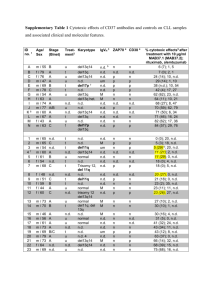
D-Hillarin, a Novel W180-Domain Protein, Affects Family Member Pnut
advertisement

D-Hillarin, a Novel W180-Domain Protein, Affects
Cytokinesis through Interaction with the Septin
Family Member Pnut
Yun Ji, Uttama Rath, Jack Girton, Kristen M. Johansen, Jørgen Johansen
Department of Biochemistry, Biophysics, and Molecular Biology, Iowa State University,
Ames, Iowa 50011
Received 11 September 2004; accepted 14 December 2004
ABSTRACT: By database searches of the
Drosophila genome project we have identified D-hil as
the fly member of a novel family of W180-domain
containing proteins. Immunocytochemistry demonstrated that D-hil is localized to the neuropil of the
embryonic CNS, to the cellular cortex of dividing
neuroblasts from larval brains, and that it is up-regulated in the cleavage furrow of S2 cells. We show that
D-hil distribution overlaps extensively with that of
the septin family member Pnut. Cross-immunoprecipitation experiments further indicated that the two
proteins may be members of the same protein com-
plex. Analysis of a severe hypomorphic P-element
mutation in the D-hil locus suggested that D-hil is a
nonessential protein. However, by creating double
mutant flies we show that the D-hil locus acts as a
modulator of Pnut function by increasing the level of
polyploidy of neuroblasts in PnutKG00478/PnutKG00478
larval brains. Based on these results we propose that
D-hil may function as a regulator of septin function
during cytokinesis in the developing nervous system. ' 2005 Wiley Periodicals, Inc. J Neurobiol 64: 157–169, 2005
Keywords: CNS; mitosis; S2 cells; LIM-domain;
Drosophila
INTRODUCTION
erate myopathy preceding kyphoscoliosis (Blanco
et al., 2001). Whereas the mouse protein is localized
in muscle and affects neuromuscular junctions
(Blanco et al., 2001), leech Hillarin is localized to the
axon hillock of CNS neurons (Ji et al., 2001). In nematode LTD-1 is found in the apical regions of the dorsal and ventral hypodermis (Vargas et al., 2002).
Furthermore, it is localized to the cleavage furrow
between mother and daughter cells in the hypodermal
syncytium, suggesting a role of LTD-1 in cell division (Vargas et al., 2002). Another potential member
of the W180-domain containing protein family implicated in cytokinesis is Cyk3 in yeast (Korinek et al.,
2000).
Here we report on the developmental expression,
localization, and possible function of D-hillarin
(D-hil), the only W180-domain containing protein
identified in database searches of the Drosophila
genome. We show that D-hil is expressed in the CNS
of Drosophila embryos and larvae as well as being
We recently characterized a nervous system specific
protein, Hillarin, in leech that is a member of a novel
family of proteins containing a W180 domain
(Ji et al., 2001). The W180 domain is defined by five
invariant tryptophans in addition to a number of other
highly conserved residues over a stretch of 180 amino
acids (Ji et al., 2001). Members of this family of proteins have been found in cyanobacteria (D90900),
lower invertebrates such as nematode (LTD-1) and
leech (Hillarin), as well as in vertebrates including
mouse (Ky) and humans (FLJ33207). In mouse,
mutations in this protein are responsible for a degenCorrespondence to: J. Johansen (jorgen@iastate.edu).
Contract grant sponsor: NIH; contract grant number: NS28857.
# 2005 Wiley Periodicals, Inc.
Published online 7 April 2005 in Wiley InterScience (www.
interscience.wiley.com).
DOI 10.1002/neu.20131
157
158
Ji et al.
localized to the cleavage furrow of dividing S2 cells.
Furthermore, we provide evidence from antibody
double labeling studies and coimmunoprecipitation
analysis that D-hil may be part of a complex with the
septin family member Pnut. Five septin family members have been identified in Drosophila: Pnut, Sep1,
Sep2, Sep4, and Sep5 (Neufeld and Rubin, 1994;
Fares et al., 1995; Field et al., 1996; Longtine et al.,
1996; Adam et al., 2000), among which Pnut is the
best characterized. Pnut was first identified as an
enhancer of sina (seven in absentia), which is essential for photoreceptor development (Carthew et al.,
1994; Neufeld and Rubin, 1994). However, Pnut has
a wide distribution pattern that includes the cleavage
furrow of dividing cells, the nervous system, the
developing photoreceptors, the advancing membrane
front of cellularizing embryos, and the ring canals of
testes (Neufeld and Rubin, 1994; Hime et al., 1996).
Pnut copurifies and colocalizes with Sep1 and Sep2,
suggesting that Drosophila septins function as a complex similar to the case for septin family members in
yeast (Neufeld and Rubin, 1994; Fares et al., 1995;
Field et al., 1996; Longtine et al., 1996; Adam et al.,
2000). Pnut loss of function mutations result in multinucleate cells in imaginal discs as well as in polyploid neuroblasts in larval brains, suggesting that
Pnut plays an important role in cytokinesis (Neufeld
and Rubin, 1994). We find that polyploidy of larval
neuroblasts is enhanced in D-hilKG03890 PnutKG00478/
D-hilKG03890 PnutKG00478 double mutants, suggesting
that D-hil may act as a modulator of septin function
during cytokinesis in the developing nervous system.
METHODS
Drosophila Stocks and Double Mutant
Generation
Fly stocks were maintained according to standard methods
(Roberts, 1986). Oregon-R or Canton-S were used for wildtype preparations. The y[1]; P{y[þmDint2] w[BR.E.BR]¼
SUPor-P}KG03890; ry[506] stock and the y[1]; P{y
[þmDint2] w[BR.E.BR]¼SUPor-P}pnut[KG00478]/SM6a
stock were obtained from the Bloomington Drosophila Stock
Center.
