product qjC L > Lc (Eq. 48) L < Lc (Eq. 49) (Eq. 50) the intrinsic specific growth rate and is - 147 - independent of the level of agitation, qi is the specific death rate due to microcarrier- eddy interactions, 7 is the average power input per unit mass, v is the fluid kinematic viscosity, a is Ke and function of the cell and microcarrier properties, and possibly the reactor geometry. To test the kinetic model presented above, microcarrier the data for the dilute culture, For each cultures were analyzed. the average specific death rate was calculated from the equation: q = (Eq. 51) Ad - Mobs where pobs and pd have been defined with equation 42. Figure 34 shows a plot of specific death rate versus eddy concentration group, (e/V for two different vessels with the same impeller. Each set of data shows a linear correlation, as predicted by the eddy length model. The intercept values near zero follow the assumption of insignificant cell death with mild agitation. For the data from the 500-ml cm) vessel, (9.6 the value of Ke from linear regression is 6.9 x 10-13 cm3 /sec with a 95% confidence interval 10-13 cm3 /sec. of ± 1.7 x value two interval values confidence of ± 1.3 x 10-13 cm3 /sec. of Ke interval, is not as statistically from determined presented in Kleinbaum and Kupper (1985). not 125-ml vessel, the of Ke from linear regression is 5.4 x 10-13 cm 3 /sec with a 95% confidence the For the data from the indicate a clear effect of vessel The difference between significant within a the statistical 148 - methods The limited data therefore do geometry. In fact, a correlation fits both vessel geometries reasonably well. - 95% single A = 5.3 cm Dt = 6.3 cm Di * Di Dt = 5.3 cm = 9.6 cm 60 50 Cl) 40 w I 30 20 U U] n~ Cl) 10 0 0 2 8 6 4 EDDY CONC. (ES/V 3 3/4 ) 10 (10 6/ML) Specific death rate vs. eddy Figure 34. concentration for dilute FS-4 cultures 149 12 The data in Figures 31-34 all indicate reasonably good correlations power with average dissipation regime, viscous strong effects of vessel Because cell death is probabilistic and involve eddies in the geometry. expect input and do not indicate which are also probabilistic, one would not In this thesis, the average value a strong effect of geometry. of 6.2 x 10-13 cm3 /sec will be used for Ke in the kinetic expression for cell death from microcarrier-eddy interactions. Future research will hopefully determine and incorporate any effects of vessel geometry in more advanced kinetic expressions. hydrodynamic The predictions the model, in effects dilute predictions the of the To further test the validity of of the eddy-length model. the followed cultures FS-4 model were also compared to the following sets of results: 1) the effects of agitation on net growth of Vero cells on Cytodex 1 microcarriers, as determined by Hirtenstein and Clark (1981) and shown in Figure 1 of this thesis, 2) the effects of agitation on net growth of FS-4 cells on Cytodex 1 microcarriers, as shown in Figure 24 of this thesis, the effects 3) of agitation on secondary disruption of protozoa in baffled stirred tanks, as determined by Midler and Finn (1966) For both the Vero and FS-4 cells, the cultures were grown in unbaffled spinner vessels, and the specific death rates were determined from equation 51. The microcarrier concentrations were 3 g/l, or 3% solids by volume, and thus were slightly out of the dilute regime. - 150 - For protozoa, the which are and about half sensitive shear size the of microcarriers, the specific death rates were calculated from the slopes of secondary disruption data. correlations The power inputs were determined from the Rushton et al presented in (1950) and Bates et al (1963) with the corrections presented in Bujalski et al (1987). Figure 35 shows a log-log plot of specific power input per unit mass for the Vero cells, and protozoa. The data indicate that Vero linear correlation with a slope near 0.75. are FS-4 cells, average the eddy-length model, all three sets of data show a As predicted by cells death versus considerably more sensitive agitation than FS-4 to cells. However, the power input calculations for the Vero cells were rough and may have been in error with regard to absolute values. The data for the protozoa was taken over a wide range of tank geometries. The ratios of impeller to tank diameter were varied between 0.24 and 0.71. wide range of geometries, the data can be reasonably single correlation based on average power input. that there are strong effects no Over this described by a This is more evidence of geometry when cell death arises through eddies in the viscous dissipation regime. a number For describes cultures. random of sets that kinetics Thus, of dissipation regime. eddy-length model the death of cell it appears interactions data, in accurately dilute or reasonably dilute that cell death truly does arise through between microcarriers and eddies in the viscous Such interactions will not lead to cell death, however, if the eddy is larger than the microcarrier. - 151 - 10 Slope= 0.82 Protozoa IU Cw 1- 10 Slope=0.76 Vero cells Slope =0.72 FS-4 cells 10 10 II I 10 AVERAGE 10 I I I I 4 Protozoa I~ 102 10 10 3 Animal cells POWER PER UNIT MASS (CM 2/S EC3 35. Specific death rate vs. power input per mass for Vero cells, FS-4 cells, and protozoa Figure - 152 Cell Damage from Time-Average Flow Fields 4F. the conditions To investigate fields shear there is these vessels, time-average a clearance with are rates shear equation 16. is rate In the of impeller rotation, strong in generated region the the between For operation at 220 RPM, the maximum time- impeller tip and tank wall. shear dilute of only 5 mm between the impeller Under high rates and vessel wall. average performed were in the 125-ml vessels with high viscosity increases. cultures tip experiments occur, might under which damage from time-average approximately 160 estimated as sec-l, from A viscosity increase to 1.5 cp or higher might therefore will lead to shear as it induce damage from time-average shear fields, stresses greater than 7 dyne/cm 2 (equation 14). Shear stresses of this value are the minimum reported to cause cell damage (Stathopoulos and Hellums, shear Accordingly, 1985). fields was onset of damage the investigated by increasing from time-average the fluid viscosity above 1.5 cp in the 125-ml vessels at 220 RPM. Figure 36 shows the results of this investigation. are presented for four conditions the 125-mL vessels. in Growth curves Because many of the conditions were investigated with duplicate cultures, and because different inocula were used for each set of duplicates, viable concentrations are reported relative to initial The cultures at 35 insignificant hydrodynamic the exhibited RPM The death. dextran had very low viable cell degree of hydrodynamic death. cell values at inoculation. cell normal cultures concentrations at 220 growth with RPM with no and exhibited a great When the viscosity was increased to 1.04 - 153 - 10 A I ~-------------- LG ~ ------ rpm No dextran 220 rpm 20 g/L dextran - e0 ~ 35 - 220 rpm 50 g/L 0 0 0.1 I 30 I 60 I 90 I 120 * I 150 TIME (HRS) Figure 36. Effect of dextran on net growth in a reactor with strong time-average shear fields dextran 220 rpm No dextran cp with 20 g/l cell dextran, death and removal were strongly reduced. When the viscosity was further increased to 1.85 cp with 50 g/l dextran, cell death was more pronounced than in the 20 g/1 cultures. stress from this flow time-average fields, Tmax, or cell death from time-average If dyne/cm2. dextran, at 220 RPM with 50 g/l culture the In the maximum shear was 9 approximately flow fields was occuring in culture, but not in the other cultures, the specific death rate should be higher power. expected than would be for the given viscosity Figure 37 shows that this hypothesis is correct. death rates from Figure 34, and and The specific the best-fit linear correlation, are 2 shown for the cultures with Tmax < 5 dyne/cm . The specific death rate 2 for the culture with Tmax = 9 dyne/cm is far above the correlation for the other cultures. a 99% confidence average shear The difference is statistically significant within interval fields. and is Thus, that appears it of cell damage from time- indicative cell damage from time- The onset of such damage can be roughly average shear fields can occur. predicted through fluid-mechanical modelling and through knowledge of a critical shear stress for cell damage. The predictions can be used to purposely avoid cell damage from time-average in many of the experiments in this thesis. flow fields, as was done The predictions can also be used to develop criteria for reactor design and scale-up. 4G. Effects of Cavitation In the turbulent flow field of a stirred tank, dynamic reductions in pressure will occur as the fluid flows around the impeller. - 155 - For * Max time-avg IT < 5 * Max time-avg - = 9 dyne/cm 2 dyne/cm2 60 a w Co 50 N 0 1~ 40 Lu I- 30 I FLu 0 20 10 2 4 6 EDDY CONG. (EIV 8 3 3/4 ) 10 12 I (10 6/ML) Specific death rat e vs. eddy Figure 37. concentration at different levels of maximum shear stress from time-average shear fields 156 - sufficiently strong agitation, the reduction in pressure may lead to the formation of vapor cavities, an explosive phenomena known as cavitation. the local pressure falls below the fluid Cavitation will arise only if If cavitation were vapor pressure at the given operating temperature. to occur in likely would result in it a microcarrier bioreactor, cell damage or lysis. The existence generally can cavitation of cavitation number (Daily and Harleman, correlated with be The cavitation number, 1966). a a, is given by (Boucher and Alves, 1973) P a = - Py (Eq. 52) 2 Pg U /2 where P is the static pressure in the undisturbed flow, Pv is the fluid vapor pressure, pf is the fluid density, and U is the free-stream fluid Cavitation will occur only if velocity. the cavitation number below a critical value characteristic of the critical values These given geometry. are gnerally in the range of 0.2 to 2.5 falls (Boucher and Alves, 1973). For a stirred tank, minimum the cavitation number might be reasonably estimated from the maximum fluid velocity, including both the For a radial flow impeller, both time-average and turbulent components. the maximum time-average generally occur in fluid the radial jet average radial velocity, Umax, is - velocity and off the impeller. turbulent intensity The maximum time- proportional to the tip speed of the 157 - impeller, as given by the data presented in Oldshue (1983): ii 0.7 = max (Eq. 53) r N D. 1 In reference to this velocity, the maximum values of relative turbulent an upper Thus, 1985). limit on the total Tavlarides, (Placek and of 60% order generally on the intensity are fluid velocity Umax can be estimated by U max u'max where U = is max the + u' max maximum (0.7 w N D.) - (Eq. 54) 1.6 turbulent mean square which exhibited root velocity fluctuation. For the cultures microcarrier removal, the maximum total less than 72 cm/sec. maximum This is values and death fluid velocities given by equation 54 were Thus, with P = 760 mm Hg and Pv = 47.1 mm Hg one can estimate a minimum cavitation number (Liley and Gambill, 1973), of 370. cell over two orders of magnitude greater than the typical for which occurs. cavitation Thus, it appears that cavitation did not occur in the experiments performed in this thesis. In general, for the levels of agitation employed cultures, cavitation should not present a problem. in microcarrier Nonetheless, for any given design and set of operating conditions, the cavitation number can be calculated. as one of the A minimum value for the cavitation number might be set The critical design criteria. incipient cavitation, however, appears equipment (Boucher and Alves, 1973). - 158 - to cavitation number depend on the for scale of the MECHANISMS OF HYDRODYNAMIC DEATH IN CONCENTRATED CULTURES CHAPTER 5. 5A. Effect of Microcarrier Concentration with High Agitation primarily through circumstances, unusual concentrated cultures encounters For fields. flow time-average cell and, cell death might also occur through hydrodynamic As between microcarriers. discussed previously, collisions, even though these they may flow between microcarriers which come fluid the involve in microcarrier-eddy under interactions will be referred to as primarily occurs agitation, under high agitation, interactions death high cultures dilute For in close but not actual contact. To with collisions microcarrier cultures The whether determine contained occurs inert microcarriers agitated at 150 RPM in 1.5 g/l Cytodex 1, inert microcarriers were through death agitation, high cultures cell microcarrier were added the 125-ml vessels. 5% (v/v) serum, added to levels of 0, 5, to All and no dextran. 10, 15, 20, 25, A control culture at 35 RPM without inert microcarriers was and 30 g/l. grown for reference purposes. Figure 38 shows the attached cell concentrations microcarrier concentrations. culture The at 150 for various inert RPM without inert microcarriers exhibited a moderate amount of cell death and removal. the inert microcarrier concentration was increased, concentrations were progressively reduced. As the attached cell A detrimental effect from adding inert microcarriers was especially apparent during the attachment - 159 - Speed 106 35 rpm 150 rpm 150 rpm z- 5 10 ..J 0-J 150 rpm 0 150 rpm z 0 4 10 60 120 180 240 TIME, (HRS) Effect of inert microcarriers on net growth with high agitation Figure 38. - 160 - 300 With high inert microcarrier concentrations, cell concentrations stage. At 30 g/l, 12 hours. dropped precipitously during the first only 54% of The debri from the unattached cells the inoculum was viably attached. was clearly evident for up to 30 hours past inoculation. A detrimental effect of adding inert microcarriers was also apparent inert microcarriers, the culture at 150 RPM grew to 45% of the maximum density observed in the control culture growth stage. during the absence In the of As inert microcarriers were added, at 35 RPM. growth progressively decreased until the culture with 30 g/l exhibited only 8% of the maximum density of the control. At high levels of agitation, the observed growth rates were clearly reduced through the addition of inert microcarriers. 