For a diprotic acid with following reactions: H
advertisement
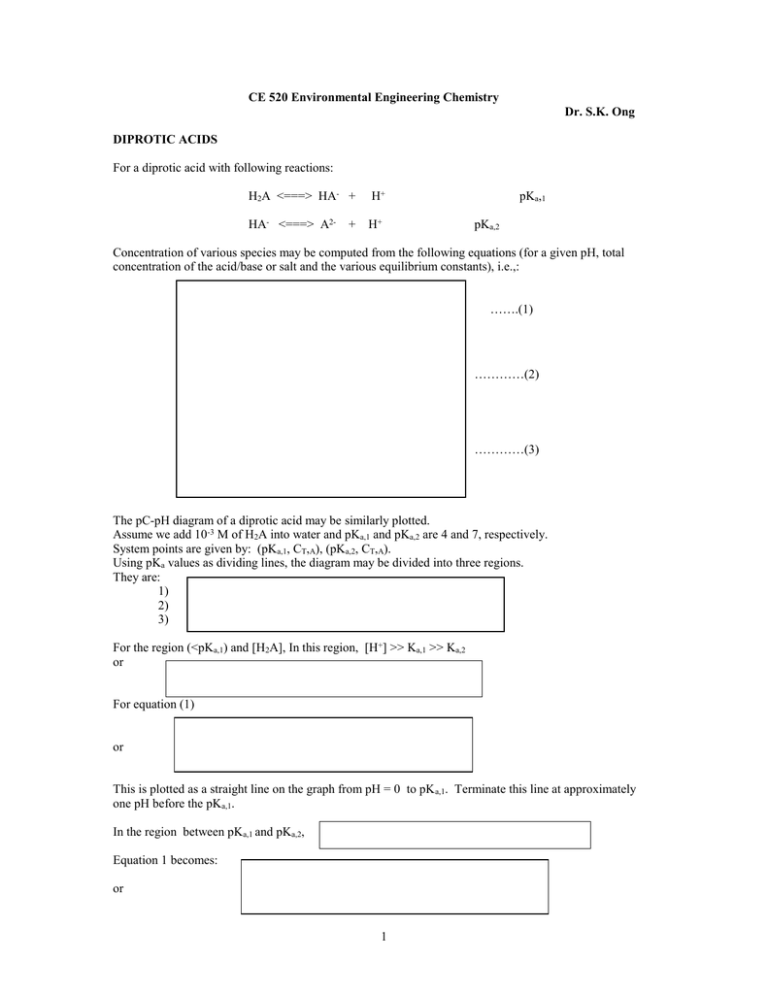
CE 520 Environmental Engineering Chemistry Dr. S.K. Ong DIPROTIC ACIDS For a diprotic acid with following reactions: H2A <===> HA- + H+ HA- <===> A2- H+ + pKa,1 pKa,2 Concentration of various species may be computed from the following equations (for a given pH, total concentration of the acid/base or salt and the various equilibrium constants), i.e.,: …….(1) …………(2) …………(3) The pC-pH diagram of a diprotic acid may be similarly plotted. Assume we add 10-3 M of H2A into water and pKa,1 and pKa,2 are 4 and 7, respectively. System points are given by: (pKa,1, CT,A), (pKa,2, CT,A). Using pKa values as dividing lines, the diagram may be divided into three regions. They are: 1) 2) 3) For the region (<pKa,1) and [H2A], In this region, [H+] >> Ka,1 >> Ka,2 or For equation (1) or This is plotted as a straight line on the graph from pH = 0 to pK a,1. Terminate this line at approximately one pH before the pKa,1. In the region between pKa,1 and pKa,2, Equation 1 becomes: or 1 p[H2A] = pCT,A + pH - pKa,1 p[H2A] vs pH for this region gives a line with a slope of 1. In the region between > pKa,2, Ka,1 > Ka,2 > [H+] or pKa,1 < pKa,2 < pH Equation 1 becomes: ……………………….(5) p[H2A] = pCT,A + 2 pH - pKa,1 - pKa,2 p[H2A] vs pH for this region gives a line with a slope of 2. The curve for [H2A] near the pKa,1and pKa,2 can be computed using equation 5. when pH = pKa,1 then At this point [HA-] = [H2A], therefore or log (CT,A) - log (2) = log[H2A] p[H2A] = pCT,A + 0.3 This equation means that at pH equal to system point pKa,1, p[H2A] is 0.3 below pKa,1 For pH equal to system point pKa,2, p[H2A] is plotted 0.3 units below the line with slope 1 and slope 2. Lines for HA- and A2- may be plotted similarly. Examples: 1. Solution with 10-3 M H2A, pKa,1and pKa,2 are 4 and 7 , respectively. Proton condition: Excess Zero Level Deficit Equilibrium pH = 3.5 2. Solution with 10-3 M NaHA, pKa,1and pKa,2 are 4 and 7 , respectively. Proton condition: Excess Zero Level Deficit 2 [H+] + [ H2A ] = [OH- ] + [ A2-] Equilibrium pH = 6.1 3. Solution with 10-3 M Na2A, pKa,1and pKa,2 are 4 and 7, respectively. Proton condition: Excess Zero Level Deficit [H+] + 2[H2A ] + [HA-] = [OH-] Equilibrium pH = 9.5 IONIZATION FRACTIONS For monoprotic acids, we can write: [ HA] CT , A 1 1 Ka [ A ] 1 1 CT , A [ H ] 1 Ka o [H ] For diprotic acid: [ H 2 A] CT , A 1 1 K a ,1 [H ] K a ,1 K a , 2 o [H ]2 [ HA ] 1 1 K a,2 CT , A [H ] 1 K a ,1 [H ] [ A 2 ] 1 2 2 CT , A [H ] [H ] 1 K a ,1 K a , 2 K a , 2 The value gives the fraction of the ion present in the solution for a given pH. An advantage of using the ionization fraction is that it is independent of the analytical concentration. Multiplying the fraction with the total concentration of the species added will give the concentration of the species. Diagrams showing the distribution of the species are known as distribution diagrams. Note subscript of gives the number of proton lost. Note that i = 