To generate a recombined chromosome carrying both
Pnut and D-hil mutations, female virgin flies of the Pnut
SUPor-P element insertion line, y[1]; P{y[þmDint2]
w[BR.E.BR]¼SUPor-P}pnut[KG00478]/SM6a, were crossed
with male flies of the D-hil SUPor-P element insertion line,
y[1]; P{y[þmDint2] w[BR.E.BR]¼SUPor-P}KG03890;
ry[506], with recombination occurring in the female progeny
(PnutKG00478/D-hilKG03890). Fifty single fly crosses were set
up in order to obtain enough independent recombined chro-
mosomes potentially carrying both PnutKG00478 and
D-hilKG0389. Homozygous double mutants of D-hilKG03890
and PnutKG00478 were identified by single fly PCR analysis
using primers to the end of the SUPor-P element and to the
genomic sequences flanking each P-element, respectively.
Lines with positive PCR results for both P element inserts
were selected and subsequently balanced with T(2,3)SM6aTM6B, which allows homozygous third instar mutant larvae
to be identified by their non-tubby phenotype.
Identification of D-hil, Sequence
Alignment, and Phylogenetic Tree
Construction
Homology searches of the Berkeley Drosophila Genome
Project with W180-domain sequence from leech Hillarin (Ji
et al., 2001) identified the Drosophila W180-domain containing protein, D-hil, which is coded for by the CG30147
locus. A full length EST (SD03168) obtained from the Berkeley Drosophila Genome Project was sequenced and used
to assemble the full-length D-hil coding sequence. The
D-hil sequence was compared with known and predicted
sequences using the National Center for Biotechnology
Information BLAST e-mail server. The sequence was
further analyzed using SMART (Simple Modular Architecture Research Tool; http://smart.embl-heidelberg.de/)
(Schultz et al., 1998) to predict the domain organization of
the protein. Homologous protein sequences were aligned
with the Clustalw version 1.7 program using default settings and initially encompassed the entire amino acid
sequences of the proteins. However, in the final analysis
any gaps in the resulting alignments were removed by
deleting residues corresponding to the gaps. Trees were
constructed by maximum parsimony using the PAUP computer program version 4.0b (Swofford, 1993) on a Power
Macintosh G4. All trees were generated by heuristic searches
and bootstrap values in percent of 1000 replications are indicated on the bootstrap majority rule consensus tree.
Antibodies
Residues 222–426 of the predicted D-hil protein were subcloned using standard techniques (Sambrook et al., 1989) into
the pGEX-4T-3 vector (Amersham Pharmacia Biotech) to
generate the construct GST-Dhil. The correct orientation
and reading frame of the insert was verified by sequencing.
GST-Dhil fusion protein was expressed in XL1-Blue cells
(Stratagene) and purified over a glutathione agarose column
(Sigma-Aldrich) according to the pGEX manufacturer’s
instructions (Amersham Pharmacia Biotech). The mAb 9E9
was generated by injection of 50 g of GST-Dhil into
BALB/c mice at 21 day intervals. After the third boost,
mouse spleen cells were fused with Sp2 myeloma cells and
monospecific hybridoma lines were established using standard procedures (Harlow and Lane, 1988). The mAb 9E9 is
of the IgG1 subtype. All procedures for mAb production
were performed by the Iowa State University Hybridoma
Facility. The Pnut mAb 4C9 (Neufeld and Rubin, 1994) and
D-Hillarin Interacts with Pnut
the Fasciclin II mAb 1D4 were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa.
The Pnut polyclonal antibody KEKK was a generous gift
from Dr. C.M. Field (Harvard Medical School). The anti-tubulin mAb (IgG1), anti--tubulin mAb (IgM), and antiGFP polyclonal antibody were obtained from commercial
sources (Sigma, AbCAM, and Molecular Probes, respectively). The Chromator mAb 6H11 has been previously
described (Rath et al., 2004).
Biochemical Analysis
SDS-PAGE and Immunoblotting. SDS-PAGE was performed according to standard procedures (Laemmli, 1970).
Electroblot transfer was performed as in Towbin et al.
(1979) with transfer buffer containing 20% methanol and in
most cases including 0.04% SDS. For these experiments we
used the Bio-Rad Mini PROTEAN II system, electroblotting to 0.2 m nitrocellulose, and using antimouse HRPconjugated secondary antibody (1:3000; Bio-Rad) for visualization of primary antibody diluted 1:1000 in Blotto. The
signal was visualized using chemiluminescent detection
methods (ECL kit; Amersham). The immunoblots were
digitized using a flatbed scanner (Epson Expression 1680).
For quantification of immunolabeling, digital images of
exposures of immunoblots on Biomax ML film (Kodak)
were analyzed using the ImageJ software as previously
described (Wang et al., 2001). In these images the grayscale
was adjusted such that only a few pixels in the wild-type
lanes were saturated. The area of each band was traced
using the outline tool and the average pixel value determined. Levels in mutant larvae and flies were determined
as a percentage relative to the level determined for wildtype controls using tubulin or Chromator levels as a loading
control. Protein extracts were prepared from S2 cells or
staged dechorionated embryos, larvae, pupae, or adults that
were homogenized in immunoprecipitation (ip) buffer
(20 mM Tris-HCl, 150 mM NaCl, 10 mM EDTA, 1 mM
EGTA, 0.1% Triton X-100, 0.1% NP-40, 2 mM Na3VO4,
pH 8.0) with added protease inhibitors 1.5 g/mL aprotinin
and 1 mM PMSF (Sigma).