5B. Cell Death through Microcarrier Collisions microcarriers Because they primarily will small and nearly neutrally buoyant, are very follow streamlines. fluid Almost all relative motion between two neighboring microcarriers will arise from the action of turbulent eddies. might The viscous forces or pressure fluctuations from a throw two neighboring microcarriers single eddy other. The volumetric frequency of such collisions, fe, order in microcarrier concentration, f where K2 is c = K against each would be second Cm, and would thus be given by: (Eq. 55) C 2 m a collision frequency constant. The amount of cell death from each collision will be proportional to the cells per unit surface - 161 - area, If and the effective surface area exposed to each collision, K 3 - T, a cell in an exposed area had a probability K4 of rate volumetric the collision, death cell of death from each from collisions, (dC/dt)coll. , would be then be given by (dC/dt) Coll. where C is q2, K 3K 4) - 2 i m volumetric cell concentration. represents - q C C (Eq. 56) The specific death constant, the combined product of the parameters K 2 , K 3 , and K4 divided by the total surface area per microcarrier, K 5 , or 4nrrp 2 , As all three parameters K2 , K3 , and K4 will vary with the level of agitation and the strength of the hydrodynamic forces involved with the collision, the combined term q2 should be a function of the of For mild agitation, there was no effect agitation and fluid viscosity. of adding level and thus the value of q2 was zero. inert microcarriers, In general, the value of q2 will depend not only on hydrodynamic variables, but also on the cell and microcarrier properties. If the eddy-length model is microcarrier collisions, the now extended to include cell death from (or viable) attached cell concentrations should follow the equations pC 1 with mild agitation (Eq. 57) with strong agitation (Eq. 58) dt dC C = pC - q 1 C - q 2CC dt where the criteria for cell death involves - 162 - the level of agitation and may not be a simple function of Kolmogorov eddy length. If cell damage occurred from a microcarrier collision, and if one of the microcarriers the resulting damage should be, was an inert microcarrier with no cells, half of that which would on average, cells. Thus, are microcarriers inert if occur if had both microcarriers added at high levels of agitation, equation 58 should be extended to dC =pC - - qlC - q2 C (Eq. 59) (q2 /2)C C - dt where Ci is the concentration of inert microcarriers. To evaluate whether microcarrier collisions levels are important at high one can analyze the data shown in of agitation, the model presented in equation 59 is correct, Figure 38. If the data should follow the relation obs = (Eq. 60) b - (q2 /2 )C. where 1 Mobs = C dC (Eq. 61) avg - dt and with constant q1 , Cm, and average y b = constant If there is = (Eq. 62) p - ql- q 2 Cm a second order damage mechanism, and if all first order mechanisms are relatively independent of microcarrier concentration, the observed growth rate should decrease in a linear fashion with the inert microcarrier concentration. The rate of damage - 163 - from the second order (involving the inert microcarriers) should be proportional mechanism(s) to of damage from the first order mechanism(s) The rate slope. the should be proportional to the difference between the intercept, b, and the value of (p - q2Cm)- Figure the average growth rate observed after attachment inert microcarrier the versus shows 39 of equations predictions concentration. 60-62 and clearly The follows the that there are at collisions. involves microcarrier concentration and thus in microcarrier concentration and first order mechanism is other indicates data One mechanism is second order in least two distinct death mechanisms. microcarrier The involves microcarrier-eddy encounters. the For cultures at 150 bulk the RPM, increased through the addition of inert microcarriers. power number correlations in presented the viscosity kinematic was According to the Materials Methods and section, the culture with 30 g/l inert microcarriers should have had an average power input 25% microcarriers at 150 RPM. than higher the culture with no inert This would have apparently resulted in a 18% increase in qi and an (as yet) undetermined increase in q2. in may have introduced minor errors the calculations This effect of qi and q2 from equations 60-62. The linearity of the data in Figure 39 does not strictly prove that the first order mechanism was independent of microcarrier concentration. Nonetheless, the data follows a model which assumes this independence - 164 - -. 4 10 Co C 1~ x 35 rpm = 150 rpm -2 0 12 6 18 24 INERT MICROCARRIER CONCENTRATION, (GM/LITER) Observed specific growth rate Figure 39. vs. inert microcarrier concentration - 165 - 30 is on the microcarrier of energy dissipation rate the The pertinent criteria range of concentrations investigated. over the If the power draw will go up, are added at constant speed, microcarriers surface. The but so will the amount of energy dissipated by other microcarriers. average Thus, relatively unchanged. be may solids offset increased the on dissipation increased remain may increased power draw from the the by microcarrier each on rate dissipation energy other This effect may account for the linearity of the data in microcarriers. Figure 39. For cultures the at spacing average the surfaces ranged from 50 to 380 microns, microcarrier length scales Kolmogorov RPM, 150 microcarrier was spacing greater than the average the while the average 60 microns. to ranged from 50 between The average Kolmogorov length scale for all but the most concentrated culture. an eddy-microcarrier mechanism to be for In order independent of microcarrier concentration, one might think that average spacing between the microcarrier surfaces would have to be greater than the eddy diameter, somewhat or the loosely, Kolmogorov length smallest scale. Strictly, however, the Kolmogorov length scale is not defined as an eddy but diameter, dissipation does not rather occurs in invalidate as the typical the absence the of distance solids. the logic behind over This which subtle eddy-length viscous difference model, but it should make one wary of comparing eddy lengths with the distance between microcarriers. For microcarrier-eddy interactions to be independent of - 166 - microcarrier concentration, it is not required that the microcarrier spacing is greater than the Kolmogorov length scale, but rather that the microcarrier spacing is greater than the dissipation occurs when an eddy interacts distance distance over which viscous with a microcarrier. should be somewhat less than the Kolmogorov This length scale, as the presence of solids is known to increase power dissipation (Hiemenz, 1977; Monin and Yaglom, 1971). 5C. Effects of Agitation Power on Cell Death from Collisions To investigate the effects of agitation power on collision damage, cultures with inert microcarriers were grown in the 125-ml vessels. cultures contained 3 g/1 Cytodex 1 g/l inert All cultures were agitated at 35 microcarriers, 5% FCS, and no dextran. RPM for a 44 hour 15 microcarriers, All The cultures were subsequently attachment period. agitated at various speeds during the growth period. The results of the experiment are shown in Figure 40. at the higher lower viable Extensive stirring speeds, especially cell death from hydrodynamic stirring speeds. 140 and 200 RPM, than the control culture cell concentrations The cultures exhibited at 35 RPM. occurred at the higher forces The amount of death increased sharply with stirring speed; the culture at 200 RPM exhibited a strong and continual decrease in attached cell shown As concentration. by a comparison between Figures 29 and 40, the rate of death for the concentrated culture at 200 RPM far exceeded that for a dilute culture at 220 RPM in the same vessel. The difference was due to death from microcarrier collisions. - 167 - 10 6 -3-35 rpm ----- 70 rpm e--- 100 rpm *- 150 rpm - - 200 rpm 10 30 60 90 120 TIME SINCE ATTACHMENT 150 (HRS) Figure 40. Net growth at different stirring speeds with 15 g/L ine rt microcarriers 168 - the between per unit pobs, rate, attached cell was constant, q2, For each culture, the specific order second agitation power growth to determine the relationship Figure 40 were analyzed The data in mass. death from calculated a linear at For the cultures concntrtins. and average observed net regression on the the high agitation, total specific death rate q was calculated from the equation: q = p - pobs (Eq. 63) where p was the observed growth rate for the control culture at 35 RPM with no hydrodynamic death. specific death The rate due to microcar- rier-eddy interactions, qi, was calculated from the correlation derived for the dilute cultures Figure 34. (6.3 cm) vessels, in the 125-ml The collision death rate constant, q2, shown in as was then calculated from the equation q - g (Eq. 64) q2 C + C./2 m i where the factor microcarriers. of for 2 accounts For each value of the lack of q2, a 95% on cells the inert confidence interval was calculated according to the statistical methods presented in Walpole and Myers (1972). Because uncertainty in the value of q2 arises from uncertainity in the values of both q and qi, the total interval for each q2 was estimated as the sum of the intervals for each qt and qi. Figure 41 shows the collision death rate constant, q2, versus The data scatters somewhat but average agitation power per unit mass. indicates that q2 increases with the level of agitation. - 169 the - The intercept A Di =5.3 cm Dt = 6.3 cm 0.14 0.12 0.10 0.08 0.07 0.05 0.03 0.01 -0.01 200 400 600 800 1000 AVG. POWER PER MASS (CM 2/SEC3 ) Second order death rate constant q2 vs. average power input per unit mass Figure 41. - 170 - further verifying the observation of falls close to the value of zero, The error bars insignificant hydrodynamic death with mild agitation. represent the in relationship which increase between an shows been graphed determined As correlation. mathematical drawn e can be and with coordinates arithmetic on q2 any For purposes of simplicity, the data through the confidence intervals. has Almost q2. of calculation the uncertainty The point. lead to tremendous of q and q the calculation combined uncertainty in data each for intervals confidence 95% regression, through simple a the line linear has a correlation coefficient of 0.85. 5D. Effects of Fluid Viscosity on Cell Death from Collisions To investigate cultures inert with supplements. the effects of fluid viscosity on collision damage, microcarriers To avoid cell damage were grown with from time-average 500-ml vessels were used with the 5.3 cm impellers. various dextran flow fields, the All five cultures contained 3 g/l Cytodex 1 microcarriers, 15 g/l inert microcarriers, and 5% FCS. All five cultures were grown at supplement for a 33 hour attachment period. 60 RPM with no dextran Four of the cultures were subsequently agitated at 190 RPM while one control culture continued to be agitated at 60 RPM. The four cultures at 190 RPM were supplemented with various levels of 78,500 MW dextran. The results of the experiment are shown in Figure 42. All four cultures at 190 RPM exhibited lower viable cell concentrations than the control culture at 60 RPM. Extensive hydrodynamic death and removal - 171 - 106 60 rpm 0m No dextran A1S------- AA ... 10 190 rpm 44 g/L dextran . ----- 5 190 rpm 59 g/L dextran 0 -190 rpm 3 1 g/L dextran -A- 190 rpm No dextran 10 4 0 30 60 90 TIME SINCE ATTACHMENT 120 150 (HRS) Effect of dextran (78,500 MW) on net growth Figure 42. at different stirring speeds with 15 g/L inert microcarriers occurred at 190 RPM. The total rate of death was the greatest in the culture with no dextran but was moderately reduced with 31 g/l dextran and strongly reduced with 44 and 59 g/l dextran. As shown by a comparison between Figure 30 and Figure 42, the rate of death in the concentrated culture with no dextran at 190 RPM far exceeded that for the dilute culture with no dextran at 185 RPM in the same vessel. The difference was again due to cell death from microcarrier collisions. The data in between Figure analyzed to determine 42 were the collision death rate constant, For viscosity. microcarrier-eddy each culture, the qi, interactions, q2, the relationship and the kinematic fluid specific death rate due to was calculated from the correlation derived for the dilute cultures in the 500-ml (9.6 cm) vessels, as shown in The collision death rate constant, q2, was calculated Figure 34. section 5C. from the methods presented in inputs, the collision rate death Due to differences in power constants were normalized by the average agitation power per unit mass. Figure 43 shows the normalized collision death rate constant, q2/E, versus kinematic The fluid viscosity. data scatters somewhat but clearly indicates that q2 decreases with the kinematic fluid viscosity. The error point. q2, bars represent the 95% confidence intervals for each data Again, there was tremendous uncertainty in the calculation of and almost any mathematical confidence intervals. For relationship purpose the of can be drawn through the simplicity, the data graphed on arithmetic coordinates with a simple linear regression. - 173 - was As * Di =5.3 cm Dt =9.6 cm 0.40 1(0 C~J 0.30 0.20 q 4p 0.10 I 4I 0.00 p -0.10 0.00 I I 1.00 0.50 KINEMATIC 1.50 I 2.00 2.50 FLUID VISC. (10- 2 CM 2/SEC) Normalized second-order death Figure 43. rate constant vs. kinematic fluid viscosity - 174 determined through regression, the line has a correlation coefficient of 0.84. Figure 44 shows the collision death rate constant, q2, as a function of E/v for both the 125 and 500-ml vessels with the same impeller. The e/v. The scatters data q2 that shows clearly but with increases The data do regressed line has a correlation coefficient of 0.84/1.0. The regressed indicate a significant effect of vessel geometry. not through line data observation the verifying the of all of has