SURFACE MODIFICATIONS OF PM STAINLESS STEELS FOR ENHANCED CORROSION RESISTANCE
advertisement

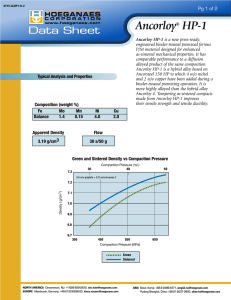
SURFACE MODIFICATIONS OF PM STAINLESS STEELS FOR ENHANCED CORROSION RESISTANCE Chris Schade Hoeganaes Corporation Cinnaminson, NJ 08077 ABSTRACT In general, PM stainless steel parts have inferior corrosion resistance to their wrought counterparts. Furthermore, the variation in surface porosity and oxide layers exhibited by sintered PM parts can lead to a considerable amount of uncertainty in corrosion resistance. In the present study addition of elements, which may modify or segregate to the surface of the powder, leading to enhanced corrosion resistance, are added prior to sintering. The effect of these elements on mechanical properties and corrosion resistance will be studied and compared to grades of stainless steel powder commercially available. The effect of processing variables such as sintering atmosphere and density will also be considered. INTRODUCTION The corrosion resistance of stainless steel is attributed to the formation of a passive layer of chromium oxides that forms readily on the surface of the steel. In general, the poor corrosion resistance of PM stainless steels (compared to wrought stainless steel) is attributed to the porosity which gives rise to crevice corrosion.1 Thus in recent years, focus has been on producing parts with higher density via higher compaction pressures and sintering temperatures as well as sintering in either vacuum or 100 volume percent hydrogen. However there exists many potential applications in which the mechanical properties of lower density PM materials is adequate and the corrosion resistance is the limiting factor which determines if the PM part is used. In early work by Klar and Ro1 it was shown that water atomized powders exhibited large differences between the bulk composition of the powder particles and the composition at the surface. The oxidation states of various elements that segregate to the particle surface were shown to lead to differences in corrosion resistance. In particular, the authors found that silicon forms an oxide film on the water-atomized powder that, due to its brittle nature can be fractured during compaction. The fractured oxide layer creates sites for crevice corrosion. If the oxide layer remained intact it would protect the surface from corrosion. It was also found that when sintering in a hydrogen atmosphere the silicon oxide layer can be reduced. If the reduction of the silicon oxide layer is not complete, leaving patches of silicon oxide, a local galvanic cell can be produced leading to poor corrosion. It is also speculated that there is a ratio of tin to silicon at the surface that must be met to keep the protective oxide layer intact. It is unclear at this time what is the proper density and sintering temperature combination that leads to optimal corrosion resistance. Several authors1-2 have modified this surface oxide with tin. It was found that tin, like silicon, segregated to the surface of the water-atomized powder, and formed tin oxide. The tin helps keep the oxide layer ductile and during compaction less damage to the oxide film occurs. In addition, due to it’s low melting point, the tin may aid in healing any cracks in the oxide layer during the sintering process. There appears to be an optimum ratio of tin to silicon on the surface of the powder.3 It is believed that tin migrates from the bulk of the powder particle to the surface, while the silicon oxide present at the surface tends to be reduced or diffuses back into the bulk particle. As a result, the ratio of tin to silicon on the surface increases with the time and temperature of sintering. Additional work by Klar4 has shown that the addition of tin may affect the nitrogen adsorption during cooling. When nitrogen is in solution in the matrix of stainless steels it can increase the pitting and corrosion resistance. However, if the solubility is decreased, the nitrogen will form chromium nitrides leading to the depletion of chromium in localized areas (particularly grain boundaries) and thus poor corrosion resistance. Klar presented data on tin bearing 316L and 304L for various cooling rates, which showed lower nitrogen values for tin bearing grades (versus conventional grades) cooled at rates typical of conventional sintering furnaces. The significance of this is that tin modified grades can provide superior corrosion resistance than conventional materials when sintered in nitrogen containing atmospheres. It also has been noted that tin acts as a barrier to carbon diffusion in ferrous materials and may prevent chromium carbide in the same fashion nitrides cause sensitization and hence, poor corrosion resistance.5 Although tin has been the most utilized element to improve the surface conditions other elements are known to become surface enriched.6 Although theory does not exist for why elements segregate to the surface of water atomized powders, it is known that elements with a high affinity for oxygen tend to promote surface oxidation during water atomizing. There are several characteristics listed by Klar et. al.4 that make an element a candidate for modifying the surface oxide layer. First, the element should have high diffusivity and low solubility in the base alloy. This will allow the element to segregate to the surface of the water-atomized powder rather than be in solution in the base alloy. The second factor is that the element should form an oxide film, in conjunction with the silicon-oxide that is ductile and provides complete coverage after sintering. This allows a passive layer to be formed on the surface of the sintered part. The element added should not adversely affect the mechanical or corrosion properties of the material. The exact mechanism allowing this to be achieved cannot be predicted so experimentation is necessary to determine the best elements available. Complicating the preceding discussion is the role of density in corrosion resistance. It has long been seen that corrosion resistance increases with decreasing density.4 Oxygen diffusion in low-density specimens with larger pores is facilitated and crevice corrosion is reduced. At intermediate densities the pores are of size and shape that passivation cannot occur due to oxygen depletion. This leads to poor corrosion resistance. At higher densities, where a greater percentage of the porosity is closed, corrosion resistance improves.7 The purpose of the present work is to study the effect of tin addition on the mechanical and corrosion properties of 304L for a series of densities, sintering atmospheres and temperatures. Using this data as a knowledge base, various oxidizable elements such calcium, magnesium, zirconium, aluminum and lanthanum are added to 304L prior to atomization to determine if these elements might be effective in enhancing corrosion resistance by forming a more tenacious oxide layer on the atomized powder. ALLOY PREPARATION AND TESTING The powders used in this study were produced by water atomization with a typical particle size distribution 100 w/o <150 µm (–100 mesh) and 38 to 48 w/o <45 µm (-325 mesh). All the alloying elements were pre-alloyed into the melt prior to atomization, unless otherwise noted. The powders were of the same base composition (304L) with only minor additions where noted. Table I list the chemistry of the individual heats along with a letter designation indicating the change from the base 304L chemistry. Alloy 304L-Sn, which has tin and copper additions, is based on commercially available material while the other alloys listed are experimental materials produced specifically for this study. Table I: Nominal Compositions of Experimental 304L Alloys (w/o) The powders were mixed with 0.75 w/o Acrawax C lubricant. Samples for transverse rupture (TR) and tensile testing were compacted uniaxially at compaction pressures 415 to 690 MPa (30 to 50 tsi). All the test pieces were sintered in a high temperature Abbott continuous-belt furnace at temperatures ranging from 1120 °C (2050 °F) to 1260 °C (2300 °F) for 30 min in either a 100% hydrogen atmosphere with a dewpoint