Immunoprecipitation Assays. For coimmunoprecipitation
experiments, anti-D-hil, anti-Pnut, or control antibodies
were coupled to protein G beads (Sigma) as follows: 1 mL
of mAb 4C9 hybridoma supernatant, 20 L of mAb 9E9
ascites, or 10 L of control ascites (Chromator mAb 6H11)
was coupled to 10 L of protein G beads (Amersham Biosciences) for 2 h at 48C on a rotating wheel in 200 L ip
buffer. Lysate from S2 cells (107 cells/mL in ip buffer) or
dissected third instar larval brains was precleared with
20 L protein G beads coupled with 10 L normal mouse
serum at 48C for 2 h. Subsequently, the appropriate antibody-coupled beads and 200 L precleared lysate were
combined and incubated overnight at 48C on a rotating
wheel. Beads were washed three times for 10 min each with
1 mL of ip buffer with low speed pelleting of beads
between washes. The resulting bead-bound immunocom-
159
plexes were analyzed by SDS-PAGE and Western blotting
according to standard techniques (Harlow and Lane, 1988)
using mAb 9E9 to detect D-hil and mAb 4C9 to detect
Pnut.
Immunohistochemistry
Antibody labelings of 0–18 h embryos were performed as
previously described (Johansen et al., 1996; Johansen and
Johansen, 2003). The embryos were dechorionated in a
50% Clorox solution, washed with 0.7 M NaCl/0.2% Triton
X-100, and fixed in a 1:1 heptane:fixative mixture for
20 min with vigorous shaking at room temperature. The fixative was either 4% paraformaldehyde in phosphate buffered saline (PBS) or Bouin’s fluid (0.66% picric acid,
9.5% formalin, 4.7% acetic acid). Vitelline membranes
were then removed by shaking embryos in heptane-methanol (Mitchison and Sedat, 1983) at room temperature for 30
s. S2 cells were affixed onto poly-L-lysine coated coverslips
and fixed with Bouin’s fluid for 10 min at 248C and methanol for 5 min at 208C. The cells on the coverslips were
permeabilized with PBS containing 0.5% Triton X-100 and
incubated with diluted primary antibody in PBS containing
0.1% Triton X-100, 0.1% sodium azide, and 1% normal
goat serum for 1.5 h. Double and triple labelings employing
epifluorescence were performed using various combinations
of antibodies against D-hil (mAb 9E9, IgG1), Pnut (mAb
4C9, IgG1, or the KEKK polyclonal serum), anti--tubulin
mouse IgG1 (Sigma) or IgM antibody (AbCAM), GFP-antibody (rabbit polyclonal serum), and Hoechst to visualize
the DNA. The appropriate TRITC- and FITC-conjugated
secondary antibodies (Cappel/ICN) were used (1:200 dilution) to visualize primary antibody labeling. Confocal
microscopy was performed with a Leica confocal TCS NT
microscope system equipped with separate argon-UV,
argon, and krypton lasers and the appropriate filter sets for
Hoechst, FITC, and TRITC imaging. A separate series of
confocal images for each fluorophor of double labeled preparations was obtained simultaneously with z-intervals of
typically 0.5 m using a PL APO 100X/1.40-0.70 oil objective. A maximum projection image for each of the image
stacks was obtained using the ImageJ software. In some
cases individual slices or projection images from only two
to three slices were obtained. Images were imported into
Photoshop where they were pseudocolored, image processed, and merged.
For light microscopy primary antibody labeled
embryos or dissected third instar larvae were incubated
with HRP-conjugated goat antimouse secondary antibody
(1:200; Bio-Rad) at room temperature for 2.5 h, and
washed with PBST followed by a PBS-only wash. The
preparations were then incubated in PBS with 0.2 mg/mL
DAB (Pierce) and 0.03% H2O2 (Sigma) at room temperature for 10 min, whereafter the reaction was stopped by
washing with PBS. The final preparations were mounted
in glycerol and viewed with a 20X objective on a Zeiss
Axioskop. Digital images were obtained using a Spot
camera (Diagnostic Instruments).
160
Ji et al.
Figure 1 Amino acid sequence of D-hil and comparison to other W180-domain containing proteins. (A) The complete predicted amino acid sequence of D-hil, which contains a LIM-domain
(underlined), a W180-domain (grey box), and an H-domain (white box). (B) Domain structure of
D-hil compared to other W180-domain containing proteins from other organisms. Sequence identity on the amino acid level between the various domains is indicated in percent. (C) Phylogenetic
relationship of D-hil with other W180-domain containing proteins. The consensus maximum parsimony tree was derived from an alignment with all gaps removed. The tree is unrooted and is
depicted with the associated bootstrap support values from 1000 iterations.
For larval brain preparations third instar larval brains
were dissected in physiological insect saline solution,
rinsed in PBS, and treated with 50 M colchicine (Sigma)
at room temperature for 1.5 h. The tissues were then treated
with a hypotonic solution (0.5% sodium citrate in H2O) for
10 min, fixed by 45% acetic acid for 8 min, and then affixed
to slides and squashed by standard methods (Sullivan et al.,
2000). The resulting slides were stained by Hoechst
(0.2 g/mL in PBS) for 2 min and mounted in 90% glycerol
containing 0.5% n-propyl gallate. The number of polyploid
and mitotic cells from at least 10 individual brain squashes
were scored blind for the double mutant as well as for each
individual mutant line. For antibody labelings the brains
were fixed with 4% PFA for 30 min, postfixed for 3 min in
45% acetic acid, and subsequently squashed in 60% acetic
acid without colchicine treatment following the protocol of
Bonaccorsi et al. (1999).
Expression of GFP-D-hil in Transfected
S2 Cells
Full-length D-hil was amplified from D-hil cDNA clone
SD03168 by PCR and cloned into the pMT/V5-HisB vector
(Invitrogen) with a SuperGFP tag in-frame at the NH2-terminus and a V5 tag in-frame at the COOH-terminus. The
construct was sequenced at the Iowa State University
Sequencing Facility to confirm its fidelity. S2 cells in the
D-Hillarin Interacts with Pnut
161
Figure 2 Alignment of the W180-domain (A) and the H-domain (B) in D-hil with the corresponding sequences in potential orthologues from human, mosquito, nematode, leech, cyanobacteria, and yeast. Identical residues are indicated in white typeface outlined in black whereas
conservative amino acid substitutions are indicated by black typeface outlined in grey. The five
conserved tryptophans defining the W180-domain are indicated by asterisks (A).
log growth phase were transfected with SuperGFP-D-hil
plasmid using a calcium phosphate transfection kit (Invitrogen). Stable lines were established by cotransfection with
pCoHYGRO (Invitrogen) to confer hygromycin resistance.