an insignificant cell further zero, near intercept mild with death agitation. The collision death rate constant increased with the power input and collisions This indicates that microcarrier the fluid viscosity. decreased with fluid. arise through the action of turbulence in the The collisions may occur primarily through turbulent eddies which are of a size comparable to spacing Cherry and Papoutsakis velocities and In viscous between microcarriers. (1986a), As discussed such eddies may lead to high relative collisions between neighboring microcarriers. microcarrier dissipation cultures, regime the eddies typically are spacing between microcarriers. This hypothesis is near the to the are in or comparable in size which It is likely that such high wave number eddies account for most of the cell death from microcarrier in Figure 44. in collisions. supported by the lack of geometric effects observed High wave number eddies exist in - 175 - states of equilbrium A Di =5.3 cm Dt 6.3 cm 0 Di = 5.3 cm Dt = 9.6 cm 0.16 0.12 0.08 0.04 0.00 -0.04 20 40 60 POWER PER MASS/KIN. 80 100 VISC. (10 3/SEC 2 Figure 44. Second-order death rate constant vs. (E/v) - 176 - 120 which are not strongly dependent on reactor geometry. Low wave number eddies and time-average flow fields are strongly influenced by reactor geometry. - 177 - CELL DAMAGE FROM DIRECT SPARGING CHAPTER 6. Effect of antifoam addition and direct sparging on cell growth 6A. It is frequently stated that direct sparging causes damage to cells 1981; (Pharmacia, microcarriers on and Spier 1984). Griffiths, Nonetheless, no published reports were found which document such damage. damage Cell is known to damage occurs from direct sparging in the damage may be more in extensive sparging direct Handa et al, (Tramper et al, 1986; cultures from occur of suspension It is likely that 1987). microcarrier cultures. given that cultures, microcarrier In fact, the cells are immobilized and are more susceptible to mechanical forces. effect The of sparging As cultures. microcarrier two experimental section, on cell described growth in was the examined FS-4 and Methods 500-ml Bellco spinner Materials cultures were grown in for flasks equipped with filter sticks for direct sparging. One experimental culture was sparged at a superficial gas velocity of 0.01 cm/sec with a Foaming was essentially eliminated through the 90:10 air-CO 2 mixture. daily addition of 20-40 ppm Medical Midland, MI). stick, and impeller. FCS, were basis, no stick, but with no sparging. complete with filter experimental third Corning, A second experimental culture was grown with antifoam in an identical vessel, A Emulsion AF antifoam (Dow culture was sparging in grown with no 500-ml a Corning antifoam, no vessel with a filter 7.8 cm All cultures contained 2.0 g/l microcarriers in DMEM with 5% replenished with nutrients on the normal interval feeding and were essentially at saturation with the 90:10 air CO2 gas - 178 - mixtures. Cell growth in the the results of the experiment. Figure 45 shows modified Bellco vessel with antifoam was essentially identical to cell This indicates that the growth in the Corning vessel with no antifoam. modifications to the Bellco vessel, including the presence of the filter It also indicates that antifoam growth. on cell had no effect stick, AF, at levels between 40 and 180 ppm, had no effect on cell growth. Aunins et al on microcarriers, tPA-CHO cells For similarly found no (1986) effect on cell growth from antifoam AF at 100 ppm. For culture the which directly was sparged, from cell damage sparging. the viable cell This is evidence of concentrations were lower than in the controls. significant the damage was The clearly evident even though the superficial gas velocity was only 0.01 cm/sec and there was no significant the first published foam formation. of evidence cell To my knowledge, this is from damage sparging in covered the microcarrier cultures. Subsequent to the described experiment above, growth period up to 180 hours after inoculation, added to the sparged culture. approximately 0.5 cm/day. which antifoam was no longer A foam layer began to form at a rate of After four days, nearly microcarriers had collected on the vessel wall above the Many microcarriers were spattered regions of the vessel. If through bubble bursts all 179 - the foam layer. into one wishes to keep the microcarriers - of distant in the 5x10 4 4 A No antifoam No sparging * Antifoam w/o sparging * Antifoam w/ sparging 4 10. 0 40 80 120 160 TIME (HRS) Figure 45. Effect of antifoam and sparging on net cell growth - 180 - 200 liquid phase, foam formation must be strictly avoided. Mechanisms of Cell Damage from Sparging in Microcarrier Cultures 6B. For on cultures, there has been little cultures In the absence of foam investigation. microcarrier in from sparging The possible mechanisms are listed below along not clear. is of microcarrier For sparging. of cell damage the mechanism amount considerable been has direct from damage cell research formation, there cultures, suspension with a review of the results for suspension cells: 1) Nutrient such as onto depletion. Required nutrients which are surface active, denatured or deactivated bubble burst at fluid phase out of the These substances could be concurrently the bubble interface. the adsorbed could be many proteins, rose with the bubble or as as they culture Aunins surface. the (1986) have et al shown that significant medium degradation can occur through direct sparging a over week long period. Such degradation may arise through protein denaturation at the liquid-gas interface. 2) Interfacial interface, forces. Upon cell contact with the cell membrane could spread and lyse, moving a bubble or non-integral membrane proteins, such as fibronectin, could be removed. If cell lysis did not occur upon initial contact, the cell may still adhere to the bubble and then lyse when the bubble bursts at the culture surface. that and Handa et al (1987) Kilburn and Webb (1968) damage of cells suspension from reduced by adding surface active agents, general, sparging is have found significantly such as Pluronic F-68. In surface active agents may form a shield between the cell - 181 - or lysis comes only that protection however, appears, cell eliminate thus and bubble, and It entrainment. from surface-active agents which are high in molecular weight (Kilburn and Webb, 1968). 3) directly the to exposed killed could be Cells Bubble bursts. bursts bubble at they damaged if or the culture are surface. Evidence for this mechanism has been given by Tramper et al (1986) and Handa et al (1987) for suspension cells. Tramper et al (1986) showed that cells caught in a bubble burst at the end of a pipette were columns, both Handa et al For bubble damaged. Tramper and Vlak the volumetric found that (1987) (1987) and rate of damage from sparging of suspension cells decreased with an increase in the bubble Tramper indicative This is height-to-diameter ratio. disengagement at the culture surface 1987) or at and Vlak, injector the of cell damage from (Handa et al, 1987; (Tramper nozzle and Vlak, 1987). 4) Hydrodynamic hydrodynamic forces bubble. could Cells forces. be killed or damaged by the fluid adjacent to a rising or bursting in This effect should increase with volumetric gas flow rate, but should decrease with fluid viscosity. An increase in viscosity should slow the bubble rise and dampen the turbulence in the fluid adjacent to the bubble. For suspension cells, essentially unaffected by cell damage increase from sparging fluid viscosity in appears to be through dextran Although the data is limited and supplementation (Handa et al, 1987). not conclusive, it appears that damage of suspension cells from sparging - 182 - in hydrodynamic be not may This nature. is as surprising, not suspension cells are more than two orders of magnitude smaller than most bubbles and are relatively resistant to damage from hydrodynamic forces (Augenstein et al, 1972; Dodge and Hu, 1986; McQueen et al, 1987; Smith et al, 1987). Cells A simple calculation readily illustrates that from hydrodynamic forces. the from observed damage hydrodynamic in nature. which column discussed in occupied Aunins are much more sensitive to damage however, on microcarriers, Figure in sparging 45 could have been In the sparged culture, the bubbles rose in a 10% approximately et al of the power (1986), the volume. culture input to As the liquid near bubbles may be approximated by the power required to match the buoyancy Thus, forces. for the sparged column region, the power sparging per unit mass of fluid, es, Q ( Pf - may be roughly calculated by: pb ) g H - Pf ( H (7rDt 2/4) 0.1 g=== Es the gas density, where Pb is input from Q is (Eq. H0) the volumetric 65) flow rate of gas in ml/sec, g is the gravitational acceleration, H is the reactor height, and H0 is the gas hold-up in the sparged region. The gas hold-up was estimated to be 20 ml from the increase in culture volume upon sparging. Equation local power into the a value 65 was used to estimate input per unit mass in of 100 cm2 /sec the sparged region. 3 for the This is well range for which death occurs from impeller-generated power, - 183 - even with the mild viscosity in bubbles. part, antifoam about by the Thus, the damage from sparging could have been due, at supplementation. least increase brought to Future power dissipation in the fluid adjacent research will hopefully elucidate to the mechanisms kinetics of cell damage from sparging in microcarrier cultures. - 184 - rising and BIOREACTOR DESIGN AND OPTIMIZATION CHAPTER 7. 7A. Mass Transfer Requirements In microcarrier cultures, agitation is required for cell-liquid mass transfer mass transfer, gas-liquid mixing. As previously (oxygenation), phase liquid and transfer can be discussed, cell-liquid mass For each chemical species approached with a Sherwood number analysis. which is consumed or produced by cells on microcarriers, the normalized gradient between the chemical concentration in the bulk medium, Xb, and the concentration at the cell surface, Xc, is given by Xb - Xc T the is (Eq. 66) Df Xb Shc Xb where Re dp I diffusion coefficient per coverage surface for the cell rate number for cell-liquid mass per unit area, The chemical species. given transport, Shc, is Df and Rc species, given chemical the for uptake cells in given by (Sano is the is the Sherwood et al, 1974; Chaudhari and Ramachandran, 1980) Sh i where is c = the (Eq. 67) 2 + 0.4(F d 4/D V )1/3 pD f per input power average unit mass and dp is the microcarrier diameter. In the design of a reactor, one might specify the chemical concentrations at the cell surface, or the acceptable gradients between the cell surface and bulk In media. - 185 - general, as long as the are microcarriers and the viscosity has not been = 2) (She suspended the gradients will be negligible. greatly increased, Given negligible the cells and bulk fluid, one gradients between should also consider the requirements for adequate mixing in the liquid transferred are chemicals in the liquid phase the throughout tions the into In regions where reactor. liquid mixing phase, should be to to eliminate cell damage from locally high concentrations. adequate If required to maintain appropriate chemical concentra- Mixing is phase. are directly added for pH control, acid or bases mixing should be adequate to minimize the pH excursion and any corresponding cell damage. If oxygen pure is used for oxygenation, mixing should be adequate to as dissolved oxygen limit the local concentration of dissolved oxygen, levels in excess of normal air saturation are frequently toxic (Kilburn and Webb, 1968; Balin et al, 1976). In general, in to only added, fluid should be supply and nutrient uptake. nutrient not the minimize the transients circulated among the regions of Adequate circulation is required in regions where chemicals are but also to ensure that adequate nutrient levels are maintained distant susceptible Oxygen is regions of the reactor. to depletion. As oxygen is often the nutrient most toxic in excess of air saturation, which represents a very low molar concentration, the oxygen content of supplied. the medium must be For sufficiently limited in the region where high cell concentrations and oxygen is low mixing rates, the limited oxygen content may be completely consumed before the - 186 - medium recirculates back to the region of oxygen supply. Liquid phase mixing requirements are frequently analyzed in terms of Mt, the mixing time, a For many reactors in the after it has been added at a single position. fully-turbulent regime, for the reactor concentration throughout reach a homogeneous to chemical required time the represents time mixing The times. mixing for the fluid phase can be estimated from (McCabe et al, 1985): M al (H/D .) = (Eq. 68) /N (Dt/D) where H is the liquid height, N is the impeller rotation rate, and al is a constant which is characteristic of the reactor and impeller geometry. The mixing time generally estimated as be can equation (McCabe et al, 1985). time required for fluid element to circulate once around a the reactor required for about time circulations of the tank contents five complete Thus, represents the From 68. consumption rate, oxygen supply this of the mixing time and the one-fifth circulation the variation in given by volumetric oxygen dissolved oxygen level between the of the and uptake regions time reactor, ADO, can be roughly estimated R 0 AD where Mt is CM t (Eq. 69) = the mixing time given by equation 68, C is the viable cell concentration, and R0 is the specific oxygen uptake rate per cell. - 187 - In the design of a microcarrier reactor, one might specify the maximum AD0 between agitation The local medium. and cells the oxygen concentration in based on the cell metabolism and any gradients should then be sufficient to provide a AD0 less than the specified maximum. Given adequate cell-liquid transport and liquid-phase mixing, one consider also should into and out of the bulk fluid. products to supply, difficult nutrient only sparingly soluble in by the cells and yet is all Nearly and waste other nutrients