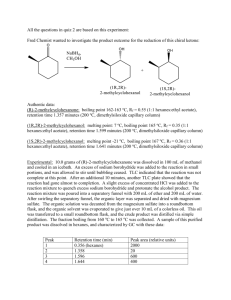
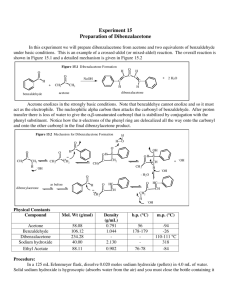
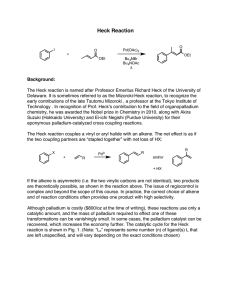
Enantioselective Reduction of a Ketone Using Enzymes Background:
advertisement

Enantioselective Reduction of a Ketone Using Enzymes carrot peels O O H 2O rt O OH Background: Enzymes are chiral biocatalysts. Some are capable of performing a reaction enantioselectively, to provide one stereoisomer as the major product. Enzymes have also been used to selectively catalyze a reaction of one enantiomer of a substrate over another. The separation of two substrates based on a difference in their reaction rates is known as “kinetic resolution”. Lipases, which catalyze the formation and hydrolysis of esters, have been widely used to resolve enantiomeric alcohols and esters. Isolated and purified enzymes can be expensive and unstable. Often, whole cells (such as baker’s yeast) are used as a convenient source of enzymes. In his week’s lab, an achiral ketone will be reduced to a chiral alcohol using the enzymes found in carrots. Hazards: All steps involving chemicals are to be performed in a fume hood while wearing gloves and goggles. Ethyl acetate, hexanes, and methanol are flammable liquids and must be disposed of in the organic waste carboy. Procedure: To a 250-mL Erlenmeyer flask containing a large magnetic stir bar add 20 mg of benzofuran-2-yl-methyl ketone followed by 75 mL of distilled water. Swirl the flask, and then add 24 g of carrot peelings to it. Clamp the flask above a magnetic stirrer but not in contact with it (Note 1) and adjust the stirrer to maintain a slow rate of stirring. Make a note of the time. Monitor the reaction by TLC. To do this, take aliquots (~1 mL) at 10, 30, 60, 100, and 120 minutes after stirring is initiated, transferring them to a centrifuge tube or test tube. Add ~1 mL of ethyl acetate, and tap the centrifuge tube repeatedly to thoroughly mix. Analyze the top (organic) layer by TLC (Rf ≅ 0.2 (alcohol); 0.4 (ketone); 85:15 hexanes:ethyl acetate). Visualize the spots using 254 nm UV light and circle the spots with a pencil. In addition, you may use p-anisaldehyde reagent (“PAA”, a solution of anisaldehyde and sulfuric acid in ethanol) as the detection agent. Quickly immerse your TLC plate into the PAA stain and remove it; blot excess stain without touching the silica surface, then char on a hot plate until spots just become visible. Make a note of the color of the spots. Decant the liquid into a 250-mL Erlenmeyer flask; wash the carrot peels with an additional 25 mL of distilled water and combine with the liquid in the Erlenmeyer flask. Extract the aqueous solution with ethyl acetate (4 × 25 mL), dry the combined organics with anhydrous Na2SO4, decant into a 250-mL round-bottom flask and concentrate in vacuo. Purify the crude product by by column chromatography, using a Pasteur pipette halffilled with silica gel as the column. Elute your product with a mixture of 15 mL of hexanes and 2.5 mL of ethyl acetate, collecting fractions in test tubes (ca. 1 column volume each fraction). Pool the fractions that are pure in your product alcohol as judged by TLC, concentrate them in vacuo, and obtain the mass of product. Obtain an IR spectrum of your product. Use a polarimeter to find the optical rotation of your 1-benzofuran-2-yl-ethanol. Dissolve at least 30 mg of your compound in 12 mL of methanol (you may have to pool material with other students in order to have enough sample). Fill the polarimeter cell with your solution and obtain the optical rotation of your sample. Use the following formula to calculate the specific rotation for 1-benzofuran-2-yl-ethanol: [α]!" ! = 𝛼 𝑐∙𝑙 where α = measured optical rotation, c = sample concentration in g/mL, and l = the cell length in dm. Notes: 1) The Erlenmeyer must not be in contact with the stirrer plate, since heat development is detrimental to the reaction progress. The yield dramatically drops off when the temperature rises above 28 °C.