The Indiana Clinical Translational Science Institute (I-CTSI)
advertisement

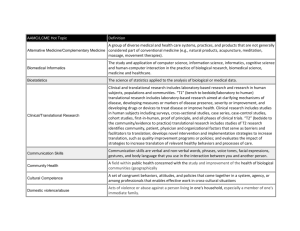
IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH The Indiana Clinical Translational Science Institute (I-CTSI) Institute Director: Anantha Shekhar, MD, PhD Bloomington director: William P. Hetrick, PhD Purdue director: Connie Weaver, PhD Notre Dame director: Gregory Crawford Crawford, PhD IndianaCTSI IU Health Information and Translational Sciences Building, Indianapolis ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH What is clinical translational science? • Science aimed at: – translating discoveries in basic science to the understanding, treatment and prevention of human disorders (T1) treatment, • translating discoveries generated during research in the laboratory, and in preclinical studies, to studies in humans and the development of human trials – developing and implementing evidence-based standards of practice and public health policy (T2) • enhancing the adoption of best practices in the community • enhancing cost-effectiveness of prevention and treatment strategies • More than simply p y interdisciplinary p y Conventional Model: g g systems y of research & training g Segregated IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Mental or Physical Health Genetics Neuroscience Cognitive Social •Behavioral •Neuroimaging •Systems •Cellular •Molecular •Animal models Health policy Psychopathology Clinical practice •Classification •Diagnosis •Phenomenology •Etiology gy •Case management •Psychotherapy •Neurophysiology •Pharmacology gy Conventional Model: g g systems y of research & training g Segregated IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Mental or Physical Health Genetics Neuroscience Cognitive Social Psychopathology Clinical practice Health policy Clinical Translational Science Model: Integrated systems of research & training IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Best Practices & Policy The Problem Area (e.g., schizophrenia) Patient Bedside Genetics Neuroscience Cognitive Social Psychopathology Clinical practice Problem focused research & training that facilitates translation Health policy Laboratory L b t Bench Clinical Translational Science: A subdiscipline to add to the landscape IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Mental &/or Physical Health Translational Science Genetics Health policy Clinical practice Neuroscience Cognitive Social Psychopathology IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Statement of justification • “At no other time has the need for a robust, bidirectional information flow between basic and translational scientists been so necessary. Advances in our understanding of biologic systems and the development of powerful new t l that tools th t can be b applied li d att b both th th the b bench h and d th the b bedside...offer d id ff unprecedented prospects for advancing knowledge of human disorders in a translational context... Today, there is good reason to believe that the scope of knowledge and expertise needed to be an effective translational or clinical scientist can no longer be acquired "on on the job, job " as was done in the past. Although we have made every effort to provide the support functions for translational and clinical research, there is a call for training in a wider range of skill sets that span the biomedical and behavioral sciences and make use of far more advanced and more complex resources and methods than h ever b before. f W We may have h ffailed il d to recognize i that h clinical li i l and d translational science is an emerging discipline that encompasses both the acquisition of new knowledge about health and disease prevention, preemption, and treatment and the methodologic research necessary to develop or improve research tools tools.” — Elias A A. Zerhouni (2005) IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Agenda 1 1. Q i k overview Quick i off the th Indiana I di CTSI a. b. c. 2. What is clinical translational science? a. b. c. d. 3. NIH’s impetus Strategic goals the CTSAs Key functions Who has a CTSA? Indiana CTSI research & training programs a. b. c. 4. What it is? Who funds it? Who are its partners and users? Research grants Education & career development p Infrastructure and resources Translational research & training on the Bloomington campus a. b. b c. d. e. What is its role? How o do we ep promote o o e it a at IUB? U How do we increased IUB participation in the I-CTSI? How can IUB strengthen the I-CTIS? Annual Bloomington InCTSI retreat IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Providing Leveraging Opportunities Throughout the State Biocrossroads Wellpoint, Inc. Indiana University Roudebush VA Medical Center IndianaCTSI Clarian Health Partners Regenstrief Institute ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Eli Lilly and Company Purdue University Richard M. Fairbanks Foundation Notre Dame Wishard Health Services Cook Group, Inc Indiana State Government IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Agenda 1 1. Q i k overview Quick i off the th Indiana I di CTSI a. b. c. 2. What is clinical translational science? a. b. c. d. 3. NIH’s impetus Strategic goals the CTSAs Key functions Who has a CTSA? Indiana CTSI research & training programs a. b. c. 4. What it is? Who funds it? Who are its partners and users? Research grants Education & career development p Infrustructure and resources Translational research & training on the Bloomington campus a. b. b c. d. e. What is its role? How o do we ep promote o o e it a at IUB? U How do we increased IUB participation in the II-CTSI? How can IUB strengthen the II--CTIS? Fall 2010 meeting in Bloomington IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH The Translational Research Gap Source: Butler D. Translational research: Crossing the valley of death. Nature. 