A Long Term Wearable Electrocardiogram (ECG) Measurement System Margaret (Maggie) K. Delano

A Long Term Wearable Electrocardiogram (ECG)
Measurement System
by
Margaret (Maggie) K. Delano
S.B., E.E., M.I.T. (2010)
Submitted to the Department of Electrical Engineering and Computer
Science in partial fulfillment of the requirements for the degree of
Masters of Engineering in Electrical Engineering and Computer
Science at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
June 2012
©
Margaret (Maggie) K. Delano, MMXII. All rights reserved.
The author hereby grants to MIT permission to reproduce and distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created.
Author . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Department of Electrical Engineering and Computer Science
May 21, 2012
Certified by . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Dr. Charles G. Sodini
Clarence J. LeBel Professor
Thesis Supervisor
Accepted by . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Prof. Dennis M. Freeman
Chairman, Masters of Engineering Thesis Committee
2
A Long Term Wearable Electrocardiogram (ECG)
Measurement System
by
Margaret (Maggie) K. Delano
Submitted to the Department of Electrical Engineering and Computer Science on May 21, 2012, in partial fulfillment of the requirements for the degree of
Masters of Engineering in Electrical Engineering and Computer Science
Abstract
In this thesis, a low-power, wearable monitoring system was developed from discrete electronic components and custom PCBs.
The device was designed to maximize comfort and minimize the footprint on the user. A single lead, clinical grade electrocardiogram (ECG), along with 3 axes of acceleration are recorded while a user wearing the device carries out his/her daily activities. The monitor itself consists of a central PCB that contains an electrode and the majority of the electronics, along with either one or two additional electrodes that are connected to smaller electrode
PCBs. The monitor can be configured such that any given electrode can act as an input or an output. The system can record ECG and acceleration for over 1 week and consumes 7.3 mW.
To confirm the quality of the ECG recorded by the device, a clinical test was performed. Individuals wore both the device discussed in this thesis and a clinical
ECG recorder while engaging in physical activities such as sitting, standing, and running. Four participants have been tested at this time. QRS sensitivity and QRS positive predictability were determined for each ECG trace. The cardiac monitor outperformed the clinical recorder in all interventions. It should be a viable alternative to conventional monitors. Additional participants will be tested as part of future work.
Thesis Supervisor: Dr. Charles G. Sodini
Title: Clarence J. LeBel Professor
3
4
Acknowledgments
I would first like to thank Professor Charlie Sodini, my thesis supervisor, for his support, mentorship and guidance throughout the MEng thesis. I look forward to working with him over the next few years as I pursue further graduate studies. I would also like to thank Eric Winokur, who was my UROP supervisor when I first started working on this project. Eric helped me get up to speed on the project and learn how to be an effective graduate student. It was his mentorship that helped me make an effective transition to graduate life and decide to pursue the research further. Collin Stultz and Thomas Heldt both provided a valuable perspective on the underlying physiology as it interacts with my work. They both helped me make sure I had the facts straight. David He, Sabino Pietrangelo, and Bruno Do Valle helped me when I had questions about anything from system level design to which microcontroller to use. I would also like to thank all the other members of our office space, who provided a great environment to work in. Bryan Haslam, Cathy Ricciardi, and Ankit Gordhandas were vital contributors to the clinical testing at MIT. It could not have happened without them. I would also like to thank Texas Instruments and
Analog Devices, Inc. for the parts samples I used for the monitor. I would like to thank all my friends and family for their support and encouragement over the past school year. Last but certainly not least, I would like to thank my parents, Mark and
Maureen Delano. Although I don’t get to see them or call them as much as I should,
I know that they will support me in whatever I do.
5
6
Contents
15
Motivation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
15
Objectives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
16
The Electrocardiogram . . . . . . . . . . . . . . . . . . . . . . . . . .
17
Using the Electrocardiogram . . . . . . . . . . . . . . . . . . . . . . .
22
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
24
29
Electrical Specifications and Challenges . . . . . . . . . . . . . . . . .
29
ECG Signal Requirements . . . . . . . . . . . . . . . . . . . .