perfusion (Nahapetian, 1986) products can levels adequate solution; aqueous Oxygen is also be cell culture medium. soluble easily maintained or medium exchange. in through Oxygen can also be through an external this technique requires high recirculation rates and immobilization. cell generally the most are highly supplied through continuous perfusion and recycle oxygenator, waste consumed at a considerable rate is as it and nutrients of transfer overall the microcarrier cultures For normal in a stirred tank, the perfusion techique is not a viable means of supplying oxygen. through supplied generally is Oxygen between the bulk fluid and a gas phase. transfer, * where KL o is transfer mass The volumetric rate of oxygen generally described by an equation of the form: NO, is N continuous = K a (D the L o - overall (Eq. 70) D ) o oxygen transfer coefficient, a is the interfacial gas-liquid surface area per unit culture volume, Do * is the dissolved oxygen concentration in equilibrium with the gas phase, and Do - 188 - is the steady average dissolved state, the in oxygen concentration transfer oxygen volumetric At the bulk media. rate the equal will volumetric oxygen consumption rate, and thus * R C o K a = (D (Eq. 71) D ) - o L o where R0 is the specific oxygen uptake rate per cell and C is the viable cell concentration. In design of a microcarrier bioreactor, the in the oxygen pressure of maximum partial pressure of oxygen in average The partial will specify the equilibrium Po, the gas phase, the gas phase and the bulk medium. in the of dissolved oxygen concentration one might specify value Do* in the liquid from the typical Henry's Law relation * D where # is P = o the 0 (Eq. 72) # Henry's Law coefficient. levels above the gas phase and air saturation. the gas the toxic effects of dissolved oxygen In setting the average concentration of dissolved oxygen in the bulk medium, one should consider the concentration at the cell between different regions surface, of phase one should consider the level of composition or equilbrium value Do*, mixing near In setting the desired the variation in dissolved oxygen vessel, and gradient in dissolved oxygen between the cells and local fluid. Specification transfer. of Do and The maximum cell Do* sets the concentration - 189 - driving is force for oxygen then determined by the If surface aeration is employed, the value of KLa values of R0 and KLa. is of equations by given generally the form (Aunins et al, 1987; Oldshue, 1983): -K KL a = a2 (Re) 6 (Sc) Re = N D. /v Sc = v/Df Fr = N Sh where s i (Eq. 75) (Eq. 76) 0 (Eq. 77) and 63 are empirical constants, surface aeration, as as (Eq. 74) (Fr) b number for surface aeration, volume (Eq. 73) /D )(1/H) D./g i 61, 62, where a2, (ShS Df 0 = Ks is the oxygen transfer coefficient is the gas-liquid surface given by the reciprocal the Sherwood Shs is for area per unit culture of the liquid height H for surface aeration, Re is the impeller Reynold's number, Sc is the Schmidt number, the Froude number, Di is the impeller diameter, rotation rate, v is the diffusion coefficient Dfo is Fr is Dt is the tank diameter, the kinematic fluid viscosity, N is vb is for oxygen, the impeller the kinematic suspension viscosity, and g is the acceleration due to gravity. tank is fully baffled, the effect If the of Froude number becomes negligible and 63 is essentially zero. Aunins et al (1986) have investigated surface aeration in baffled cell culture reactors containing medium with 10% serum and no thickening - 190 - agents. From their results, one can determine a value of 0.61 for a2 and The value of 62 is generally considered to be 0.33 (Aunins 0.73 for 61. et al, 1986). is oxygen If tubing surface and the value of KLa is determined by the sum: aeration, = K La K a K a (Eq. 78) from surface aeration, as given the contribution from silicone and Ktat represents 73-77, equation + the contribution where Ksas represents by silicone both supplied through tubing aeration, as determined by the tubing mass transfer coefficient, Kt, baffled tubing surface the and culture cell tubing mass transfer area unit per reactors which coefficient is are volume, culture geometrically given by For at. similar, the an equation of the form (Aunins et al,1986): 1/Kt where a3, = a3 (dt/D + c 64, 65, ) (Re)-64 (dt/D -65 (Sc)-66 and 66 are empirical constants, c is (Eq. 79) the conduction resistance through the tubing wall, dt is the tubing diameter, Dt is the tank diameter, Df,o is the coefficient diffusion for oxygen in the medium, Re is the impeller Reynold's number as given by equation 75, and the Schmidt number as given by equation 76. Sc is have found values values of 0.055 for a3 and 0.69 for 64, Aunins et al (1986) and have estimated For 0.03 cm silastic silicone of 0.53 for 65 and 0.33 for 66. tubing, the value of c was estimated to be 140 sec/cm. - 191 - 7B. Basic Principles in Bioreactor Design and Optimization design The of is bioreactors maximum cell concentration the Upon scale-up, optimization problem. engineering chemical classic a will be determined by the effects of several independent variables, such fluid viscosity, as the microcarrier concentration, and sparging rate. increase An in microcarrier cell death from collisions. concentration will may also lead to more it provide more surface area for growth; however, level of agitation, An increase in fluid viscosity will reduce An increase in hydrodynamic death, but will also reduce mass transfer. will agitation hydrodynamic death. transfer, mass increase may but also increase An increase in sparging rate will increase mass transfer, but may also lead to more cell death. In general, there will be a trade-off between mass transfer and hydrodynamic death. To accurately incorporate these trade-offs in bioreactor design, one needs quantitative death. hydrodynamic quantitative In this death expressions thesis, for expressions In the previous the rates section for the rates of mass quantitative expressions of transfer mass of this and chapter, transfer were reviewed. for the rates of hydrodynamic in microcarrier cultures have been derived for the first time. The cell population kinetics have been found to follow the expression: dC_ = - C - qC - (Eq. 80) q2 mC dt 6.2 x 10-13 q= q = (Eq. 81) 3 3/4 (Eq. 82) 7.6 x 10-16 (T/V) - 192 - where p is independent of the level of the inherent growth rate and is agitation, qi is the first-order specific death rate in sec-1, and q2 is For low levels of the collision death rate constant in micro/ml-sec. agitation, insignificant, essentially is death hydrodynamic the and values of qi and q2 are generally negligible. Equations 81 microcarrier-eddy processes relatively kinetics the describe interactions appear to and microcarrier flow fields is appears damage to cell from death These collisions. 2 approximately 5-9 dyne/cm . In contrast, cell damage highly dependent on reactor geometry. occur when from surface microcarrier of involve high wave number eddies and appear to be independent of reactor geometry. from time-average Such 82 and the time-average maximum shear fields, flow the stress on Tmax, exceeds This fact can be used as a criterion for the avoidence of cell damage from time-average flow fields. Kinetic expressions for the rates of mass transfer and hydrodynamic death can be used to optimize the reactor performance according to many different death, objectives. The the yield maximize nutrients, or maximize productivity. to minimize generally be objective may be of a given to minimize hydrodynamic product the cell concentration on a given and volumetric set of reactor In the most general sense, the overall objective will be the cost As of production. significant, kinetic the economies expressions greatest use in the design of large scale units. - 193 - of may scale find will their one might consider To illustrate the use of the kinetic expressions, problem the scale concentration the maximum cell bioreactors, In such bioreactor. in a large cell concentration the of maximizing is frequently limited by the rate of oxygen transfer (Aunins et al, 1986). The oxygen limitation will occur as oxygen level of Specification Do,min, value, minimum this or minimally a critical below drops the cells grow and the dissolved acceptable value. gas phase and the composition will specify the maximum driving force for oxygen transfer. Specification of the reactor geometry, agitation power input, and fluid At the point of maximum oxygen viscosity will specify the value of KLa. transfer, an oxygen balance gives: * R o C max K a (D = L - o D . o,min (Eq. 83) ) where R. is the specific oxygen uptake rate and Cmax is the maximum cell concentration which can be adequately supplied with oxygen. For many associated cells, uptake oxygen (Tyo and Wang, 1980; is both Goldstein, growth 1986). and maintenance The specific oxygen uptake rate can generally be described by an equation of the form (Pirt, 1975): R 0 where Yn/o is + = (Eq. 84) M n/o0 the growth yield of cells on oxygen and Mo is maintainence coefficient, or the specific oxygen uptake growth. - 194 - the oxygen rate under no to are utilized given concentration cell maximum the extent, fullest their of a reactor transfer capabilites oxygen If the by the limitations of oxygen transfer will equal the maximum cell concentration contact maximum cell and specific growth rate drops to When the inhibited. This grow toward confluence the cells concentration will be reached as become death. of hydrodynamic limitations the given by a value equal to the total specific death rate, the rate of change in cell concentration will be zero, and the cells will have reached the maximum cell concentration: dC dt or = 0 = q, + = pC - q1 C - (Eq. 85) (Eq. 86) q2 m between Substituting q 2 GmG 84, 83, equations and 86, one arrives at the result: * K Y C max n/o - where Cmax is Yn/o M a L - (D + D . ) o,min q2 Cm o q, + (Eq. 87) the maximum cell concentration as determined by the rate of oxygen transfer and hydrodynamic death. Equation 87 quantitatively transfer and hydrodynamic sets forth the trade-off between oxygen specifies a quantitative relation It death. for maximixing cell concentration. Through information on the rates of oxygen transfer and hydrodynamic cell death, equation 87 can be used to determine which reactor fluid geometry, - 195 - viscosity, microcarrier and concentration, These concentration. for the other mass transfer requirements. consideration must be sufficient suspend the microcarriers to cell maximum performed, however, with must be calculations the to lead agitation of level The agitation adequate and provide liquid-phase mixing. in As shown Equation 87, oxygen transfer without a in an increase concurrent increase in hydrodynamic death will lead to a higher maximum cell appears involve only the to cultures, microcarrier For concentration. smallest eddies, In contrast, mass dependent on geometry. damage hydrodynamic strongly which are not transfer involves not only small eddies, but also large eddies, which are strongly dependent on By manipulating the effects of geometry on the large eddies, geometry. significant increases in mass attainable should be transfer without concurrent increases in hydrodynamic damage. 7C. Illustration of Optimization Calculations design can be approached To fully illustrate how bioreactor optimization optimization calculations The culture. rate quantitative involving problem as an expressions, are described below for an FS-4 microcarrier optimum combination of agitation, microcarrier concentration, and fluid viscosity were determined for a given reactor. The objective general, such was an to maximize optimization different reactor geometries. maximum the be might cell concentration. performed for a number In of The geometry which leads to the highest maximum cell concentration would be chosen as the best. - 196 - The geometry of the reactor for this optimization is shown in Figure 46 and in Table 3. The reactor has a liquid volume of 100-liters and is a pitched-blade equipped with baffles, The (FBT). turbine is reactor turbine reactors the to similar geometrically which have been extensively studied by Aunins et al (1986). For this it was assumed that oxygen transfer came solely through optimization, The oxygen transfer coefficient aeration. surface and a flat-blade (PBT), equations 73-77 with a2 = 0.61, 61 = 0.73, 62 = then given by is 0.33, and 63 = 0 (Aunins et al, 1986). From the results of Bates et al (1963) and Rushton et al (1950), one can estimate a power turbine and a number of 1 for the pitched-blade power number of 4 for the flat-blade turbine, with both turbines in The spacing between the turbines fully-turbulent regime. 60% of their independently. that such, the Thus, operation in the for required for microcarrier E, was estimated by: e = where N impellers may less than not operate From the results in Richards (1963), one could estimate the power draw would be only 80% operation. mass, As diameter. is the 0.8 N p N3 D.5/ i suspension, of the value for independent complete turbulent regime, as the average power input per unit (Eq. 88) V c is the combined power number with an estimated value of five, Vc is the culture volume, and N is the impeller rotation rate, with all terms in cgs units. - 197 - Baffles D.O. Probe 4-FBT Figure 46. 