1. whereby 3 CARBONATE SYSTEMS Major anions present in natural waters are bicarbonate, sulfate and chloride. Of the above three anions, bicarbonate is of higher concentration than the other two anions. Therefore, being a conjugate base of a weak acid, the pH of natural waters is regulated by the carbonates present. The species of the carbonate systems include: CO2 (g); CO2 (aq); H2CO3 (carbonic acid); HCO3- (bicarbonate); CO32- (carbonate) Equations relating them are as follows: Since, it is difficult to distinguish between CO2 (aq) and H2CO3 by analytical methods such as acid-base titration, a hypothetical species [H2CO3 *] is used, i.e., However, the fraction of [H2CO3 ] present is small in comparison to [CO2 (aq)]. Therefore, we can write [H2CO3 *] [CO2 (aq)] Using [H2CO3 *] in the equilibrium equation instead of [CO2 (aq)] and [H2CO3 ], we have: The three equations that we will use frequently in our calculations for a carbonate system are: To generalize the various situations in the environment, the systems can be categorized as follows: 1. An open system with no solids present, e.g., an aeration basin of an activated sludge plant 2. An open system with solids present, e.g., lime softening process with lime present and exchange of CO2 with atmosphere. 3. A closed system with no solids present, e.g., titration of a solution containing carbonates species in a closed vessel 4. A closed system with solids present, e.g., bottom of a lake in equilibrium with calcium carbonate but with no exchange of CO2. Focus will be on systems 1 and 3. For system 3, a closed system, the construction of the pC-pH diagram follows that of a hypothetical acid H2A. What is the final pH for a 10-5 M solution of H2CO3*, NaHCO3 and Na2CO3? Proton condition for H2CO3*: [H+] = [OH-] + [HCO3-] + 2[ CO32-] or approximately [H+] = [HCO3- ] Equilibrium pH 5.7 Proton condition for NaHCO3: [H+] + [H2CO3 *] = [OH-] + [CO32-] 4 or approximately Equilibrium pH ≈ 7.6 Proton condition for Na2CO3: [ H2CO3*] = [OH-] [H+] + 2[ H2CO3*] + [HCO3-] = [OH-] or approximately [HCO3-] = [OH-] Equilibrium pH ≈ 9.0 For an open system (system 1), the carbonate species in solution are in equilibrium with CO 2 in the atmosphere. The normal atmosphere contains 10-3.5 atm of CO2. Then we have: [ H2CO3 *] = CO2 (aq) = or p [ H2CO3*] = 5 For [HCO3- ],then: K a ,1 [H ][ HCO 3 ] [H 2 CO 3 * ] [H ][ HCO 3 ] 10 5 therefore For [ CO32-]: K a ,2 [H ][CO 3 2 ] [HCO 3 ] [CO 3 2 ] 10 5 K a ,1 K a , 2 [H ] 2 See Diagram (Note CT,CO3 line) What is the final pH for a 10-5 M solution of H2CO3*, NaHCO3 and Na2CO3 in equilibrium with atmosphere? Proton condition for H2CO3*: [H+] = [OH-] + [HCO3-] + 2[ CO32-] or approximately [H+] = [HCO3-] Equilibrium pH ≈ 5.7 Charge condition for NaHCO3: [H+] + [Na+] = [OH-] + 2[ CO32-] + [HCO3-] where [Na+] = 10-5 M or approximately 10-5 = [HCO3-] Equilibrium pH ≈ 6.3 Charge condition for Na2CO3: [H+] + [Na+] = [OH-] + 2[ CO32-] + [HCO3- ] where [Na+] = 2 x 10-5 M or approximately 2 x 10-5 = [HCO3- ] Equilibrium pH ≈ 6.6 5