of –40 oC (40 °F), or a mixed atmosphere of 90% nitrogen and 10% hydrogen (90/10). Prior to mechanical testing, green and sintered density, dimensional change (DC), and apparent hardness, were determined on the tensile and TR samples. Five tensile specimens and five TR specimens were tested for each composition. The densities of the green and sintered steels were determined in accordance with MPIF Standard 42. Tensile testing followed MPIF Standard 10 and apparent hardness measurements were conducted on tensile and TR specimens, following MPIF Standard 43. Metallographic specimens of the test materials were examined by optical microscopy in the polished and etched (glyceregia) conditions. Salt spray testing on TR bars was performed in accordance with ASTM Standard B 11703. Five TR bars per alloy (prepared as previously described) were tested. The percent area of the bars covered by red rust was recorded as a function of time. The level of corrosion was documented photographically. RESULTS AND DISCUSSION Mechanical Properties of 304L with Tin The mechanical properties of the 304L with tin additions are shown in Figures 1a - j . The properties are shown for various sintering temperatures and compaction pressures sintered in 100 volume percent hydrogen and 90 volume percent nitrogen/10 volume percent hydrogen (90/10). As expected, the mechanical properties improve with compaction pressure (i.e. density) and the denisty of the 304L with tin addition sintered in 100% hydrogen is slightly higher than when it is sintered in 90/10. Both the ultimate tensile strength and the yield strength are higher for the material sintered in 90/10 due to the formation of nitrides in the microstructure. Figure 2 shows the mirostructure of 304L with tin sintered in hydrogen and 90/10 atmospheres. The microstructure of the hydrogen sintered materials is austenite with twins while the microstructure of the materail sintered in nitrogen contains a large amount of nitrides. These nitrides act as second phase particles which enhance the strength of the material. 90/10 Hydrogen Compaction Pressure (MPa) Compaction Pressure (MPa) 346 7 415 484 553 o o o o o o 622 692 346 7 761 2050 F (1121 C) 484 o o o o 553 622 691 761 40 45 50 55 2050 F (1121 C) 6.9 2100 F (1149 C) 2100 F (1149 C) 3 Sintered Density (g/cm ) 2300 F (1260 C) 3 Sintered Density (g/cm ) 6.9 415 6.8 6.7 6.6 6.5 6.4 o o 2300 F(1260 C) 6.8 6.7 6.6 6.5 6.4 6.3 6.2 6.3 25 30 35 40 45 Compaction Pressure (tsi) (a) 50 55 25 30 35 Compaction Pressure (tsi) (b) Compaction Pressure (MPa) 484 553 o o o o o o 622 Compaction Pressure (MPa) 691 761 621 346 90 415 2050 F (1121 C) 80 80 o o o o o 70 483 60 414 50 40 30 35 345 50 345 276 40 276 40 45 50 207 30 3 UTS (x 10 psi) 414 UTS (MPa) 60 55 207 25 30 35 40 484 o o o o o 622 761 414 346 60 415 2300 F (1260 C) 55 YS (x 10 psi) 345 o o o o o o 622 691 761 414 380 2100 F (1149 C) 50 345 45 310 40 276 40 276 35 241 35 241 30 207 30 207 172 25 25 30 35 40 45 50 3 310 YS (MPa) 45 25 55 172 25 30 35 Compaction Pressure (tsi) 415 484 553 o o o o o o 45 50 55 (f) Compaction Pressure (MPa) Compaction Pressure (MPa) 346 65 40 Compaction Pressure (tsi) (e) 622 691 346 65 761 415 484 o o o o o o 553 622 691 761 40 45 50 55 2050 F (1121 C) 2050 F (1121 C) 60 2100 F (1149 C) Apparent Hardness (HRB) Apparent Hardness (HRB) 553 2300 F (1260 C) 3 YS (x 10 psi) 484 2050 F (1121 C) 380 2100 F (1149 C) 60 55 Compaction Pressure (MPa) 691 2050 F (1121 C) 50 50 (d) 553 o 45 Compaction Pressure (tsi) Compaction Pressure (MPa) 55 552 483 (c) 415 761 621 70 Compaction Pressure (tsi) 346 60 691 2300 F (1260 C) 3 UTS (x 10 psi) o 622 2100 F (1149 C) 2300 F (1260 C) 30 553 2050 F (1121 C) 552 2100 F (1149 C) 25 484 2300 F (1260 C) 55 50 45 2100 F (1149 C) 2300 F (1260 C) 55 50 45 40 40 35 35 25 30 35 40 45 Compaction Pressure (tsi) (g) UTS (MPa) 415 50 55 25 30 35 Compaction Pressure (tsi) (h) YS (MPa) 346 90 Compaction Pressure (MPa) Compaction Pressure (MPa) 346 25 415 484 553 o o o o o o 622 691 346 25 761 o o o o o 553 622 691 761 40 45 50 55 2100 F (1149 C) 2100 F (1149 C) 20 