Stable lines were maintained with 300 g/mL hygromycin
(Invitrogen) in the culture medium. The expression of GFPD-hil was induced with 0.5 mM CuSO4 for 24 h. Cells were
cytospun onto poly-L-lysine coated coverslips in a 24-well
plate and processed for immunocytochemistry or extracted
for immunoblot analysis as described above.
162
Ji et al.
Figure 3 Localization and developmental expression of D-hil. (A) and (B) Labelings of 12–18 h
Drosophila embryos with the D-hil specific mAb 9E9 show that D-hil protein localizes to axons
(arrows) within the CNS. The labeling was visualized with HRP-conjugated secondary antibody. A
lateral view is shown in (A) whereas (B) is a ventral view. (C) Protein extracts from selected stages
of Drosophila development were fractionated by SDS-PAGE, immunoblotted, and labeled with the
D-hil specific mAb 9E9 (upper bands) and with tubulin antibody as a loading control (lower bands).
D-hil migrates as a 97 kD protein and its expression is strongly up-regulated in 12–24 h embryos and
persists during larval, pupal, and adult stages. However, faint traces of D-hil protein can be detected
at early embryonic stages as well (0–2 and 2–6 h). Tubulin (Tub) migrates as a 66 kD protein.
Analysis of D-hil SUPor-P Element
Mutants
Mapping of the SUPor-P Element Insertion Site. Genomic DNA flanking the SUPor-P element KG03890 was
amplified by the single fly PCR technique (Preston and
Engels, 1996) using primers, of which one is within the end
of the SUPor-P element and the other is within the nearby
genomic region. PCR products were purified using the PCR
Purification Kit (Qiagen) followed by direct sequencing.
Sequences were aligned with fly genomic sequence to map
the insertion site.
Hatch Rate Assays. The viability of the SUPor-P element
insertion line was tested by comparing the hatch rates of
progeny from y[1]; P{y[þmDint2] w[BR.E.BR]¼SUPorP}KG03890; ry[506] or Oregon-R flies. Eggs were collected on yeasted agar plates and kept at room temperature
for 48 h. Hatching viability was measured by comparing
the number of unhatched fertilized eggs with the total number of fertilized eggs.
RESULTS
Identification and Phylogenetic Analysis
of D-hil
In order to identify W180-domain (Ji et al., 2001)
containing proteins in Drosophila we searched the
Drosophila genome databases with leech Hillarin
W180 domain sequences. We identified one such protein corresponding to the CG30147 locus, which we
have named D-hil. We obtained and sequenced an
EST (SD03168) from this locus, which contained the
complete 818 amino acid sequence of the gene
[Fig. 1(A)]. The predicted molecular mass of D-hil is
94 kD and it contains an NH2-terminal LIM-domain,
a W180 domain, and an H domain. The W180 and H
domains were first described in leech Hillarin
(Ji et al., 2001), and an alignment of known and predicted sequences from proteins of other organisms
containing these domains are shown in Figure 2. The
W180 domain is characterized by five tryptophans in
invariant positions [Fig. 2(A), asterisks] that are conserved in proteins from cyanobacteria to humans.
Yeast Cyk3 contains a somewhat divergent W180
domain where only two of the tryptophans are conserved. The level of amino acid identity among the H
domains from higher eukaryotes is less extensive than
for the W180 domains, and the presence of an H
domain cannot be conclusively verified in cyanobacteria and yeast [Fig. 2(B)]. The domain structure of
D-hil is most closely related to nematode LTD-1, as
they are the only two proteins with NH2-terminal
LIM-domains [Fig. 2(B)]. Yeast Cyk3 has an NH2terminal SH3 domain and leech Hillarin has a cassette of W180 and H domains repeated twice
[Fig. 2(B)]. The organization of leech Hillarin is
likely to have been derived from a duplication event
D-Hillarin Interacts with Pnut
163
Figure 4 Localization and expression of D-hil in S2 cells. (A) Confocal section of S2 cells
labeled with the D-hil specific mAb 9E9. D-hil (green) is localized to the cortex of the cellular rim.
(B) Confocal section of an S2 cell at metaphase double labeled with D-hil mAb 9E9 (green) and
with tubulin antibody (red). (C) Confocal section of an S2 cell at telophase double labeled with Dhil mAb 9E9 (green) and with tubulin antibody (red). During cytokinesis D-hil expression is concentrated at the cleavage furrow (arrow). (D) Confocal section of an S2 cell transfected with a
GFP-tagged D-hil expression construct (GFP-D-hil) and labeled with an anti-GFP polyclonal antibody (green). Even when overexpressed the GFP-D-hil construct localizes to the cortex of the cellular rim. (E) Immunoblot of protein extract from S2 cells overexpressing the GFP-D-hil construct
labeled with mAb 9E9. Native D-hil is recognized as a 97 kD band whereas the GFP-D-hil expression construct due to the GFP and V5 tags migrates as a 123 kD protein. [Color scheme can be
viewed in the online issue, which is available at http://www.interscience.wiley.com]
of an ancestral protein with single cassette structure
(Ji et al., 2001). The evolutionary relationship
between the W180-domain containing proteins is
illustrated in Figure 1(C), where nematode LTD-1
and D-hil form a clade with 100% bootstrap support.
Localization and Developmental
Expression of D-hil
In order to determine the localization and developmental expression of D-hil we generated a monoclo-
nal antibody (mAb 9E9) against a D-hil fusion
protein-containing sequence from amino acid 222–
426. Labeling with mAb 9E9 of 0–18 h Drosophila
embryos revealed that D-hil is localized to the neuropil of the CNS [Fig. 3(A,B)]. On immunoblots of protein extracts from different stages of development
D-hil expression is up-regulated after 12 h of embryogenesis and persists into adult stages [Fig. 3(C)].