100-Liter reactor with 4-bladed flat-blade turbine (4-FBT) and pitched-blade turbine (4-PBT) - 198 - TABLE 3. GEOMETRY OF 100-LITER BIOREACTOR Dimension Size (cm) Tank diameter 53.3 Liquid height 44.8 Impeller diameter 26.7 Baffle width 6.1 Radius to baffle inner edge 15.6 Impeller blade width 8.0 Height of center of pitched-blade turbine 15.0 Height of center of flat-blade turbine 30.0 - 199 - 10-liter reactor For the al investigated by Aunins et (1986), the minimum speed for complete microcarrier suspension was approximately 30 RPM 1986) (Goldstein, This correponds to an estimated value of 16 and no thickening agents. Upon scale up to for the average power input per unit mass. cm2/sec3 the 10 g/l microcarriers, for medium with 10% serum, agitation power per required for suspension should be reduced according to the 100-liter geometrically-similar unit mass the reactor, equation (Nienow, 1985): 1 sl where average the is (Eq. 89) 0.28 i, / Di2 2 unit per input power mass required for microcarrier suspension in the 10-liter reactor, T2 is the average power per mass unit reactor, Di,1 Di,2 is 89, required is the suspension for microcarrier the impeller diameter for in the 100-liter 10-liter reactor, and From equation the impeller diameter for the 100-liter reactor. one can estimate a minimum power per unit mass of 13 cm2/sec3 for microcarrier complete unthickened medium. incorporate the minor in suspension From the the 100-liter of Zweitering results with vessel (1958), effects of fluid viscosity and volume one can fraction solids on the power required for microcarrier suspension. optimization This liquid-phase was performed The criteria 200 - adequate dissolved oxygen was less than 2% of saturation cell-liquid transport was - of The liquid phase mixing the estimated variation in as determined from equation 69, air. the under mixing and cell-liquid transport. considered adequate if content, with was considered adequate if the fluid, determined as increase viscosity from was as so limited to than less was 66, equation and local 0.10. The the cells between in oxygen gradient estimated normalized introduce not cell-mass transport limitations at a Sherwood number of two, or the minimum value for complete suspension. Cell damage the viscosity limiting flow from time-average fields was easily avoided by For all conditions investigated, the increase. maximum shear stress on the microcarrier surface from time-average flow fields was less than 1 dyne/cm 2 This optimization requires knowledge of the mathematical dependence of specific growth rate on cell and microcarrier concentration. 4 the cells, mathematical inhibition and contact inhibition contact on cell-derived growth growth was inhibitors. with incorporated of effects the both involves dependence For FS- The effect of the following empirical equations which fit the data presented in Hu et al (1985) for FS-4 cells: where Z p = ymax (100 - Z)/60 for Z > 40 (Eq. 90) A = Amax for Z < 40 (Eq. 91) The growth rate free of contact the percent confluence. is inhibition, pmax, and the maximum surface coverage depend weakly on the volumetric cells are cell concentration, as inoculated microcarrier, at a shown in Chapter constant roughly the weak dependence - on volumetric 201 - 1. number Because of cells FS-4 per cell concentration can microcarrier is concentrations dependence optimization, this For concentration. empirical a by described roughly be microcarrier on range relevant the between 5 and 15 g/l. of For this range, the data in Figures 12-14 for 5% serum can be described by the empirical relations: pmax = Z = 4.3 x 10 5 /C 0 26 2 (Eq. 92) 100 C 0.80 500 (Cm ) (Eq. 93) where Cm is the microcarrier concentration in microcarriers/ml, C is the cell concentration in cells/ml, pmax is the maximum growth rate in sec- , and Z is the percent confluence. This optimization was performed under the following assumptions: 1) the headspace gas contained a 90:10 air-CO 2 mixture, 2) the medium was supplemented with 5% (v/v) fetal calf serum 3) the diffusion coefficient for oxygen in unthickened medium with 5% serum was 2.5 x 10-5 cm2 /sec, oxygen diffusion in as given in and water corrected (1977) Reid et al for minor for temperature effects from the correlation of Wilke and Chang (1955) 4) the diffusion coefficent for oxygen was inversely proportional to fluid viscosity, as given by Wilke and Chang (1955) 5) the Henry's law coefficient for oxygen was 0.86 mM/L-atm (Fleischaker and Sinskey, 1981) 6) the minimum acceptable level of average - 202 - dissolved oxygen in the Below this value, oxygen can fluid was 10% of saturation with air. become growth-limiting (Miller et al, 1987; Balin et al, 1976). The mmole/cell-hr. 10-11 x 1.3 maintenance the and cells/mmole Yn/o, oxygen, on cells of yield growth the 7) 3.8 was x 108 was coefficient on oxygen, Mo, estimation of these values is described in Appendix 2. making After of number power the independent to variables fluid viscosity two: reduced and calculation optimization involves The input per unit. one has above, listed assumptions the average of the concentration for each combination of fluid viscosity and maximum cell average power input. The combination which gives the highest maximum cell concentration would be chosen as the optimum. For combination each of calculation microcarrier concentration and concentration and cell the maximum viscosity fluid of involved an iterative agitation the power, corresponding procedure. At the maximum cell concentration, the specific growth rate, p, should equal the total viscosity and agitation power, from qi specific death rate, equations microcarrier 81 and the values of qi and q2 were calculated If 82. then one maximum the concentration, For each value of fluid + q2Cm. guesses cell a value concentration for can the be determined from equation 87, and the corresponding specific growth rate This procedure can be repeated can be determined from equations 90-93. until the specific growth rate given by equations 90-93 is equal to the total specific death rate, q The corresponding maximum cell + q2Cm. - 203 - concentration represents the solution to the iteration. they concentration cell liquid mixing and cell-liquid transport were were and concentration, one can determine whether the criteria of microcarrier adequate maximum the calculated having After the not, power agitation criteria are fulfilled. should be fulfilled. If until the increased For this optimization, the calculations were extended into a range of power levels far above the minimum values for adequate liquid mixing and cell-liquid transport. Figure 47 shows the of results the calculations. optimization Maximum cell concentration is plotted against power input per unit mass. are shown for three different viscosities: Calculations (0.74 cp), and medium slightly thickened cell concentrations normal medium and 1.09 cp). (0.92 Maximum are shown only for power levels which are equal to or greater than the minimum power for complete microcarrier suspension. For particular this the optimization, required agitation for microcarrier suspension more than fulfilled the requirements for liquid mixing and cell-liquid transport. The results show some in Figure 47 For all interesting trends. three viscosities, the maximum cell concentration initially rises as the agitation is suspension. capabilities. increased These The increases highest minimum the above are cell due to increased concentration is microcarrier for level oxygen obtained with lowest viscosity, again due to oxygen transfer capabilities. - 204 - transfer If the the 2.25 0.74 cp 2.00 0.9 2 cp 1.09 cp 1.75 N 1.50 1.25 IIIL-I I 40 80 I 120 AVG. POWER PER MASS (CM I_ 160 2 _ 200 /SEC 3 ) Estimated maximum cell concentration vs. average power input per unit mass at different fluid viscosities Figure 47. - 205 - agitation is increased above the optimum at the lowest viscosity, the maximum cell concentrations drop sharply. Eventually, at a power input of approximately from excessive agitation. 125 cm2 /sec 3 is due to cell death This death rate is of 0.74 cp, the specific for the viscosity The attached cell greater than the maximum growth rate of the cells. concentrations will decrease from the time of inoculation. If the flatter and less increased is viscosity above sensitive to agitation. the cp, 0.74 curves become In general, an increase in viscosity leads to a reduction in both oxygen transfer and hydrodynamic death. For optimum viscosity. increases. input in a given any given power As the power input increases, for allow Higher viscosities reactor, is an there the optimum viscosity operation at higher power inputs. For this particular optimization, the highest cell concentration was If this optimization was performed for obtained with lowest viscosity. a different reactor, concentration might be The trends shown in or a cell different line, obtained with a slightly Figure 47, would still however, the highest cell increased viscosity. be present. There would still be a trade-off between mass transfer and hydrodynamic death. Protection through Polymer Adsorption and Viscoelasticity As previously discussed the in Literature Review section, many investigators have used polymers to protect cells from fluid-mechanical disruption. The mechanism of this protective effect was not previously - 206 - in this thesis, it from turbulent damage by Although viscosity. medium the increasing presented results the polymers may protect cells that appears the of light In identified. viscous reduction of turbulent damage most likely does account for some of the protection, it does not appear for all the results presented in to fully account the literature. In instances, many with qualitatively, great of protection deal percent. very low of degree great polymer is observed, or viscosity protection concentrations instance, serum supplementation apparently provides a For increases. a and yet increases the viscosity only a few Methylcellulose supplementation, at a typical concentration of approximately 1 g/l and with a typical molecular weight of approximately 15000, al, also apparently provides a great deal of protection 1960; leads Bryant, 1966; Holmstrom, to a viscosity 1968; Birch and Pirt, increase of only 18%. (Kuchler et 1969) and yet For a dilute microcarrier culture, an 18% viscosity increase would provide only a 31% reduction in hydrodynamic death. accurately represents ture. It polymers is appears, It to say whether a 31% reduction is difficult results presented in the qualitative however, that the protection the litera- provided by some more than would be expected through only viscous reduction of turbulent damage. The mechanism of the protective effect is therefore open to more investigation. Kuchler et al (1960), Bryant (1966), and Mizrahi (1984) have hypothesized that the polymers adsorb onto the cells and form a - 207 - This adsorption would occur along with a concurrent protective shell. the in concentration in polymer reduction For supernatant. culture methylcellulose, Bryant (1966) observed a steady decrease in the culture viscosity, indicating polymer potentially and pluronic cells. polyols, found a clear protective effect even though over 99% of (1984) Mizrahi starch, hydroxyethyl For carboxymethylcellulose, the onto attachment the added polymer remained in the supernatant. there has directly measured whether To my knowledge, no one any adsorption of the polymers is From the data currently available, the protective shell onto the cells. hypothesis is neither directly supported nor refuted. protection of mechanism Another interactions between the polymers and the discussed in the Materials and Methods occur flow fields. As these interactions can turbulent section, viscoelastic involve may even with very low polymer concentrations. In the experiments presented in this thesis, low molecular weight dextrans were used as the Viscoelastic interactions were purposefully avoided thickening agents. to effects the isolate such polymers, as of For viscosity. methylcellulose and some of the protective carboxymethylcellulose, viscoelastic behavior has been reported (Amari and Nakamura, 1973, 1974; Hoyt, 1985). There protect cells hydrodynamic mass great is from damage potential turbulent appears the behind damage. use For of viscoelasticity microcarrier to cultures, to involve only the smallest eddies while transfer involves both small - and large eddies. 208 - If viscoelastic polymers are added that the the small eddies but not interfere with large eddies, a strong decrease in hydrodynamic damage may be attainable without concurrently a viscoelastic polymers should in decrease strong a have characteristic relaxation equivalent to the burst duration for the smallest eddies. might not strongly indicated by the should be interfere results performed to with the of Quraishi et al investigate the protecting cells from turbulent damage. - 209 - transfer mass (1977). use of The transfer. mass time Such polymers processes, as New experiments viscoelasticity in SUMMARY AND CONCLUSIONS approach has been fundamental and quantitative thesis, a this In The effects of initiated toward the design of microcarrier bioreactors. forces hydrodynamic on growth cell death and death. significant hydrodynamic no Growth was inert microcarrier fluid viscosity, in changes under studied With mild agitation, there conditions of both mild and high agitation. was were not affected by concentration, or level of agitation, and thus appeared to be unaffected by the hydrodynamic environment per se. With high agitation, a reduction in net growth was observed and was found to be entirely due to cell death and removal and not inhibition. growth Cell was growth still by the agitation was unaffected hydrodynamic enviroment per se. For FS-4 cultures, irreversible and lethal. which lethal. from excessive from removal excessive Secondary growth did not occur over areas from had been previously cells removal cell agitation removed. In frequently was -CHO cultures, reversible cell and not Secondary growth readily occurred over areas from which cells had been previously removed. cell In fact, growth was areas were left void through hydrodynamic cell removal. stimulated as For a moderate level of agitation, the secondary growth was fast enough to overcome any reduction in attached cell removal. Secondary growth concentrations from hydrodynamic can clearly lead sensitivity. - 210 - to death or reduced hydrodynamic Through cultures. microcarrier found to occur fluid power, cell viscosity, in death through microcarrier-eddy in unusual Microcarrier-eddy fields. flow time-average and, collisions, of effects agitation and microcarrier bioreactors was interactions, the on experiments concentration, microcarrier investigated with FS-4 of hydrodynamic damage were The mechanisms circumstances, were interactions predominant in dilute cultures and led to cell death when the turbulence For dilute generated eddies which were smaller than the microcarriers. to the death rate was found to be proportional the specific cultures, concentration of eddies For both dissipation regime. in the viscous cell death was found to increase with dilute and concentrated cultures, agitation power and decrease with fluid viscosity. A population kinetics viscosity, and the to and and cell death microcarrier trade-off the transfer upon scale-up. The kinematic included the which was not a function of hydrodynamic microcarrier-eddy interactions from both The kinetic expression hydrodynamic between An expression cell the related concentration, microcarrier collisions. quantify which derived power. agitation contributions from cell growth, environment, was expression quantitative illustration was cell was death presented used and to to oxygen show how bioreactor design can be approached as an optimization problem involving quantitative rate expressions. - 211 - RECOMMENDATIONS FOR FUTURE RESEARCH The results in this thesis point to several interesting areas for The topics have been discussed throughout the thesis future research. and include: 1) Effects of normal forces on animal cells. In many cell culture bioreactors, the flow field is turbulent. cells are exposed not only to shear forces, To bioreactor optimize is it designs, effects of normal forces on cells. The but also to normal forces. important understand to the A literature survey indicates that the effects of well-defined normal forces on animal cells have not been investigated. the typically The undiscovered role of normal forces may account for poor agreement shear between effects in laminar flow fields and global hydrodynamic effects in turbulent stirred tanks, such as by reported microcarriers, Rosenberg et al For (1987). cell removal from the observed lack of selectivity for mitotic cells may indicate that cell removal occurs primarily through normal forces. 