2300 F (1260 C) Elongation (%) Elongation (%) 484 o 2050 F (1121 C) 2050 F (1121 C) 20 415 15 10 2300 F (1260 C) 15 10 5 5 0 0 25 30 35 40 45 Compaction Pressure (tsi) (i) 50 55 25 30 35 Compaction Pressure (tsi) (j) Figure 1. Properties of 304L-Sn as a function of compaction pressure. Left side of figure sintered in hydrogen. Right side of figure sintered in 90/10. The hardness of the material sintered in a 90/10 atmosphere is higher than the materials sintered in hydrogen. This is likely due to the nitrides in the microstructure. The apparent hardness of the material sintered in hydrogen at 1260 oC (2300 oF) decreases despite and increase in density. This is most likely due to tin diffusing back into the bulk of the alloy or the tin forming a low melting phase at the higher temperatures. The ductility of the materials is correlated with the density and yield strength for the two different sintering atmospheres. (a) (b) Figure 2. Representative microstructures: (a) Hydrogen sintered 304L-Sn; (b) 304L-Sn sintered in 90/10. Corrosion Properties of 304L with Tin It has been shown in the preceding section that 304L with tin sintered in a nitrogen bearing atmosphere has higher strength than material sintered in hydrogen. In general, if the nitrogen is soluble in the austenite, corrosion resistance is not negatively impacted. The nitrides that form as a second phase in the matrix, increasing the strength of the material, also deplete the matrix of chromium by the formation of chromium nitrides. This leaves areas depleted of chromium and generally leads to poor corrosion resistance. Since PM stainless steels are already at a disadvantage in corrosion resistance to their wrought counterparts, due to porosity, it is hard to justify the use of nitriding to increase the strength of PM. However, if the corrosion resistance of austenitic PM stainless steels sintered in nitrogen can be improved, the use of nitrogen as a strengthening agent is more viable. Figure 3 and 4 show results of salt spray testing for 240 hrs of TRS specimens sintered at various compaction pressures and sintering temperatures in hydrogen and 90/10 respectively. For the TRS specimens sintered in hydrogen, the corrosion resistance (indicated by the level of red rust) decreases with both compaction pressure and sintering temperature (i.e. density). Conversely, the corrosion resistance of the TRS specimens sintered in 90/10 increases with increasing compaction pressure and sintering temperature. The results of the sintering in hydrogen support the earlier discussion in which the corrosion resistance of PM materials decreases with increasing density. The poor oxygen flow in smaller pores leads to increased crevice corrosion. In addition, the hydrogen atmosphere reduces the protective silicon oxide film, leaving the surface exposed to the salt spray solution. 2050 oF (1121 oC) 2200 oF (1200 oC) 30 tsi (414 MPa) 40 tsi (552 MPa) 50 tsi (690 MPa) Figure 3. Salt Spray testing coupons of 304L-Sn sintered in Hydrogen (240 hrs). 2300 oF (1260 oC) 2050 oF (1121 oC) 2200 oF (1200 oC) 2300 oF (1260 oC) 30 tsi (414 MPa) 40 tsi (552 MPa) 50 tsi (690 MPa) Figure 4. Salt Spray testing coupons of 304L-Sn sintered in 90/10 (240 hrs). The reason for the corrosion behavior in the nitrogen-sintered materials is less clear. In general, the corrosion resistance of the nitrided TRS specimens is better than the TRS specimens sintered in hydrogen. This overall better corrosion resistance could be attributed to the non-reducing 90/10 atmosphere. In this case, the protective silicon oxide layer on the surface is not reduced and the tin/silicon ratio remains optimum. The behavior of the material versus density (via compaction pressure and sintering temperature) is opposite from most all PM materials which decrease in corrosion resistance with increasing density. This may be explained by the fact that the nitrogen, carbon and oxygen levels of the 90/10 sintered materials decrease with increasing sintering temperature (see Table II). The solubility of nitrogen increases with decreasing sintering temperature