D-hil is recognized by mAb 9E9 as a 97 kD band,
which is close to the predicted molecular mass of
D-hil of 94 kD. Occasionally lower bands were also
observed; however, they are likely to represent degra-
164
Ji et al.
dation fragments. The 12–24 h window for up-regulation of D-hil expression correlates well with the onset
of nervous system development, which commences at
about 12 h of embryogenesis. However, it should be
noted that very low levels of D-hil can be detected on
immunoblots from 0–6 h embryos, possibly representing maternal product and indicating a putative
early embryonic role for D-hil.
In S2 cells D-hil is localized to the rim of the cellular cortex. This is illustrated in Figure 4(A), which
shows a confocal section of S2 cells labeled with
mAb 9E9. At metaphase D-hil is still localized to the
cellular cortex [Fig. 4(B)]; however, at telophase
D-hil is concentrated at the cleavage furrow [Fig.
4(C), arrow]. To further verify the localization of
D-hil we generated an S2 cell line stably transfected
with a full length D-hil construct with an in-frame
GFP-tag at the NH2-terminus (GFP-D-hil). As shown
in Figure 4(D) this construct also localized to the rim
of the cell cortex. To test whether the GFP-D-hil construct would have a dominant negative effect on S2
cell morphology or cell division we overexpressed
the construct. However, even in S2 cell cultures
where GFP-D-hil was overexpressed by an order of
magnitude [Fig. 4(E)] no obvious phenotypes were
observed.
D-hil Interacts with the Septin Family
Member Pnut
The distribution pattern of D-hil in the embryonic
CNS and in the cleavage furrow of S2 cells is reminiscent of the Drosophila septin family member Pnut
(Neufeld and Rubin, 1994), suggesting a possible
interaction between these proteins. We therefore double labeled S2 cells with the D-hil mAb 9E9 and the
Pnut polyclonal antibody KEKK (Field et al., 1996).
In such double labelings D-hil and Pnut staining was
found to overlap at the cell cortex as indicated by the
yellow color in the composite image of Figure 5(A).
To further explore the possible interaction between
D-hil and Pnut we performed coimmunoprecipitation
experiments. For these experiments proteins were
extracted from S2 cell lysate, immunoprecipitated
using either D-hil mAb 9E9 or Pnut mAb 4C9 (Neufeld and Rubin, 1994), fractionated on SDS-PAGE
after the immunoprecipitation, immunoblotted, and
probed with antibodies to Pnut and D-hil, respectively. Figure 5(B) shows that the D-hil mAb 9E9
pulled down Pnut protein as a 60 kDa band also
detected in the S2 cell lysate and not present in the
control immunoprecipitated with immunobeads
coupled to the Chromator mAb 6H11. Figure 5(C)
Figure 5 Colocalization and cross-immunoprecipitation
experiments with D-hil and Pnut antibodies. (A) Confocal
section of an S2 cell double labeled with D-hil mAb 9E9
(green) and the Pnut polyclonal antiserum KEKK (red).
Both D-hil and Pnut localization show extensive overlap at
the cellular cortex as indicated by the yellow color in the
composite image (comp). (B) Immunoprecipitation (ip) of
lysates from S2 cells was performed using D-hil antibody
(mAb 9E9, lane 1) coupled to immunobeads or with immunobeads coupled to a control antibody (Chromator mAb
6H11, lane 3). The immunoprecipitations were analyzed by
SDS-PAGE and Western blotting using Pnut mAb 4C9 for
detection. Pnut antibody staining of S2 cell lysate is shown
in lane 2. Pnut is detected in the D-hil immunoprecipitation
sample and in the lysate as a 60 kD band (lane 1 and 2,
respectively) but not in the control sample (lane 3). (C)
Immunoprecipitation (ip) of lysates from S2 cells was performed using Pnut antibody (mAb 4C9, lane 1) coupled to
immunobeads or with immunobeads coupled to a control
antibody (Chromator mAb 6H11, lane 3). The immunoprecipitations were analyzed by SDS-PAGE and Western blotting using D-hil mAb 9E9 for detection. D-hil antibody
staining of S2 cell lysate is shown in lane 2. D-hil is
detected in the Pnut immunoprecipitation sample and in the
lysate as a 97 kD band (lane 1 and 2, respectively) but not
in the control sample (lane 3). [Color scheme can be viewed
in the online issue, which is available at http://www.
interscience.wiley.com]
shows the converse experiment where Pnut mAb 4C9
pulled down a 97 kDa band detected by D-hil mAb
9E9, which was also present in the cell lysate but not
in the control immunoprecipitated with Chromator
mAb 6H11. These results indicate that D-hil and Pnut
are present in the same protein complex.
D-Hillarin Interacts with Pnut
165
Figure 6 P-element insertion in the D-hil gene. (A) Diagram of the D-hil genomic locus. The
locus has nine exons separated by eight introns. The P-element insertion site of line KG03890 14
bp upstream of the D-hil transcription start site is indicated by the triangle. The ORF coding for the
D-hil protein including the position of the LIM-, W180-, and H-domains is depicted underneath.
(B) D-hil protein expression in homozygous KG03890 mutant flies as compared to wild-type flies.
The immunoblots were labeled with the D-hil mAb 9E9 and with antitubulin antibody (Tub) as a
loading control. D-hil is detected as a 97 kD protein by mAb 9E9. The level of D-hil protein in
adult homozygous KG03890 mutant flies is approximately 4% that of the level in wild-type flies.
Characterization of Hypomorphic
P-Element Mutation in the D-hil Locus
A SUPor-P element (Roseman et al., 1995) inserted
in the 50 D-hil genomic region was obtained from the
Bloomington Drosophila Stock Center. The P-element (D-hilKG03890) is inserted 14 base pairs upstream
of the transcription start site of D-hil [Fig. 6(A)] as
determined by PCR amplification of flanking
sequence of the SUPor-P element followed by direct
sequencing. Homozygous mutants are viable with
approximately a 98.4% hatch rate, which is very close
to the hatch rate of Oregon-R wild-type flies (data not
shown). However, the level of D-hil protein in homozygous adult D-hilKG03890 flies is only 3.9 6 1.3%
(n ¼ 5) that of wild-type levels as determined by
immunoblot analysis [Fig. 6(B)]. Phenotypically
homozygous mutant animals have no apparent
defects in their CNS as determined by FasII antibody
labeling of 12–18 h embryo collections from homozygous D-hilKG3890 parents, and they behave normally (data not shown). These data suggest that D-hil
is a nonessential gene.