2) Effects of reactor geometry on the kinetics of hydrodynamic death. In this thesis, hydrodynamic phenomena were correlated with average power input per unit mass. The results did not indicate a strong effect of reactor geometry on the kinetics of hydrodynamic cell death, although a limited number of reactor geometries - 212 - was investigated. Future should dissipation. with of rates and power to involve only the smallest eddies, which In constrast, mass strongly dependent on geometry. small only eddies, involves not strongly dependent on geometry. involves large eddies geometry (Nienow, 1985). are local geometries manipulated, For microcarrier cultures, of hydrodynamic death. hydrodynamic death appears are not on reactor The kinetics of mass transfer should be considered along kinetics the information quantitative incorporate range of a wider investigate should research high and also but large transfer which eddies, are Suspension of the microcarrier also strongly again is dependent on reactor If the effects of geometry on the large eddies transfer mass of rates should be obtainable without high rates of hydrodynamic death. 3) Microscopic fluid flow fields. In microcarrier Future the hydrodynamic forces experienced by the cells in this thesis, research direction of the extensive in only an cultures were calculated should determine forces experienced by the cells. modelling microcarrier-eddy precisely more of the microscopic interactions flow approximate manner. the magnitude and This will require fields which and microcarrier collisions. thorough models might incorporate the protrusion of the cells flow field along with the mechanics of cell deformation. arise in The most into the The results of these models will allow for more a more precise and thorough approach to bioreactor design and optimization. - 213 - 4) Reversible cell removal and secondary growth. y-CHO For from which areas in contrast, cultures, had been previously cells cell removal was secondary growth did not occur. For FS-4 removed. irreversible and lethal, and At a moderate level of agitation, the y-CHO cultures was fast enough to overcome any secondary growth in the from hydrodynamic death and reduction in attached cell concentrations Secondary growth thus led to reduced hydrodynamic sensitivity removal. and was agitation excessive Secondary growth readily occurred frequently reversible and non-lethal. over from removal cell cultures, were cells the occured because have may Such removed whole. reversible removal may have been related to the attachment properties of thoroughly Future research should be performed to more y-CHO cells. the aneuploid elucidate removal and of cell the nature its relation to secondary growth. 5) Hydrodynamic effects on cell metabolism. cell animal Industrial culture frequently will be used for the The performance of these industrial processes production of proteins. will depend not only on the cell concentration and growth rate, but also on the cell metabolism. hydrodynamic It is therefore important to understand the effects on both cell metabolism and cell growth. In thesis, the hydrodynamic effects on cell growth were investigated. growth of the cells hydrodynamic therefore not forces. appeared The to be neither inhibited nor enhanced by The metabolic significantly this events required altered by - 214 - agitation. for growth were Nonetheless, all events metabolic influenced by agitation. growth not were which associated In fact, the specific glucose uptake rate was significantly reduced at high levels of not growth associated and was There are numerous other limited but agitation. effects on cell metabolism, regarding hydrodynamic been have could Literature Review section. interesting results as discussed in the Future research will hopefully extend the current information on the hydrodynamic effects of cell metabolism. 6) Mechanisms of cell damage from direct sparging. often lead sparging can however, prematurely sparging. Direct and for many suspension cultures, cultures, For microcarrier rule damage. extensive cell to the out sparging One represents potentially should not, of usefulness potential a direct direct simple and inexpensive method for oxygenation of large-scale cell culture reactors. If the mechanisms of cell damage from sparging are elucidated, a viable In general, the use of sparging sparging technique may become apparent. should be considered in and cell The kinetics death. quantitatively light of the trade-off between oxygen transfer of cell damage described along the kinetics from sparging should be of oxygen transfer. The kinetic expressions can be used to determine the sparging conditions and reactor bubbles design which lead to optimum performance. involves turbulence, the use of If cell damage from thickening viscoelastic polymers may reduce the amount of damage. - 215 - agents or 7) Viscoelastic reduction of hydrodynamic death. Polymers are frequently added to cell culture medium to The mechanism of protection may cells from fluid-mechanical disruption. involve viscoelastic In flow fields. between interactions general, for is there the protect the polymers and turbulent great potential behind the use reduction of hydrodynamic death of from viscoelastic agents turbulence. Hydrodynamic damage appears to involve only the smallest turbulent eddies, while mass transfer involves both the small and large eddies. added that If viscoelastic polymers are interfere with the small eddies but not the large eddies, a strong decrease in hydrodynamic death should be attainable without a concurrently strong mass transfer. - 216 - decrease in NOMENCLATURE Roman a gas-liquid interfacial area per unit culture volume, cm-1 as culture surface area per unit culture volume, cm-1 at silicone tubing surface area per unit culture volume, cm-1 A parameter in power number correlation, dimensionless b intercept value in equation 60, sec-' B parameter in power number correlation, dimensionless c conduction resistance through silicone tubing, sec/cm C 3 volumetric concentration of attached cells, cells/cm Cmax 3 maximum volumetric concentration of attached cells, cells/cm Ci 3 vol. concentration of attached cells at time ti, cells/cm Cl, 3 5 3 C1 for culture at 35 RPM, cells/cm Cl, 1 5 0 3 C1 for culture at 150 RPM, cells/cm C2 3 vol. concentration of attached cells at time t2 , cells/cm C 2 ,3 5 3 C2 for culture at 35 RPM, cells/cm C 2 ,1 5 0 3 C2 for culture at 150 RPM, cells/cm Ci 3 inert microcarrier concentration, microcarriers/cm Cm 3 microcarrier concentration, microcarriers/cm D 3 concentration of DNA in culture fluid, diploid equiv./cm DI 3 D at time t1 in 150 RPM culture, diploid equivalents/cm D2 3 D at time t2 in 150 RPM culture, diploid equivalents/cm Df diffusion coefficient, cm2 /sec Df, o 2 diffusion coefficient for oxygen in culture medium, cm /sec Di impeller diameter, cm - 217 - Di, i impeller diameter for reactor one, cm Di, 2 impeller diameter for reactor two, cm Dt inner diameter of vessel, cm Do 3 dissolved oxygen concentration in liquid, mmoles/cm Do,min 3 minimum acceptable DO, mmoles/cm Do* D dp microcarrier diameter, cm dt silicone tubing diameter, cm E number of hemacytometer squares scored fe frequency of microcarrier collisions, #collision/cm3-sec f(t) distribution function of cell age, t, since mitosis F ratio of initial to final sample volume for nuclei counts Fn 2 normal force per unit area on microcarrier surface, dyne/cm Fr Froude number, dimensionless g 2 acceleration due to gravity, 980 cm/sec G glucose concentration, g/liter Gi glucose concentration at time ti, g/liter G2 glucose concentration at time t2 , g/liter h cell height above growth surface, cm H liquid height in reactor, cm Ho 3 gas hold-up in reactor, cm IL interval length between exchanges of medium, sec IGo fraction of cell population in Go resting state, dmsnless. IM fraction of cell population in mitotic phase, dimensionless ISF integrated shear factor, sec ITS impeller tip speed, cm/sec 3 at saturation with gas phase, mmoles/cm - 218 - 1 Jw specific rate of whole cell removal, sec-l K1 ratio of time-average shear rate to impeller tip speed, cm-1 K2 3 collision frequency constant, #collisions-cm /micro.2-sec K3 2 average effective surface area exposed to each collision, cm K4 probability of death for a cell in the collision-exposed area K5 total surface area per microcarrier (- 4wrrp2), cm2/micro. K6 specific fluorescence of FS-4 DNA, fluor./(dip. equiv./cm3) Ke 3 first-order specific death rate constant, cm /sec KL overall oxygen transfer coefficient, cm/sec Ks oxygen transfer coefficient for surface aeration, cm/sec Kt oxygen transfer coefficient for tubing aeration, cm/sec L length scale for eddies in viscous dissipation regime, cm Lc critical eddy length scale for cell death, cm Mo specific oxygen uptake rate with no growth, mmoles/cell-sec Mt mixing time for fluid phase in reactor, sec Mw molecular weight of polymer, daltons N rotation rate of impeller or viscometer, rotations/sec No volumetric rate of oxygen transfer into liquid, mmole/cm3-sec Ngppower number for impeller, dimensionless Nu Nusselt number for cell-liquid heat transport, dimensionless p parameter in power number correlation, dimensionless P 2 static pressure in undisturbed flow, dyne/cm Pv vapor pressure of fluid, dyne/cm 2 P' 2 intensity of turbulent pressure fluctuation, dyne/cm P'vdr 2 P' due solely to eddies in viscous diss. regime, dyne/cm q 1 total specific death rate, sec- - 219 - qi specific death rate due to micro.-eddy interactions, sec-' q2 3 second-order death rate constant, cm /microcarrier-sec Q 3 flow rate of gas for sparging, cm /sec ri radius of inner viscometer cylinder, cm ro radius of outer viscometer cylinder, cm rp microcarrier radius, cm R 2 ideal gas constant, 8.31 x 107 g-cm 2 /mole-*K-sec Rc specific uptake rate of a given chemical, mmole/cell-sec Re Reynolds number for flow, dimensionless Rg specific glucose uptake rate, g/cell-sec RO specific oxygen uptake rate, mmole/cell-sec Sa average DNA content of cell population, Sr average DNA content of cells removed, diploid equiv./cell Sc Schmidt number, dimensionless Shc Sherwood number for cell-liquid mass transport, dimensionless Shs Sherwood number for surface aeration, dimensionless T temperature, degrees Kelvin ti time at beginning of interval, sec t2 time at end of interval, sec Tc total time of cell cycle with constant TG1, sec Td doubling time of cell population, sec TG2 duration of G2 phase, sec TM duration of mitotic phase, sec TS duration of DNA synthesis (S) phase, sec Sa average DNA content per cell, u' root mean square turbulent velocity component, cm/sec - 220 - diploid equiv./cell diploid equivalents/cell u'max maximum root mean square turbulent velocity component, cm/sec U time-average fluid velocity component, cm/sec U total fluid velocity component, cm/sec Umax maximum total fluid velocity component, cm/sec Umax maximum time-average fluid velocity component, cm/sec v velocity scale for eddies in viscous diss. regime, cm/sec Vc 3 average culture volume, cm w impeller width, cm W 3 volumetric concentration of unattached whole cells, cells/cm x 3 polymer concentration, g/cm Xb 3 concentration of given nutrient in bulk liquid, mmole/cm Xc 3 concentration of given nutrient at cell surface, mmole/cm Y shear rate in flow field undisturbed by microcarrier, sec-1 Yn/o growth yield of cells on oxygen, cells/mmole oxygen Z percent confluence, dimensionless Greek al parameter in fluid mixing-time relation, dimensionless a2 parameter in gas-liquid mass transfer relation, dimsnlss. a3 parameter in tubing-liquid mass transfer relation, dimsnlss. Henry's law coefficient for oxygen solubility, mmole/cm3-atm F shear rate at surface of inner viscometer cylinder, sec-l 61 exponent on Reynolds number for surface aeration, dimsnlss. 52 exponent on Schmidt number for surface aeration, dimsnlss. 63 exponent on Froude number for surface aeration, dimsnlss. 