2008;453:840–2. Impetus for the CTSA Program Integrative Across health disciplines p Between scientific areas Into the community engagement/participation Translational Laboratory to Clinic to Community to Laboratory Educational Scientists Health care providers Health care providers Community Provides resource infrastructure NIH funded research Non‐NIH funded research Public‐private partnership CTSA Strategic Goals (1) Enhancing National Clinical and Translational Research Capability • Clinical research management • Research R h iinfrastructure f t t • Phenotyping – human and preclinical models (2) Enhancing the Training and Career Development of Clinical and Translational Scientists (3) Enhancing Consortium-Wide Consortium Wide Collaborations • Social networking • Inventory of resources • Data sharing g (4) Enhancing the Health of Our Communities and the Nation • National Model for Community Engagement • Inform Public Health Policy Through Research Re--engineering Clinical Research Re T1 Translation B Bench h T2 Translation B d id Bedside Building B ildi Blocks Bl k and d Pathways P h • Molecular Libraries • Bioinformatics Translational • Computational Biology R Research h • Nanomedicine Initiatives • • • • C Community it Practice Integrated Research Networks Clinical Research Informatics NIH Clinical Research Associates Clinical Outcomes Cross-cutting: Harmonization, Training Clinical and Translational Science Awards (CTSAs) Educate the next generation of clinical researchers Improve clinical research management Build diversity in leadership Assemble interdisciplinary teams Enhance public trust Forge partnerships with private and public health care organizations CTSAWeb.org Key Functions NIH & other government government agencies Cli i l R hI i Clinical Clinical Research Innovation Education and Research Career Development Ethics Translational Biomedical Informatics Community Engagement CTSA Academic Center Clinical Resources Industry Communications Evaluation Public Private Partnerships Public Private Partnerships Comparative Effectiveness Healthcare organizations Regulatory Support Biostatistics Building a National CTSA Consortium Case Western Reserve University of Illinois at Chicago Northwestern University of Washington g Albert Einstein OSU University of Michigan University of Pittsburgh University of Rochester WA MT ND OHSU OR ID WY Univ. of Utah UC, Davis NV Univ. of Colorado UCSF UT CO Stanford MN WI Mayo Clinic SD University of Wisconsin y IA University of Iowa NE University of Chicago Washington University, St. Louis KS CA VT Columbia Scripps IL MO OK UT Southwestern AR NM UT MB LA NJ PA TN NH MA Duke UNC UT San Antonio University of Cincinnati University of Florida UT, Houston HI Since 2006 Since 2007 Since 2008 Since 2009 NYU VA SC Medical University of SC Emory GA AL MS Univ of Alabama at Birmingham Participating Institutions Yale Weill Cornell Mt. Sinai School of Med. Rockefeller University of Pennsylvania TX AK Harvard Boston University Tufts University Johns Hopkins IN Indiana University OH WV KY Vanderbilt University of Arkansas AZ NY MI ME FL IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Agenda 1 1. Q i k overview Quick i off the th Indiana I di CTSI a. b. c. 2. What is clinical translational science? a. b. c. d. 3. NIH’s impetus Strategic goals the CTSAs Key functions Who has a CTSA? Indiana CTSI research & training programs a. b. c. 4. What it is? Who funds it? Who are its partners and users? Research grants p Education & career development Infrustructure and resources Translational research & training on the Bloomington campus a. b. b c. d. e. What is its role? How o do we ep promote o o e it a at IUB? U How do we increased IUB participation in the II-CTSI? How can IUB strengthen the II--CTIS? Fall 2010 meeting in Bloomington IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH I. Translational research acceleration programs: A Research awards A. 1. 2. 3. 4. Collaboration in Translational Research Pilot Grant Program (CTR) • Goal: Foster collaborations between investigators at IUB, Purdue, ND, IUSM, and IUPUI in translational research p projects j with strong g potential to develop into externally funded programs or IP. Project Development Team (PDT) Pilot Funding • Goal: Provides funding to support development of preliminary data for external grant submissions. submissions Pilot Core Funding • Promotes use of CTSI cores across CTSI partner institutions and supports science with a high potential for external funding or IP. Research Inventions and Scientific Commercialization (RISC) • Pilot program to support potential gaps in research that leads to IP generation at IU in collaboration with IURTC. IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH I. Translational research acceleration programs: B Education & career development awards B. 1. 