29
Accelerometer Signal Considerations
. . . . . . . . . . . . . .
36
System Level Requirements . . . . . . . . . . . . . . . . . . .
36
Software Specifications and Challenges . . . . . . . . . . . . . . . . .
37
Mechanical Specifications and Challenges . . . . . . . . . . . . . . . .
38
41
Final System Overview . . . . . . . . . . . . . . . . . . . . . . . . . .
41
Central Board . . . . . . . . . . . . . . . . . . . . . . . . . . .
42
Electrode boards . . . . . . . . . . . . . . . . . . . . . . . . .
43
Cardiac Monitor Placement . . . . . . . . . . . . . . . . . . .
43
Hardware . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
45
Analog Front End . . . . . . . . . . . . . . . . . . . . . . . . .
47
Digital Back End . . . . . . . . . . . . . . . . . . . . . . . . .
49
7
Power Management Circuitry . . . . . . . . . . . . . . . . . .
51
Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
53
Final Algorithm . . . . . . . . . . . . . . . . . . . . . . . . . .
53
Allocate space and structures . . . . . . . . . . . . . . . . . .
53
Initializing the System . . . . . . . . . . . . . . . . . . . . . .
56
Checking the micro SD card . . . . . . . . . . . . . . . . . . .
58
Selecting the available flash memory
. . . . . . . . . . . . . .
58
Sampling Timer Interrupt . . . . . . . . . . . . . . . . . . . .
59
Saving Data to Serial Flash . . . . . . . . . . . . . . . . . . .
60
Saving Data to micro SD . . . . . . . . . . . . . . . . . . . . .
61
Importing the data . . . . . . . . . . . . . . . . . . . . . . . .
61
Cardiac Monitor Specifications . . . . . . . . . . . . . . . . . . . . . .
62
ECG Specifications . . . . . . . . . . . . . . . . . . . . . . . .
62
Accelerometer Specifications . . . . . . . . . . . . . . . . . . .
63
System Specifications . . . . . . . . . . . . . . . . . . . . . . .
64
67
Motivation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
67
. . . . . . . . . . . . . . . . . . . . . . .
68
Comfort Level . . . . . . . . . . . . . . . . . . . . . . . . . . .
68
Activity Level . . . . . . . . . . . . . . . . . . . . . . . . . . .
68
Creating a Suite of Physiological Signals . . . . . . . . . . . .
68
Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
68
Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
71
Cardiac Monitor Signal Quality . . . . . . . . . . . . . . . . .
71
Cardiac Monitor Comfort Level . . . . . . . . . . . . . . . . .
73
Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
73
Cardiac Monitor Signal Quality . . . . . . . . . . . . . . . . .
73
Cardiac Monitor Comfort Level . . . . . . . . . . . . . . . . .
74
Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
75
8
77
Discussion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
77
Future Work . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
79
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
80
81
. . . . . . . . . . . . . . . . . . . . . . .
81
A.2 Micro SD .C File . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
84
9
10
List of Figures
1-1 The ECG waveform . . . . . . . . . . . . . . . . . . . . . . . . . . . .
18
1-2 12 Lead ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
19
1-3 Einthoven’s Triangle . . . . . . . . . . . . . . . . . . . . . . . . . . .
20
1-4 The Frontal Axis . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
21
1-5 Precordial Electrode Placement . . . . . . . . . . . . . . . . . . . . .
21
1-6 Dynamic Time Warping . . . . . . . . . . . . . . . . . . . . . . . . .
23
1-7 A Holter Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
23
1-8 The Netguard Device . . . . . . . . . . . . . . . . . . . . . . . . . . .
24
1-9 The V Patch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
25
1-10 The IMEC Patch . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
26
1-11 Wearable ECG Patch dimensions . . . . . . . . . . . . . . . . . . . .
27
2-1 Three Electrode Placement . . . . . . . . . . . . . . . . . . . . . . . .
31
. . . . . . . . . . . . . . . . . . . . . . . .
32
2-3 The Instrumentation Amplifier . . . . . . . . . . . . . . . . . . . . . .