over the range of sintering temperatures used in this study. Although the protective silicon oxide/tin layer may still exist, the increasing levels of carbon and nitrogen can make the material more susceptible to sensitization by the formation of chromium carbides and nitrides. In this regard, there may be an optimum level of nitrogen for both corrosion resistance and mechanical properties. Table II: Sintered Chemistry of 304L-Sn for various sintering temperatures and compaction pressures. (90/10) Compaction Pressure 30 tsi (414 MPa) 40 tsi (552 MPa) 50 tsi (690 MPa) Sintering Temperature 2050°F o (1121 C) 2200°F o (1200 C) 2300°F o (1260 C) 2050°F o (1121 C) 2200°F o (1200 C) 2300°F o (1260 C) 2050°F o (1121 C) 2200°F o (1200 C) 2300°F o (1260 C) Carbon (w/o) Sulfur (w/o) Oxygen (w/o) Nitrogen (w/o) .033 .001 .24 .90 .031 .001 .29 .66 .018 .001 .27 .62 .037 .001 .30 .87 .031 .001 .29 .65 .027 .001 .28 .60 .038 .001 .30 .84 .031 .001 .28 .62 .027 .001 .27 .57 Experiments with Oxides Since the role of the silicon oxide layer (and its modification by tin) seems to play a dominant role in the corrosion of PM alloys it was decided to explore whether other oxides could play a similar role in corrosion protection. Taking advantage of the oxidizing nature of water atomization several test materials were produced using high oxygen potential elements of both high solubility (aluminum, zirconium and lanthanum) as well as elements with low solubility (magnesium and calcium). Table I shows the chemistry of the experimental materials. Since it was uncertain how well the oxide layer would form during atomizing high levels of each element were used. The effectiveness of the elements was judged based on oxygen level in the powder (Table I) and examination of the oxide layer on sintered TRS specimens. Metallography was used to determine if the oxide layer would exist after being exposed to a reducing atmosphere during sintering. The powders made with aluminum, zirconium and lanthanum all exhibited oxygen levels, prior to sintering above 1% while the oxygen levels of the magnesium and calcium doped 304L were much closer to the typical level of stainless steels (.30%). The thickness of the oxide layer (post sintering) correlated with the powder oxygen level in Table I. Figure 3(a) shows a typical oxide film after sintering 100% hydrogen. A thin oxide layer coats the inter-particle boundaries. One interesting feature of the powders is the surface of the sintered bars for the 304L-Al and 304L-Zr sintered in a 90/10 atmosphere. Figure 3(b) shows the surface of the 304L-Zr sintered under these conditions. A thick nitride layer exists at the surface at the particle boundaries, but the nitrides in the core of the particles are much less than the other powders sintered in 90/10 (see 304L-Sn in Figure 2(b)). Since both zirconium and aluminum are strong nitride formers the nitrogen cannot diffuse to the interior of the particles. (a) (b) Figure 5. Representative microstructures: (a) Hydrogen sintered 304-Mg; (b) 304-Zr sintered in 90/10. For normal PM stainless steels the silicon oxide layer on the surface of the atomized powder is thin and does not negatively impact the green properties of the powder. This is a key feature since the powder still needs to be compacted into parts. The green density and green strength of the experimental powders were measured to determine if they were suitable for compaction and these results are shown in Table III. The green density and green strength tracked with the powder oxygen level. The 304L-Al would not be acceptable and the 304L-Zr borderline for producing parts. Since these powders were atomized in a nitrogen-purged chamber and they are susceptible to nitride formation, this may also have led to the poor compaction of these powders. Table III: Green Properties of Experimental 304L Alloys Green Green Strength Density 3 (MPa) (g/cm ) (psi) (MPa) Pressure Pressure Green Density 3 Material (tsi) 304-Sn 304L-Al 304L-Zr 304L-La 304L-Mg 304L-Ca 30 414 6.24 713 4.9 30 414 5.56 48 0.3 40 552 30 414 5.94 296 2.0 40 30 