D-hil Genetically Interacts with Pnut
To further characterize the interaction between D-hil
and Pnut in vivo, we did genetic interaction studies
between mutant alleles of D-hil and Pnut by generating double mutant individuals homozygous for both
D-hilKG03890 and the Pnut allele PnutKG00478, which
was obtained from the Bloomington Drosophila
Stock Center. By PCR amplification of the flanking
region of PnutKG00478 followed by direct sequencing,
the KG00478 SUPor-P element was found to be
inserted 67 base pairs downstream of the starting
methionine of Pnut, decreasing the expression level
166
Ji et al.
Figure 7 Genetic interaction between D-hilKG03890 and
PnutKG00478. (A) Pnut protein expression in homozygous
KG00478 mutant third instar larvae as compared to wildtype third instar larvae. The immunoblots were labeled with
the Pnut KEKK antiserum and with the Chromator antibody
mAb 6H11 (Chro) as a loading control. Pnut is detected as
a 60 kD protein by the KEKK antiserum and Chromator as
a 130 kD protein by mAb 6H11. The level of Pnut protein
in adult homozygous KG03890 mutant larvae is less than
1% that of the level in wild-type larvae. (B) D-hil and Pnut
protein
expression
in
homozygous
D-hilKG03890
KG00478
KG03890
KG00478
Pnut
/D-hil
Pnut
double mutant third
instar larvae as compared to wild-type third instar larvae.
The immunoblots were labeled with the Pnut KEKK antiserum, the D-hil mAb 9E9, and with the Chromator antibody
mAb 6H11 (Chro) as a loading control. Pnut is detected as
a 60 kD protein by the KEKK antiserum, D-hil as a 97 kD
protein by mAb 9E9, and Chromator as a 130 kD protein by
mAb 6H11. The level of D-hil and Pnut protein in homozygous double mutant larvae was severely reduced. (C) Histograms of the average ratio with standard deviation of
polyploid neuroblast cells from D-hilKG03890/D-hilKG03890
(0.0 6 0.0; n ¼ 11), PnutKG00478/PnutKG00478 (0.062 6
0.023; n ¼ 12), and D-hilKG03890 PnutKG00478/D-hilKG03890
PnutKG00478 (0.100 6 0.026; n ¼ 13) third instar larvae
brain squash preparations. The numbers of polyploid neuroblast cells at metaphase found among the total number of
neuroblasts in metaphase examined is indicated at the bottom of the histograms. No polyploid neuroblasts were found
among 1109 neuroblasts examined in homozygous DhilKG03890 third instar larvae. However, the presence of the
of Pnut protein to less than 1% of wild-type
[Fig. 7(A)]. Because Pnut and D-hil both are located
on the second chromosome we generated five independent homozygous double mutant lines by recombining both mutations onto the same chromosome.
While D-hil homozygous mutants and D-hilKG03890/
PnutKG00478 animals are viable, both PnutKG00478
homozygous
and
D-hilKG03890
PnutKG00478/
KG03890
KG00478
D-hil
Pnut
double mutants die at the
larval/pupal transition stage. However, because it has
been reported that homozygous Pnut null mutants
show increased polyploidy of neuroblast cells in third
instar larval brains (Neufeld and Rubin, 1994;
Somma et al., 2002), the late lethality allowed us to
examine whether this Pnut phenotype would be
affected by the D-hil mutation. PnutKG00478 single
mutants and D-hilKG03890 PnutKG00478 double mutants
are both balanced by T(2,3)SM6a-TM6B, wherefore
the homozygous third instar larvae double mutants
can be identified by their non-tubby phenotype.
Immunoblots of larval protein extracts verified that
both Pnut and D-hil protein levels were greatly
reduced in the homozygous double mutants
[Fig. 7(B)]. We found no obvious developmental
delay or lethality stage change of the D-hilKG03890
PnutKG00478/D-hilKG03890 PnutKG00478 double mutants
compared with the PnutKG00478 single mutant (data not
shown). However, while we did not observe any polyploid cells in third instar larval brains of homozygous
D-hilKG03890 mutants (0/1109), we found a 9.9%
percentage of polyploid neuroblasts (127/1284) in
D-hilKG03890 PnutKG00478/D-hilKG03890 PnutKG00478
double mutants, nearly double the percentage of 5.7%
(71/1251) in the homozygous PnutKG00478 single
mutant [Fig. 7(C)]. The percentage of polyploid neuroblasts was determined in squashes of larval brains
where the ploidy of dividing neuroblasts can be readily ascertained. Examples of polyploid neuroblasts
from
D-hilKG03890
PnutKG00478/D-hilKG03890
KG00478
Pnut
double mutants are shown in Figure 7(D).
The difference in percentage of polyploid cells between
the homozygous Pnut single mutant and D-hilKG03890
PnutKG00478/D-hilKG03890 PnutKG00478 double mutants
homozygous D-hilKG03890 allele increased the number of
polyploid neuroblast cells in homozygous PnutKG00478 third
instar larvae almost twofold. This difference was statistically significant (p < 0.0005, 2 test and p < 0.001, Student’s two-tailed t test). (D) Hoechst labeling of neuroblast
chromosomes from brain squashes from D-hilKG03890
PnutKG00478/D-hilKG03890 PnutKG00478 third instar larvae.
A1 shows a normal diploid cell, A2 a tetraploid cell, and
A3 an octaploid cell.
D-Hillarin Interacts with Pnut
167
both D-hil and Pnut protein are present in the CNS at
this developmental stage. Furthermore, we labeled
neuroblasts from squashes of third instar larval brains
with D-hil antibody. As in S2 cells, D-hil was localized to the rim of the cellular cortex as well as to the
cleavage furrow of dividing neuroblasts [Fig.