34
2-4 Displacement Currents . . . . . . . . . . . . . . . . . . . . . . . . . .
34
2-5 A Right Leg Drive Circuit . . . . . . . . . . . . . . . . . . . . . . . .
35
2-6 Wearable ECG Patch on subject . . . . . . . . . . . . . . . . . . . . .
39
3-1 Three Electrode Wearable, Long Term Cardiac Monitor . . . . . . . .
42
3-2 The Central Board . . . . . . . . . . . . . . . . . . . . . . . . . . . .
43
3-3 The Electrode Board . . . . . . . . . . . . . . . . . . . . . . . . . . .
44
3-4 Electrode Board as Input . . . . . . . . . . . . . . . . . . . . . . . . .
44
3-5 Electrode Board as Output . . . . . . . . . . . . . . . . . . . . . . . .
44
11
3-6 Three Electrode Cardiac Monitor Worn . . . . . . . . . . . . . . . . .
45
3-7 Two Electrode Cardiac Monitor Worn
. . . . . . . . . . . . . . . . .
46
3-8 Monitor Worn Under Shirt . . . . . . . . . . . . . . . . . . . . . . . .
46
3-9 Block Diagram of Overall System . . . . . . . . . . . . . . . . . . . .
47
3-10 Analog Front End . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
48
3-11 Three Electrode Board With Battery . . . . . . . . . . . . . . . . . .
52
3-12 Final Algorithm Flowchart . . . . . . . . . . . . . . . . . . . . . . . .
54
3-13 An Example Flash Block . . . . . . . . . . . . . . . . . . . . . . . . .
60
3-14 Saving to Flash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
61
4-1 Participant Ready for Testing . . . . . . . . . . . . . . . . . . . . . .
69
4-2 Sequence of Interventions . . . . . . . . . . . . . . . . . . . . . . . . .
70
4-3 Recording Times . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
70
4-4 Participant on treadmill . . . . . . . . . . . . . . . . . . . . . . . . .
71
4-5 ECG While Running . . . . . . . . . . . . . . . . . . . . . . . . . . .
75
4-6 ECG While Standing . . . . . . . . . . . . . . . . . . . . . . . . . . .
76
4-7 ECG While Supine . . . . . . . . . . . . . . . . . . . . . . . . . . . .
76
5-1 First Revision of Electrode Board . . . . . . . . . . . . . . . . . . . .
78
12
List of Tables
Existing device specifications . . . . . . . . . . . . . . . . . . . . . . .
26
ECG Signal Quality Requirements . . . . . . . . . . . . . . . . . . . .
30
Accelerometer Signal Quality Requirements
. . . . . . . . . . . . . .
36
Overall system specifications . . . . . . . . . . . . . . . . . . . . . . .
37
GPIO configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . .
56
. . . . . . . . . . . . . . . . . . . . . . . . . . . .
57
ECG Signal Quality Requirements and Measurements . . . . . . . . .
62
ECG Signal Quality Specifications by Design . . . . . . . . . . . . . .
62
Accelerometer Signal Quality Requirements
. . . . . . . . . . . . . .
63
Overall system specifications . . . . . . . . . . . . . . . . . . . . . . .
64
Current Dissipation . . . . . . . . . . . . . . . . . . . . . . . . . . . .
64
Write/ Read Times . . . . . . . . . . . . . . . . . . . . . . . . . . . .
65
QRS Sensitivity and Positive Predictivity . . . . . . . . . . . . . . . .
74
13
14
Chapter 1
Introduction
A wearable, long term cardiac monitor has been developed. In this chapter, the motivation behind wearable monitors will be presented. Specific objectives of this thesis will be described, along with previous work in the field.
1.1
Motivation
Cardiovascular disease is the leading cause of death in the United States, accounting
for 32.8% of total deaths in the United States in 2008 [1]. The United States spent al-
most
$
300 billion on the direct and indirect costs of cardiovascular disease and stroke.
Although the rate of death attributable to cardiovascular disease has declined 30.6% since 1999, the prevalence of traditional risk factors such as hypertension, obesity and smoking remains an issue. As the United States population ages, enormous pressure is being placed on the health care delivery system to maintain quality of care while reducing overall costs. The current quality of care can only be maintained if the health care delivery system becomes significantly more efficient.