64 exponent on Reynolds number for tubing aeration, dimsnlss. - 221 - 65 exponent on diameter ratio for tubing aeration, dimensionless 66 exponent on Schmidt number for tubing aeration, dimensionless ADO 3 spatial variation in dissolved oxygen level, mmole/cm power input or dissipation per unit mass, cm2/sec3 3 2 average power input or dissipation per unit mass, cm /sec El 2 average power input per unit mass in reactor 1, cm /sec 3 e2 2 average power input per unit mass in reactor 2, cm /sec 3 emax 2 maximum local power dissipation per unit mass, cm /sec Es 3 2 power input from sparging per unit mass of fluid, cm /sec A response time of polymer molecule, sec 77 viscosity of fluid, gm/cm-sec A Hoechst-dye fluorescence of culture fluid, fluor. units Al, 3 5 A for 35 RPM culture at time ti, fluorescence units A1 ,1 5 0 A for 150 RPM culture at time ti, fluorescence units A2 ,3 5 A for 35 RPM culture at time t A2 ,1 5 0 A for 150 RPM culture at time t 77S viscosity of solvent, gm/cm-sec ?7sp specific viscosity, dimensionless [?7] 3 intrinsic viscosity, cm /gm 0 time scale for eddies in viscous dissipation regime, sec p 1 specific growth rate, sec- Ad p under mild agitation for a given dextran conc., sec-1 pmax maximum growth rate with no contact inhibition, sec-1 pobs difference between actual growth rate and death rate, sec-1 Pr relative observed specific growth rate, dimensionless V 2 kinematic viscosity of fluid, cm /sec - 222 - 2 3 , fluorescence units 2 , fluorescence units L/b 2 kinematic viscosity of suspension, cm /sec number of nuclei counted in hemacytometer Pb 3 density of bubbles, gm/cm pf 3 density of fluid, gm/cm Pm density of hydrated microcarriers, gm/cm 3 a- cavitation number, dimensionless r shear stress on microcarrier surface, dyne/cm 2 Tmax 2 maximum shear stress from time-average flow fields, dyne/cm volume fraction of solids, dimensionless 2 surface coverage of microcarriers or T-flasks, cell/cm correction factor for DNA release calculations, dimsnlss. 2 correction factor for DNA release calculations, dimsnlss. - 223 - REFERENCES Aherne, W.A., Camplejohn, R.S., and Wright, N.A., 1977. An Introduction to Cell Population Kinetics (Edward Arnold, London, 1977) p. 58 Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K., Watson, J.D., 1983. Molecular Biology of the Cell (Garland Publishing, NY), pp. 611621 Amari, T., and Nakamura, M., solution of methylcellulose. Viscoelastic properties of aqueous 1973. J. Applied Polymer Sci. , 17, 589-603 Viscoelastic properties of dilute Amari, T. , and Nakamura, M., 1974. J. aqueous solution of methylcellulose at ultrasonic frequencies. Applied Polymer Sci., 18, 3329-3344 Effect of Augenstein, D.C., Sinskey, A.J., and Wang, D.I.C., 1971. Biotech. cells. shear on the death of two strains of mammalian tissue Bioeng., 13, 409-418 Aunins, J.G., Croughan, M.S., Wang, D.I.C., and Goldstein, J.M., 1986. Engineering developments in the homogeneous culture of animal cells: oxygenation of reactors and scale-up. Biotech. Bioeng. Symp. Series, No 17, 699-723 Balin, A.K., Goodman, D.B., Rasmussen, H., Christofalo, V.J., 1976. The effect of oxygen tension on the growth and metabolism of WI-38 cells. J. Cell Physiol., 89, 235-250. Multiplication Baserga, R., 1976. (Marcel Dekker, NY) pp. 27, 35, 50 and Division of Mammalian Cells Bates, R.L, Fondy, P.L, and Corpstein, R.R., some geometric parameters of impeller power. 2, 310-314 An examination of 1963. I & EC Proc. Design Dev., The choline and serum Birch, J.R., and Pirt, S.J., 1969. LS) in culture. (strain cells fibroblast of mouse requirements 5, 135-142 Science, protein J. Cell Bohmer, F-D., Lehmann, W., Noll, F., Samtleben, R., Langen, P., and Grosse, R., 1985. Specific neutralizing antiserum against a polypeptide growth inhibitor for mammary cells purified from bovine mammary gland. Biochemica et Biophysica Acta, 846, 145-154 Boraston, R., Thompson, P.W., Garland, S., and Birch, J.R., 1984. Growth and oxygen requirements of antibody producing mouse hybridoma cells in suspension culture. Devel. Biol. Stand., 55, 103-111 - 224 - Boucher, D.F. and Alves, G.E., Handbook, Chemical Engineers' p 5-59 NY) (McGraw-Hill, Fluid and particle mechanics, in 1973. Eds., Perry, R.H. and Chilton, C.H., Brunk, C.F., Jones, K.C., and James, T.W., 1979. Assay for nanogram quantities of DNA in cellular homogenates. Anal. Biochem., 92, 497-500 Bryant, J.C., 1966. suspension cultures. Mammalian cells in chemically defined media in Annals NY Acad. Sci. , 139, 143-161 Methylcellulose effect on cell proliferation and Bryant, J.C., 1969. in chemically defined medium in large stationary glucose utilization cultures. Biotech. Bioeng., 11, 155-179 1987. and Cooke, M., Chatwin, S., Nienow, A.W., Bujalski, W., dependency on scale of power numbers of Rushton disc turbines. Eng. Sci., 42, 317-326 Serum-free media, in Mammalian Cell 1986. Butler, M., Thilly, W.G., Ed., (Butterworths, Boston, MA) pp. 91-104 Chaudhari, R.V., and Ramachandran, reactors. AIChE J., 26, 177-201 P.A., 1980. Three The Chem. Technology phase slurry Deformation analyses in cell and developmental Cheng, L.Y., 1987. biology. Part 1 - formal methodology, Part 2 - mechanical experiments on cells. J. Biomech. Eng., 109, 10-24 Hydrodynamic effects on Cherry, R.S. and Papoutsakis, E.T., 1986a. cells in agitated tissue culture reactors. Bioprocess Engineering, 1, 29-41 Cherry, R.S. and Papoutsakis, E.T., 1986b. Physical mechanisms of cell damage in microcarrier cell culture bioreactors. Presented at the 1986 National Meeting of the American Chemical Society, Anaheim, CA, accepted for publication in Biotechnology and Bioengineering Clark, J.M. and Hirtenstein, M.D., 1981. Optimizing culture conditions Ann. N.Y. for the production of animal cells in microcarrier culture. Acad. Sci., 369, 33-46 Crouch, C.F., Fowler, H.W., and Spier, R.E., 1985. the measurement of critical animal cells to surfaces: stress permitting attachment or causing detachment. Biotechnol., 35B, 273-281 The adhesion of surface shear J. Chem. Tech. Hydrodynamic Croughan, M.S., Hamel, J-F., and Wang, D.I.C., 1987. Biotech. Bioeng., effects on animals grown in mircocarrier cultures. 29, 130-141 Daily, J.W. and Harleman, Reading, MA) pp 395-401 Fluid Dynamics (Addison-Wesley, D.R.F. , 1966. - 225 - Cell 148, Molecular Darnell, J., Lodish, H., and Baltimore, D., 1986. Biology (Scientific American Books, W.H. Freeman, NY), pp. 147, 1035-1046 Analysis of Defendi, V. and Manson, L.A., 1963. mammalian cells. Nature, 198, 359-361 the life-cycle in Relationship between low DeForrest, J.M. and Hollis, T.M., 1980. intensity shear stress, aortic histamine formation, and aortic albumin uptake. Exp. Molecular Path., 32, 217-225 Process Denn, M.M., 1980. Cliffs, NJ) pp 358-363 (Prentice Hall, Fluid Mechanics Englewood Dewey, C.F., Bussolari, S.R., Gimbrone, M.A., and Davies, P.F., The dynamic response of vascular endothelial cells to fluid stress. J. Biomech. Eng., 103, 177-185 1981. shear hybridoma cells 8, 683-686 under Dodge, T.C. and Hu, W.S., 1986. Growth of different agitation conditions. Biotech. Lett., Handbook on Methocel. Dow, 1985. Company, Midland, MI Technical literature, Dow Chemical New evidence that growth in Dunn, G.A. and Ireland, G.W., 1984. cell cultures is a diffusion-limited process. Nature, 312, 63-65 3T3 1983. Binding elastic modulus of red blood cell membrane Evans, E.A., derived from bucking instability in micropipet aspiration tests. Biophys. J., 43, 27-30 The Feder, J. and Tolbert, W.R., 1983. mammalian cells. Sci. Amer., 248, 36-43 large-scale cultivation of the use of An experimental study in 1982. Fleischaker, R.J., instrumentation to analyze metabolism and product formation in cell culture. Doctoral thesis, Dept. Applied Biol. Sci., M.I.T., Cambridge, MA Fleischaker, R.J. Biotech., 12, 193 and 1981. A.J., Sinskey, Eur. J. Appl. Microb. Frangos, J.A., Eskin, S.G., McIntire, L.V., and Ives, C.L., 1985. Flow effects on prostacyclin production by cultured human endothelial cells. Science, 227, 1477-1479 Freshney, R.I., 168, 245 1983. Culture of Animal Cells (Alan R. Liss, NY) pp. Fry, D.L., 1968. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ. Res., 22, 165-197 - 226 - Giard, D.J., Loeb, D.H., Thilly, W.G., Wang, D.I.C., and Levine, D.W., Human interferon production with diploid fibroblast cells grown 1979. Biotech. Bioeng., 21, 433-442 microcarriers. on Scale-up of membrane oxygenated recombinant Goldstein, J.M., 1986. Masters thesis, Dept. Applied Biol. Sci., animal cell bioreactors. M.I.T., Cambridge, MA Detrimental effects of and Spier, R.E., 1987. Handa, A., Emery, A.N., sparger aeration on suspended mammalian cell cultures - and their prevention. Proc. 4th European Congress on Biotech., 3, 601-604 Heisenberg, W., 1948. Z. Phys., 124, 168 Principles Hiemenz, P.C., 1977. (Marcel Dekker, NY) pp. 62-80 Hinze, J.O., 1975. of Colloid and Surface Chemistry Turbulence (McGraw-Hill, NY) pp. 222-227, 309-310 Tissue Culture in Medical 1980. Hirtenstein, M. and Clark, J., Oxford, England) p. (Pergamon, Research, R. Richards and K. Rajan, Eds. 97 Uniaxial loading of the red-cell Hochmuth, R.M. and Mohandas, N., 1972. membrane. J. Biomechanics, 5, 501-509 Measurement and Blackshear, P.L., 1973. Hochmuth, R.M., Mohandas, N., a fluidusing membrane cell red the for of the elastic modulus 747-762 13, J., Biophys. mechanical technique. Effects of shearing stress on Hollis, T.M. and Ferrone, R.A., 1974. aortic histamine synthesis. Exp. and Molec. Path., 20, 1-10 Continuous flow cultures of a HeLa cell line as a Holmstrom, B., 1968. of Rubella virus. Biotech. Bioeng., 10, 373supply steady basis for a 384 Hoyt, J.W., 1985. Drag reduction in polysaccharide solutions. in Biotechnology, 3, 17-21 Trends Growth control in cultured 3T3 Hsu, Y-M. and Wang, J.L., 1986. polypeptide responsible for 13,000 Mr an of purification v. fibroblasts 362-369 102, Biol., Cell J. activity. growth inhibitory Hu, W.S., 1983. Quantitative and mechanistic analysis of mammalian cell cultivation on microcarriers. Doctoral dissertation, Dep. Appl. Biol. Sci., M.I.T., Cambridge, MA, pp. 204-211 Hu, W.S., Meier, J., and Wang, D.I.C., 1985. A mechanistic analysis of the inoculum requirement for the cultivation of mammalian cells on microcarriers. Biotech. Bioeng., 27, 585-595 - 227 - Hyman, W.A., 1972a. Shear flow over a protrusion from a plane wall. J. Biomechanics, 5, 45-48 Hyman, W.A., 1972b. Shear flow over a protrusion from a plane wall: addendum. J. Biomechanics, 5, 643 Johnson, H.A., 1961. Some problems associated with the histological study of cell proliferation kinetics. Cytologia, 26, 32-41 Kilburn, D.G., and Webb, F.C., 1968. controlled dissolved oxygen partial 801-814 The cultivation of animal cells at Biotech. Bioeng., 10, pressure. Kleinbaum, D.G. and Kupper, L.L., 1985. Applied Regression Analysis and Other Multivariable Methods (Duxbury Press, North Scituate, MA) pp 95106 Komasawa, I., Kuboi, R., and Otake, T., 1974. Fluid and particle motion turbulent dispersion: Measurement of turbulence of liquid by in continual pursuit of tracer particle motion. Chem. Eng. Sci., 29, 641650. Fluid and particle motion in Otake, T., 1974. Kuboi, R., Komasawa, I., of liquid on the motion of turbulence of turbulent dispersion: Influence 651-657. 29, suspended particles. Chem. Eng. Sci., Kuchler, R.J., Marlowe, M.L, and Merchant, D.J., 1960. The mechanism of cell binding and cell sheet formation in L strain fibroblasts. Experimental Cell Res., 20, 428-437 Landry, J., 106, 23-32 Freyer, J.P., and Sutherland, R.M., 1981. J. Cell Phys., Turbulent flow of dilute polymer solutions - studies Lee, T-S., 1966. Doctoral dissertation, Dept. Chem. Eng., M.I.T., in couette flow. Cambridge, MA, pp. 39-51 Levesque, M.J. and Nerem, R.M., 1985. The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress. J. Biomechanical Eng., 107, 341-347 Physical and chemical data, in R.H. and Chilton, C.H., Eds., Liley, P.E. and Gambill, W.R., 1973. Chemical Engineers' Handbook, Perry, (McGraw-Hill, NY) p 3-45 Lin, C-J., Peery, J.H., Schowalter, W.R., 1970. Simple shear flow round J. Fluid inertial effects and suspension rheology. a rigid sphere: Mech., 44, 1-17 Macieira-Coelho, A., Ponten, J., and Philipson, L., 1966. The division cycle and RNA-synthesis in diploid human cells at different passage levels in vitro. Exp. Cell Res., 42, 673-684 - 228 - Microbial polysaccharides, in Margaritas, A. and Pace, G.W., 1984. H.W., Drew, S., and Wang, Blanch, 3, Volume Comprehensive Biotechnology, 1015 p. York), New Press, (Pergamon D.I.C., Eds., Maroudas, N.G., 219 1974. Short-range diffusion gradients. Cell, 3, 217- Matsuo, T., and Unno, H., 1981. Forces acting on a floc and strength of floc. J. Environ. Eng. Div., ASCE, 107, 527-545 McCabe, W.L., Smith, J.C., and Harriot, P., 1985. Chemical Engineering (McGraw-Hill, NY) p. 229-231 Unit Operations of Purification and McMahon, J.B., Farrelly, J.G., Iype, P.T., 1982. properties of a rat liver protein that specifically inhibits the Proc. proliferation of nonmalignant epithelial cells from rat liver. Natl. Acad. Sci. USA, 79, 456-460 Flow effects on the McQueen, A., Meilhoc, E., and Bailey, J., 1987. Biotech. Lett., 9, and lysis of suspended mammalian cells. viability 831-836 Cell 1980. and Hopps, H.E., Albrecht, P., Mered, B., optimization in microcarrier culture. In Vitro, 16, 859-865 growth Midler, M. and Finn, R.K., 1966. A model system for evaluating shear in the design of stirred fermentors. Biotech. Bioeng., 8, 71-84 The effects of Miller, W.M., Wilke, C.R., and Blanch, H.W., 1987. dissolved oxygen concentration on hybridoma growth and metabolism in continuous culture. Submitted to J. Cellular Physiol. Mitotic cell populations obtained Mitchell, K.J., and Wray, W., 1979. Exp. Cell Res., 123, 452-455 from a micro-carrierculturing system. Oxygen in human lymphoblastoid cell line cultures Mizrahi, A., 1984. Devel. Biol. and effect of polymers in agitated and aerated cultures. Standard., 55, 93-102 Statistical Fluid Mechanics: Monin, A.S., and Yaglom, A.M., 1971. Mechanics of Turbulence, Volume 1 (MIT Press, Cambridge, MA) pp. 412-415 Mixing: Nagata, S., 1975. pp. 24-32, 126-129, 149-163 Principles and Applications (Wiley, NY) Growth and Maintenance of Anchorage -Dependent Nahapetian, A.T., 1986. Mammalian Cells in Perfused Systems and Metabolism of Nutrients, in (Butterworth, Stoneham, Ed., Mammalian Cell Technology, Thilly, W.G., MA), pp. 151-165 - 229 - Ng, J.J.Y., Crespi, C.L., and Thilly, W.G., 1980. chinese hamster ovary cells from microcarriers. 