8(B,C)]. Figure 8(D) shows an immunoprecipitation
experiment with protein extracts from dissected third
instar larval brains where the D-hil mAb 9E9 pulled
down Pnut protein as a 60 kDa band also detected in
brain lysate but not in the beads-only control lane.
DISCUSSION
Figure 8 Localization and expression of D-hil in third
instar larval brains. (A) Immunoblot of protein extracts
from dissected larval brains. The immunoblots were labeled
with the Pnut mAb 4C9, the D-hil mAb 9E9, and with the
Chromator antibody mAb 6H11 (Chro) as a loading control.
Pnut is detected as a 60 kD protein by mAb 4C9, D-hil as a
97 kD protein by mAb 9E9, and Chromator as a 130 kD
protein by mAb 6H11. The migration of molecular weight
markers is indicated to the right. (B) Dividing larval neuroblast at anaphase labeled with D-hil mAb 9E9 (green) and
Hoechst (red). (C) Asymmetrically dividing larval neuroblast labeled with D-hil mAb 9E9. The arrow indicates the
cleavage furrow. (D) Immunoprecipitation (ip) of lysates
from dissected third instar larval brains was performed
using D-hil antibody (mAb 9E9, lane 3) coupled to immunobeads or with immunobeads only (lane 2). In addition,
lane 1 shows Pnut protein detected in brain lysate. The
immunoprecipitations were analyzed by SDS-PAGE and
Western blotting using the Pnut KEKK antiserum for detection. [Color scheme can be viewed in the online issue,
which is available at http://www.interscience.wiley.com]
is statistically significant (p < 0.0005, 2 test). These
findings strongly suggest that D-hilKG03890 genetically
interacts with PnutKG00478 and that D-hilKG03890 functions as an enhancer of the phenotype of PnutKG00478.
In order to verify that D-hil is expressed in larval
brain tissue we assayed protein extracts from dissected third instar larval brains by SDS-PAGE and
immunoblot analysis. Figure 8(A) demonstrates that
In this study we report the identification and characterization of D-hil, a novel member of the family of
W180-domain containing proteins (Ji et al., 2001).
W180-domain containing proteins have been identified in a variety of organisms including cyanobacteria, leech, nematode, fly, mouse, and humans. The
W180-domain is defined by five invariant tryptophans
and other conserved residues over a stretch of 180
amino acids (Ji et al., 2001). A limited region of this
domain has been reported to have similarity to the
catalytic domain of animal transglutaminases
(Makarova et al., 1999; Blanco et al., 2001). However, in a number of W180-domain proteins, including D-hil and LTD-1, several key residues for
transglutaminase activity have not been conserved,
making it unlikely that they posses this enzymatic
function (Makarova et al., 1999; Blanco et al., 2001).
This suggests that W180-domain proteins, while containing a structural protein fold similar to active
transglutaminases, nonetheless exhibit different functional properties from this group of proteins.
Antibody labeling studies demonstrated that D-hil
is expressed in the developing CNS, in larval neuroblasts, as well as in the rim of the cellular cortex in
S2 cells. During mitosis of S2 cells D-hil accumulated at the cleavage furrow suggesting a role in cell
division. However, D-hil appears to be a nonessential
gene. We identified a severe hypomorphic P-element
mutation in the D-hil locus that reduced protein levels
to less than 4% of wild-type. Nonetheless, this mutation could be maintained as a homozygous viable
stock with normal hatching rates and no obvious CNS
defects. A caveat is that it is still possible that this
low level of D-hil protein may be sufficient to maintain essential functions.
We further present evidence that the Drosophila
septin family member Pnut is a likely interacting
partner of D-hil. Pnut is important for cytokinesis in
many cell types (Neufeld and Rubin, 1994; Somma
168
Ji et al.
et al., 2002) and has a broad expression profile (Neufeld and Rubin, 1994; Fares et al., 1995). However,
the distribution pattern of Pnut protein in the cleavage
furrow of dividing S2 cells and the pattern in the
CNS are quite similar to the pattern of D-hil, suggesting that they may associate with each other at these
locations. This hypothesis was supported by coimmunoprecipitation experiments that provided evidence
for a molecular interaction between D-hil and Pnut.
Furthermore, direct double labeling experiments in
S2 cells indicated that the distribution of D-hil and
Pnut in these cells largely overlaps although their distributions are not identical. To further document this
interaction we conducted genetic experiments. Our
data indicated that there is a genetic interaction
between the mutant Pnut allele KG00478 and the
mutant D-hil allele KG03890. Whereas approximately 6% of the cells were polyploid in neuroblast
squashes from homozygous PnutKG00478 brains, in
homozygous double mutants of D-hilKG03890 and
PnutKG00478 this ratio almost doubled. This suggests
that loss of D-hil increases the severity of cytokinesis
defects in Pnut mutants and that D-hil acts as a modifier of Pnut during the process of neuroblasts division. This genetic result further supports the
interaction between D-hil and Pnut. However, the relatively modest increase in the polyploid ratio in
D-hilKG03890 PnutKG00478/D-hilKG03890 PnutKG00478
double mutants compared to homozygous Pnut single
mutants suggests that D-hil is not a major determining factor in the cytokinetic process in which Pnut is
involved. Although Pnut is an essential gene (Neufeld
and Rubin, 1994) the mechanism of its functions in
cytokinesis is still not clear. Drosophila has five
known septins that share high sequence homology
(Field et al., 1996; Adam et al., 2000). Thus the
depletion of Pnut may be compensated for by other
septins as is the case in mammals (Peng et al., 2002).
At present we do not know whether the interaction
between D-hil and Pnut is direct or mediated by other
proteins, and it is possible that D-hil may associate
with other members of the septin complex rather than
interact with Pnut alone. The loss of D-hil in the Pnut
mutant background enhances the mitotic defects but
does not completely eliminate the pathway, suggesting overlapping and partially redundant functions of
the members of the complex. Thus, it will be informative in future experiments to further explore the
relationship among D-hil, Pnut, and other Drosophila
septins in order to clarify their respective contributions to the process of cytokinesis.