2. 3. 4. 5. T Trainee awards • The T32 focuses on pre-doctoral training in translational research. K Clinical Scholar awards • The K12 program focuses on junior faculty pursuing clinical or translational research. K Basic Science Scholar awards • The K12 program focuses on junior faculty pursuing Basic Science research. Notre Dame Innovation Postdoc (ND only) • Post-doc funding to facilitate movement of research inventions/concepts to commercialization. Purdue’s Trask Venture Fund (Purdue only) • Goal: To assist faculty to further clinical commercial potential of IP disclosed to the Office of Technology Commercialization Commercialization. IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH I-CTSI p programs g that facilitate research: • • • • • • • • • • • • Novel Translational Methodologies and Pilot Studies Program, Project Development Teams (PDTs) g Biomedical Informatics Program Design and Biostatistics Program (DBP) Regulatory Knowledge and Support (RKS) Clinical Research Resource Center (CRRC) Community Health Engagement Program (CHEP) Translational Technologies and Resources (TTR) Cores Program Research Education, Education Training and Career Development Bioethics and Subject Advocacy Program (BSAP) Disease and Therapeutic Modeling Program Biomedical Engineering and Bionanotechnology Program Indiana CTSI HUB (http://www.indianactsi.org) IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Project Development Teams: • Purpose: – “one-stop p shop” p for study y development p by y providing investigators access to multidisciplinary research expertise, biostatistics, IRB/regulatory services, services nursing support support, and pilot funds – Comprised of experienced researchers who discuss ideas and concepts with investigators to assist in developing high quality, feasible, f d bl clinical/translational fundable li i l/t l ti l research h projects. j t IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Project Development Teams: • Eight PDTs address a broad spectrum of research focuses 1. Preclinical PDT (TRAC1) focuses on translational studies in animal and cellular models. models 2. Pediatric PDT (PRAT) facilitates research in children with emphasis on interactions with basic scientists to design and implement bench-to-bedside studies. 3. Adult PDT (TRAC2) focuses on early translational studies related to adult medicine. 4 Behavioral/Population Science PDT (BEHV) helps investigators design and 4. implement epidemiological and behavioral research. 5. Purdue PDT has particular strengths in assisting investigators with a special emphasis on bioengineering, nutritional, and veterinary medicine. 6. Translating Research into Practice PDT (TRIP) focuses on projects that evaluate and d iimplement l t evidence-based id b d practice ti and d other th h health lth services i research. h 7. Notre Dame PDT provides access to broad biomedical expertise particularly in basic research. 8. Imaging PDT provides expertise in the application of anatomical, functional, and molecular imaging methodologies and technologies technologies. IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH PDT projects - portfolio by stage BEDSIDE BENCH Basic Science Research Preclinical Studies Animal Research Proof of Concept Model Dev Drug Dev TRAC1 4 14 1 3 6 7 11 2 10 9 16 20 12 17 18 5 23 8 13 15 21 24 19 25 22 TRAC2 46 49 54 44 PRAT 73 81 63 53 Beh/Pop 97 TRIP PRACTICE Clinical Practice Delivery of Care Identification of New Clinical Questions and Gaps in Care 26 27 29 31 33 35 37 39 41 43 45 48105123 51 28 30 32 34 36 38 40 42 47 50 52 65 66 68 70 79 82 55 57 59 61 64 69 72 75 77 56 58 60 62 67 71 74 76 78 84 86 88 90 92 94 96 83 85 87 89 91 93 95 98 106 99 101103104 100 102 Imaging Purdue Human Clinical Research Controlled Observational Studies Phase 3 Clinical Trials T1 Case Series Phase 1 and 2 Clinical Trials T2 Guideline Development Meta‐analyses Systematic Reviews T3 Dissemination & Implementation Research Health Systems Redesign Applied Health IT 108 109 113114 110117 122 107 111 112 116 119 121 115 118 120 Notre Dame IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Agenda 1 1. Q i k overview Quick i off the th Indiana I di CTSI a. b. c. 2. What is clinical translational science? a. b. c. d. 3. NIH’s impetus Strategic goals the CTSAs Key functions Who has a CTSA? Indiana CTSI research & training programs a. b. c. 4. What it is? Who funds it? Who are its partners and users? Research grants Education & career development p Infrustructure and resources Translational research & training on the Bloomington campus a. b. b c. d. e. What is its role? How o do we ep promote o o e it a at IUB? U How do we increased IUB participation in the I-CTSI? How can IUB strengthen the I-CTIS? Annual Bloomington InCTSI retreat IndianaCTSI ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH Questions? Q estions? Suggestions? gg Future directions?