231-238 Selection of mitotic 109, Anal. Biochem., Nicodemo, L., Nicolais, L. and Landel, R.F., 1974. Shear rate dependent viscosity of suspensions in Newtonian and non-Newtonian liquids. Chem. Eng. Sci., 29, 729-735 The dispersion of solids in liquids, in Mixing of Nienow, A.W., 1985. Liquids by Mechanical Agitation, Ulbrecht, J.J. and Patterson, G.K., Eds., (Gordon and Breach Science Publishers, NY) p 273-307 Okamoto, Y., Nishikawa, M., and Hashimoto, K., rate distribution in mixing vessels and its mass transfer. Int. Chem. Eng., 21, 88-96 Oldshue, J.Y. , 1983. 31, 170, 198, 213 1981. Energy dissipation effects on liquid-liquid Fluid Mixing Technology (McGraw-Hill, Pardee, A.B., Dubrow, R., Hamlin, J.L., and Kletzein, R.F., cell cycle. Ann. Rev. Biochem., 47, 715-750 NY) pp. 27- 1978. Animal Packed fiber bed reactor design for animal cell Perry, S.D., 1987. culture. Masters thesis, Depart. Chem. Eng., M.I.T., Cambridge, MA Microcarrier cell culture - principles and methods. Pharmacia, 1981. Technical literature, Pharmacia Fine Chemicals, Uppsala, Sweden. Pirt, S.J., 1975. Principles of Microbe and Cell Cultivation (Blackwell Scientific Publications, London) Placek, J. and Tavlarides, L.L., 1985. Turbulent flow in stirred tanks, Turbulent flow in the turbine impeller region. AIChE J., 31, Part 1: 1113-1120 Prescott, D.M., 1976. Reproduction of Eukaryotic Cells (Academic Press, NY) pp. 24, 25, 37, 38 Quraishi, A.Q., Mashelkar, R.A., and Ulbrecht, J.J., 1977. Influence of drag reducing additives on mixing and dispersing in agitated vessels. AIChE J., 23, 487-492 Reid, R.C., Prausnitz, J.M., and Sherwood, T.K., of Gases and Liquids (McGraw-Hill, NY) p 577 1977. The Properties Power input to fermentors and similar vessels. Richards, J.W., 1963. British Chem. Eng., 8, 158-163 Rodriquez, 162 F., 1982. Principles of Polymer Systems (McGraw-Hill, - 230 - NY) p. Biological 1987. and Dunlop, E.H., Rosenberg, M.Z., Kargi, F., at the Presented stress. shear responses of plant cells to hydrodynamic LA Orleans, New Society, Chemical Annual Conference of the American Rosen, L.A., Hollis, T.M., and Sharma, M.G., 1974. Alterations in bovine endothelial histidine decarboxylase activity following exposure to shearing stress. Exp. Molecular Path., 20, 329-343 Growth of L cell suspensions on a Runyan, W.S. and Geyer, R.P., 1963. Med., 112, 1027-1030 Biol. Exp. Soc. Proc. apparatus. Warburg Power 1950. and Everett, H.J., Costick, E.W., Rushton, J.H., characteristics of mixing impellers; part 2. Chem. Eng. Prog., 46, 467476 and Adachi, Yagamuchi, N., Sano, Y., coefficients for suspended particles in J. Chem. Eng. Japan, 7, 255-261 columns. Horie, Y., Kamiwano, M., Sato, Y., Turbulent flow in a stirred vessel. transfer Mass 1974. T., agitated vessels and bubble Yamamoto, K., and Ishii, K., Kagaku-Kogaku, 31, 275-281 1967. Sato, M., Levesque, M.J., and Nerem, R.M., 1987. An application of the micropipette technique to the measurement of the mechanical properties of cultured bovine aortic endothelial cells. J. Biomech. Eng., 109, 2734 Micropipette aspiration Sato, M., Levesque, M., and Nerem, R.M., 1987. of culutured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis, 7, 276-286 Schulz, R., Krafft, H., and Lehmann, J., 1986. Experiences with a new type of microcarrier. Biotech. Lett., 8, 557-560 Shiigi, S.M. and Mishell, R.I., 1975. Sera and the in vitro induction of immune responses, Part 1: Bacterial contamination and the generation of good fetal bovine sera. J. Immun., 115, 741-744 and Nibler, J.W. Shoemaker, D.P., Garland, C.W., Steinfeld, J.I., Experiments in Physical Chemistry (McGraw-Hill, NY) pp. 32-38 1981. Sinskey, A.J., Fleischaker, R.J., Tyo M.A., Giard, D.J., and Wang, D.I.C., 1981. Production of cell-derived products: virus and interferon. Ann. N.Y. Acad. Sci., 369, 47-59 Smith, C.G., Greenfield, P.F., and Randerson, D.H., 1987. A technique for determining the shear sensitivity of mammalian cells in suspension culture. Biotech. Techniques, 1, 39-44 An examination of the data and Spier, R.E., and Griffiths, B., 1984. Devel. concepts germane to the oxygenation of cultured animal cells. Biol. Stand., 55, 81-92 - 231 - Sprague, E.A. , Steinbach, B.L. , Nerem, R.M. , and Schwartz, C.J. , 1987. Influence of a laminar steady-state fluid-imposed wall shear stress on low-density of degradation and internalization, binding, the 76, 648-656 Circulation, endothelium. lipoproteins by cultured arterial Stathopoulos, N.A. and Hellums, J.D., 1985. Shear stress effects on human embryonic kidney cells in vitro. Biotech. Bioeng., 27, 1021-1026 Role of diffusion Stoker, M.G.P., 1973. 246, 200-203 Nature, growth. of inhibition boundary layer in contact Stoker, M. and Piggott, D. , 1974. Shaking 3T3 cells: further studies on diffusion boundary effects. Cell, 3, 207-215 Swim, H.F., and Parker, R.F., 1960. Effect of Pluronic F68 on growth of fibroblasts in suspension on rotary shaker. Proc. Soc. Exp. Biol. Med., 103, 252-254 A First Tennekes, H. and Lumley, J.L., 1985. (M.I.T. Press, Cambridge, MA) pp 19-20, 262-264 Course in Turbulence Terasima, T., and Tolmach, L.J., 1962. Growth and nucleic acid synthesis Exp. Cell Res., in synchronously dividing populations of HeLa cells. 30, 344-362 Thomas, J.A. and Johnson, M.J., 1967. Trace-metal requirements of NCTC clone 929 strain L cells. J. Natl. Cancer Inst., 39, 337-345 Bioreactor design for growth of Tramper, J., and Vlak, J.M., 1987. shear-sensitive mammalian and insect cells. Advances in Biotechnological Processes, in press Tramper, J., Williams, J.B., and Joustra, D., 1986. of insect cells in suspension. Enzyme Microb. Tech., Shear sensitivity 8, 33-36 Tyo, M. and Wang, D.I.C., 1980. Engineering characterization of animal cell and virus production using controlled charge microcarriers, in Scientific and Engineering Advances in Biotechnology, Volume 1: Principles, Moo-Young, M., Ed., (Pergamon Press, NY) pp 141-146 Vaishnav, R.N., Patel, D.J., Atabek, H.B., Deshpande, M.D. , Plowman, 1983. J. Biomech. Eng., 105, 77-83 and Vossoughi, J., F. , Growth of cell-strains and primary cells on van Wezel, A.L., 1967. micro-carriers in homogeneous culture. Nature, 216, 64-65 (1967) Viggers, R.F., Wechezak, A.R., and Sauvage, L.R., 1986. An apparatus to study the response of cultured endothelium to shear stress. J. Biomech. Eng., 108, 332-337 Virk, P.S., 1975. 656 Drag reduction fundamentals. - 232 - AIChE Journal, 21, 625- Doctoral dissertation, Department of Chem. Eng., Wadia, P.H., 1975. M.I.T., Cambridge, MA Probability and Statistics Walpole, R.E. and Myers, R.H., 1978. pp 280-308, 514 NY) (MacMillan, Engineers and Scientists AIChE J., Wilke, C.R., and Chang, P., 1955. Wolf, A.V., properties of Chemistry and Raton, FA) p. for 1, 264 Concentrative Brown, M.G., and Prentiss, P.B., 1979. aqueous solutions: conversion tables, in CRC Handbook of Physics, Eds. R.C. Weast and M.J. Astle (CRC Press, Boca D-235 Dynamics of polymer molecules in Zimm, B.H., 1956. viscoelasticity, flow birefringence and dielectric Phys., 24, 269 Suspending of Zwietering, T.N., 1958. agitators. Chem. Eng. Sci., 8, 244-253 - 233 - dilute solutions: J. Chem. loss. solid particles in liquid by APPENDIX 1 CALCULATION OF THE RELATIVE DNA RELEASE UNDER HIGH AGITATION in described As release the into DNA of high under fluid culture to performed was experiment an 2, Chapter investigate the agitation. Cultures of FS-4 cells were grown on 5 g/l microcarriers in two 500-ml vessels with 7.8 cm impellers. The cultures were operated at 45 RPM to eliminate microcarrier agglomeration. were pooled, the microcarriers to two agitated concentrated One of identical 125-ml vessels. at 35 RPM while other was the When nearly confluent, and transferred to 15 g/l, these g/l 15 agitated at cultures was RPM. The 150 attached cell concentrations were shown in Figure 21. Figure 48 shows the cumulative change in Hoechst-dye fluorescence for samples taken from the culture fluid of the 15 g/L cultures. culture at 150 RPM, into suspension. fluorescence, In the there was extensive cell death and removal of cells The data or DNA content, show strong a increase the culture fluid. in 35 RPM, there was very little removal of cells into in Hoechst-dye In the culture at suspension. The data show a moderate decrease in Hoechst-dye fluorescence. The cause of the decrease in fluorescence for the 35 RPM culture was not determined. The culture medium contained approximately 1 pg/ml DNA The background fluorescence from from the 5% (v/v) serum supplement. This decrease could have come this DNA may have decreased over time. - 234 - + 35 ----- rpm ---150 rpm -2 -4 20 40 60 TIME SINCE TRANSFER (HRS) Figure 48. Cumulative chang e in Hoechst-dye fluorescence for culture fluid from 15 g/L cultures - 235 - 80 about through: 1) cellular uptake of DNA polymers (unlikely) a substance which production of 2) cellular specific reduced the fluorescence of the DNA-Hoechst dye complexes 3) of uptake cellular substance a which otherwise enhanced the specific fluorescence of the DNA-Hoechst dye complexes 4) degradation of the DNA into smaller molecules or single chains with a concurrent reduction of the specific fluorescence To calculate the absolute release of DNA into the culture fluid at one must correct 150 RPM, for the decrease in the control at 35 RPM. There are at least three reasonable methods to perform this correction: the simplest correction. This represents Absolute correction. 1) The change in fluorescence at 35 RPM is simply subtraced from the change in fluorescence at 150 RPM. For each time interval ti to t 2 , the absolute release of DNA into the culture fluid at 150 RPM, D 2 - Dl, is given by: (\ 2 ,1 150 D - where , 1 K6 2 K6 represents the fluorescence of specific , 1 5 0 and A2 , 1 5 0 represent the fluorescence in RPM culture represent the A1 ,3 5 ) ,3 5 (Eq. 94) = D 1 2 ( 150) at times t1 and t 2 , fluorescence in the DNA, the samples from the 150 respectively, and Al, 35 and A2 , 3 5 samples from the 35 RPM culture at times ti and t2 , respectively. - FS-4 cellular 236 - 2) Correction proportional to volumetric method of correction would be appropriate at 35 RPM was reduced which the due specific otherwise uptake of DNA. one to: of these a) cellular if concentration. the fluorescence production of fluorescence, b) increased the cell a decrease substance which cellular uptake specific This subtance or c) cellular fluorescence, of a If one corrects for the decrease in fluorescence due to cellular processes, the absolute release of DNA into the culture fluid at 150 RPM, D2 - D1 , is given by: (-X2,150 ~ D - D gi ~ A1 ,3 5 ) (Eq. 95) 1 K6 is a correction factor roughly given by: C1,150 1,35 C1 ,1 5 0 and + ft C where 1 (A2 ,3 5 = 2 where A1 ,1 5 0 ) + C2,150 C2 ,1 5 0 (Eq. 96) = C 2,35 represent the attached, or concentrations in the 150 RPM culture at times t1 and t and C1 , 3 5 and C2 , 3 5 represent the attached, or viable, 2 viable, cell , respectively, cell concentra- tions in the 35 RPM culture at times ti and t2 , respectively. 3) Correction proportional to DNA if correction would be appropriate concentration. the fluorescence was due to degradation and breakdown of the DNA. first order process. This method of decrease at 35 RPM This would likely be a The amount of DNA breakdown would be proportional to the amount of DNA present. If one corrects for this breakdown, the - 237 - absolute release of DNA into the culture fluid at 150 RPM, D2 - Dl, is given by: ~ 2 1, 5 0 D where - ~ A1 ,3 5 ) 2 (X 2 , 3 5 ,1 5 0 (Eq. 97) = D (2 is 1 a correction factor roughly given by: 1,150 + 2,150 (Eq. 98) - g 2,35 P1,35 Figure 49 shows the cumulative release of DNA into the culture fluid at 150 Data RPM. calculation. is the for shown three different correction proportional to DNA concentration (Method 3) gives the highest release. not a large difference of three methods Chapter 2. The the in results from Nonetheless, there the three different For the purposes of this thesis, the average methods of calculation. value of The correction proportional to cell concentration (Method 2) gives the lowest release while the is methods is plotted in used and is error bars in Figures 22 Figures 22 and 23 in and 23 cover the results from the three different methods of calculation. range of The error bars are given by the range of results plus twice the standard deviation of the assay. - 238 - +-Method - one Method Method - two three 1.80 1.50 W7 1.20 LU /7, ZA W/ U 0.90 --- z / (I ~ /,' - O.30 0.60 / 0.30 0.00. 1 0 20 1 40 TIME SINCE TRANSFER 60 80 (HRS) Cumulative release of DNA into Figure 49. suspension for 15 g/L culture at 150 RPM. Data is shown for 3 different methods of calculation. - 239 APPENDIX 2. ESTIMATION OF THE GROWTH YIELD AND MAINTENANCE COEFFICIENT FOR OXYGEN For FS-4 cells, the growth yield of cells on oxygen was estimated from the results of Balin et al (1976) for oxygen-limited growth of WI38 cells. These cells are human diploid fibroblasts and are similar to FS-4 cells. At a dissolved oxygen level equivalent to 7.8 mm Hg 02, the WI-38 had cells specific a specific growth rate, y, 0.032 hr-1 , a total of oxygen uptake rate, Ro, of 1.3 x 10-10 mmole/cell-hr, and a maintenance-associated oxygen uptake rate, Mo, mmole/cell-hr, as estimated through interpolation. of 2.8 x 10-11 The growth yield of cells on oxygen, Yn/o, was then calculated from the equation Y and was n/o p / = (R o - M ) (Eq. 99) o thus estimated to be 3 x 108 cell/mmole. This yield was used for the optimization calculations in Chapter 7. The results from Fleischaker and Sinskey (1981) were used to estimate the maintainence coefficient on oxygen, Mo, for FS-4 cells. At a specific growth rate of approximately 0.011 hr-1, the FS-4 cells had a specific oxygen uptake rate, of 5 x 10-11 mmole/cell-hr. Ro, Using equation 99 and the yield of 3 x 108 cells/mmole estimate above, one can calculate coefficient a value of on oxygen, 1.3 x Mo. 10-11 mmole/cell-hr for the maintenance This maintenance coefficient was used for the optimization calculations in Chapter 7. - 240 -