414 5.97 684 4.7 30 414 6.00 1057 30 414 6.02 1142 (tsi) (MPa) (g/cm ) 40 552 6.54 Green Strength Pressure (tsi) (MPa) Green Density 3 Green Strength (psi) (MPa) (MPa) 7.9 50 690 (g/cm ) 6.76 (psi) 1142 1555 10.7 5.83 138 1.0 50 690 6.04 238 1.6 552 6.25 549 3.8 50 690 6.48 821 5.7 40 552 6.28 1167 8.0 50 690 6.52 1716 11.8 7.3 40 552 6.33 1653 11.4 50 690 6.58 2029 14.0 7.9 40 552 6.34 1725 11.9 50 690 6.58 2314 16.0 Since the green properties of the 304L-Mg and 304L-Ca powders were comparable to 304L-Sn and the metallography confirmed an oxide coating on the particle boundaries in the sintered condition, these two powders were chosen for salt spray testing. Unlike the 304L-Sn these two materials exhibited better corrosion resistance with increasing density for both the hydrogen and 90/10 atmospheres. With the 304L-Sn the tin aids the silicon oxide layer and additional strength is gained by nitriding. However the nitriding makes the material brittle. These new oxide layers, unlike the tin addition, allow for better corrosion resistance at higher densities. Higher densities can provide strength without the sacrifice in ductility, which is important for many PM stainless parts. This technique may also allow for other strengthening methods, such as dual phase microstructures and precipitation hardening, to be used which would be affected by sintering in a nitrogen rich atmosphere. 2050 oF (1121 oC) 2200 oF (1200 oC) 40 tsi (552 MPa) 50 tsi (690 MPa) Figure 6. Salt Spray testing coupons of 304L-Mg sintered in hydrogen (240 hrs). 2300 oF (1260 oC) 2050 oF (1121 oC) 2200 oF (1200 oC) 2300 oF (1260 oC) 40 tsi (552 MPa) 50 tsi (690 MPa) Figure 7. Salt Spray testing coupons of 304L-Ca sintered in hydrogen (240 hrs). CONCLUSIONS • The corrosion resistance of 304L-Sn sintered in hydrogen decreases with increasing compaction pressure and sintering temperature (i.e. density). • The corrosion resistance of 304L-Sn sintered in 90/10 increases with increasing compaction pressure and sintering temperature (i.e. density). • Adding oxidizable elements to the melt prior to atomization, such as calcium and magnesium, lead to an oxide film on the powder that remains at inter-particle boundaries after sintering in a reducing atmosphere. • The oxide layer formed during atomization leads to lower green density than conventional atomized material. • The oxide layer formed by calcium and magnesium allowed for better corrosion resistance with increasing compaction pressure and sintering temperatures (i.e. density). REFERENCES 1. D.H.Ro and E. Klar, “Corrosion Behavior of P/M Austenitic Stainless Steels,” Modern Developments in Powder Metallurgy, vol. 13, 1981 pp.247-287. 2. J.H. Reinshagen and R.F. Mason, “Improved Corrosion Resistant Stainless Steel Based P/M Alloys,” Advances in Powder Metallurgy and Particulate Materials, compiled by J.M. Capus and R.M. German, Metal Powder Industries Federation, Princeton, NJ, 1992, vol. 5, pp. 385-397. 3. D.H. Ro, E. Klar and C.I. Whitman, “Maximizing the Corrosion Resistance of Tin Containing Stainless Steel Powder Compacts,” U.S. Patent No. 4,314,849. February 9, 1982. 4. E. Klar and M.A. Pao, “Process of Improving Corrosion Resistance in Porous Stainless Steel Bodies and Article,” US Patent 4,420,336 December 13, 1983. 5. S.K. Chatterjee and M.E. Warwick, “The Effect of Tin, Copper, Nickel and Molybdenum on the Mechanical Properties and Corrosion Resistance of Sintered Stainless Steel (AISI 304L),” Modern Developments in Powder Metallurgy, compiled by E.N. Aqua and C.I. Whitman, Metal Powder Industries Federation, Princeton, NJ, 1984, vol. 16, pp. 277-293. 6. D.H. Ro, E. Klar and C.I. Whitman, “Corrosion Resistant Powder Metallurgy Stainless Steel Powders and Compacts Therefrom,” U.S Patent 4,240,831 December 23,1980. 7. E. Klar and P.K. Samal, “Optimization of Vacuum Sintering Parameters for Improved Corrosion Resistance of P/M Stainless Steels,” Advances in Powder Metallurgy and Particulate Materials, compiled by C. Lall and A. Neupaver, Metal Powder Industries Federation, Princeton, NJ, 1994, vol. 7, pp. 239-251.