We thank members of the laboratory for discussion and
critical reading of the manuscript. We also wish to
acknowledge Ms. V. Lephart for maintenance of fly stocks.
We thank L. Ambrosio and the Bloomington Stock Center
for providing fly stocks, Dr. C.M. Field for generously providing the KEKK polyclonal antiserum, and the Developmental Studies Hybridoma Bank at the University of Iowa
for providing the Pnut (deposited by Dr. G.M. Rubin) and
Fasciclin II (deposited by Dr. C. Goodman) monoclonal
antibodies.
REFERENCES
Adam JC, Pringle JR, Peifer M. 2000. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell 11:3123–3135.
Blanco G, Coulton GR, Biggin A, Grainge C, Moss J,
Barrett M, Berquin A, Marechal G, Skynner M, van Mier
P, et al. 2001. The kyphoscoliosis (ky) mouse is deficient
in hypertrophic responses and is caused by a mutation in
a novel muscle-specific protein. Hum Mol Genet 10:
9–16.
Bonaccorsi S, Giansanti MG, Gatti M. 1999. Spindle
assembly in Drosophila neuroblasts and ganglion mother
cells. Nature Cell Biol 2:54–56.
Carthew RW, Neufeld TP, Rubin GM. 1994. Identification
of genes that interact with the sina gene in Drosophila
eye development. Proc Natl Acad Sci USA 91:11689–
11693.
Fares H, Peifer M, Pringle JR. 1995. Localization and possible functions of Drosophila septins. Mol Biol Cell
6:1843–1859.
Field CM, al-Awar O, Rosenblatt J, Wong ML, Alberts B,
Mitchison TJ. 1996. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell
Biol 133:605–616.
Harlow E, Lane E. 1988. Antibodies: A Laboratory Manual.
Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.726 pp.
Hime GR, Brill JA, Fuller MT. 1996. Assembly of ring
canals in the male germ line from structural components
of the contractile ring. J Cell Sci 10:2779–2788.
Ji Y, Schroeder D, Byrne D, Zipser B, Jellies J, Johansen
KM, Johansen J. 2001. Molecular identification and
sequence analysis of Hillarin, a novel protein localized at
the axon hillock. Biochim Biophys Acta 1519:246–249.
Johansen KM, Johansen J. 2003. Studying nuclear organization in embryos using antibody tools. In: Henderson
DS, editor. Drosophila Cytogenetics Protocols. Totowa,
NJ: Humana Press, p 215–234.
Johansen KM, Johansen J, Baek K-H, Jin Y. 1996. Remodeling of nuclear architecture during the cell cycle in Drosophila embryos. J Cell Biochem 63:268–279.
Korinek WS, Bi E, Epp JA, Wang L, Ho J, Chant J. 2000.
Cyk3, a novel SH3-domain protein, affects cytokinesis
in yeast. Curr Biol 10:947–950.
Laemmli UK. 1970. Cleavage of structural proteins during
assembly of the head of bacteriophage T4. Nature
227:680–685.
D-Hillarin Interacts with Pnut
Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS,
Fares H, De Virgilio C, Pringle JR. 1996. The septins:
roles in cytokinesis and other processes. Curr Opin Cell
Biol 8:106–119.
Makarova KS, Aravind L, Koonin EV. 1999. A superfamily of archaeal, bacterial, and eukaryotic proteins
homologous to animal transglutaminases. Prot Sci 8:
1714–1719.
Mitchison TJ, Sedat J. 1983. Localization of antigenic
determinants in whole Drosophila embryos. Dev Biol
99:261–264.
Neufeld TP, Rubin GM. 1994. The Drosophila peanut gene
is required for cytokinesis and encodes a protein similar
to yeast putative bud neck filament proteins. Cell
77:371–379.
Peng XR, Jia Z, Zhang Y, Ware J, Trimble WS. 2002.
The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol Cell Biol 22:
378–387.
Preston CR, Engels WR. 1996. P-element induced male
recombination and gene conversion in Drosophila.
Genetics 144:1611–1622.
Rath U, Wang D, Ding Y, Xu Y-Z, Qi H, Blacketer MJ,
Girton J, Johansen J, Johansen KM. 2004. Chromator, a
novel and essential chromodomain protein interacts
directly with the putative spindle matrix protein Skeletor.
J Cell Biochem 93:1033–1047.
Roberts DB. 1986. Drosophila: A Practical Approach.
Oxford, UK: IRL Press. 295 p.
Roseman RR, Johnson EA, Rodesch CK, Bjerke M,
Nagoshi RN, Geyer PK. 1995. A P element containing
169
suppressor of hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster.
Genetics 141:1061–1074.
Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY:
Cold Spring Harbor Laboratory Press. 545 p.
Schultz J, Milpetz F, Bork P, Ponting CP. 1998. SMART, a
simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA
95:5857–5864.
Somma MP, Fasulo B, Cenci G, Cundari E, Gatti M. 2002.
Molecular dissection of cytokinesis by RNA interference
in Drosophila cultured cells. Mol Biol Cell 13:2448–2460.
Sullivan W, Ashburner M, Hawley RS. 2000. Drosophila
Protocols. Cold Spring Harbor, NY: Cold Spring Harbor
Laboratory Press. 305 p.
Swofford DL. 1993. Phylogenetic analysis using parsimony.
Champaign, IL: Illinois Natural History Survey. 132 p.
Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic
transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA 9:4350–4354.
Vargas JD, Culetto E, Ponting CP, Miguel-Aliaga I, Davies
KE, Sattelle DB. 2002. Cloning and developmental
expression analysis of ltd-1, the Caenorhabditis elegans
homologue of the mouse kyphoscoliosis (ky) gene. Mech
Dev 117:289–292.
Wang Y, Zhang W, Jin Y, Johansen J, Johansen KM. 2001.
The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin
structure in Drosophila. Cell 105:433–443.