One way to reduce costs associated with health services while maintaining quality of care is to provide systems that monitor an individual as they go about their daily activities, reducing the number of doctor’s visits required. Physicians can use these systems to monitor individuals recovering from an existing cardiac condition, those at risk, and those experiencing cardiac discomfort. Monitoring an individual at home
15
and over longer periods of time can help a physician track disease progression. Earlier detection of a disease state can then lead to earlier intervention and treatment. In addition to monitoring disease progression, physiological monitoring on a day-to-day basis can help establish normal physiology for an individual and can provide feedback for more targeted treatments.
Recent studies suggest that risk of death in patients who have suffered heart
attacks can be predicted using the electrocardiogram (ECG) [2] [3]. These studies
analyzed up to 24 hours of continuous ECG data from patients in their hospital beds.
They developed computational methods that analyze the similarity of neighboring heart beats and used these methods to predict whether a patient would live or die.
These studies are currently limited to data taken from individuals who have already had a cardiac event and whose ECG is recorded shortly thereafter the event while they are recovering. Collecting long term ECG data from both patients with existing cardiovascular conditions and those at risk individuals may help further development of these risk stratification methods. Additionally, collecting long term ECG data along with an estimate of activity level (such as from an accelerometer) while an individual carries out his or her daily activities may give a physician a better estimate of that individual’s cardiac health than ECG alone.
1.2
Objectives
The goal of this thesis is to develop and validate a wearable, long term cardiac monitor. The system should be able to record ECG and acceleration data continuously for at least one week. It must be able to withstand every day physical activity and be as comfortable and unobtrusive as possible. The device must perform on par or better than the devices that exist today. It could then be used as an alternative to clinically available monitors, but would have a much smaller form factor and fewer wires. The monitor can be used to collect data from patients who have suffered a cardiac event and those at risk as they go about their daily activities. This will allow physicians to track disease progression and allow researchers to develop methods to predict and
16
prevent serious cardiac events.
The cardiac monitor will be validated in a clinical test conducted at the Clinical
Research Center (CRC) at MIT. Healthy individuals will perform a series of different interventions while wearing both the monitor developed in this thesis and a commercially available monitor. Interventions will include activities ranging from lying down to running in order to simulate what wearing the monitor throughout the day would be like. The cardiac monitor will be validated if it performs as well (if not better) than the commercially available monitor and is able to withstand every day activity while being both reasonably comfortable and unobtrusive.
1.3
The Electrocardiogram
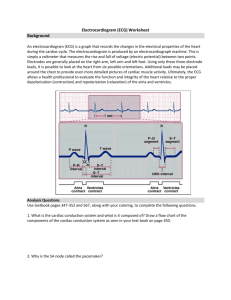
Before discussing previous work in wearable heart monitor technology, it is important to understand the basics of the electrocardiogram (ECG) itself. The ECG has become a routine part of any complete medical evaluation and has been used as a diagnostic
test since its discovery over 70 years ago [4]. Because electricity is conducted through
the heart muscle (known as the myocardium), many (but not all) types of damage to the heart tissue can be detected with an ECG. The ECG waveform allows one to infer information about electrical activity associated with different aspects of a heart beat and is therefore of particular value for assessing an individual’s cardiac rhythm and heart health.
A normal cardiac rhythm is referred to as a “sinus” rhythm because of its origination in the sinoatrial node (S-A node). Disruptions of this normal sinus rhythm are known as arrhythmias. Each heart beat is triggered by “pacemaker” cells in the
S-A node. These cells generate action potentials at 60 – 100 beats per minute (while at rest). Action potentials depolarize the tissue as they propagate throughout the myocardium in a predefined way, causing muscle tissue to contract. After the action potentials leave the S-A node, they depolarize the two upper chambers of the heart known as the atria, and then move to the atrioventricular node (A-V node). This node sits between the atria and the lower two chambers of the heart known as the
17
ventricles. It acts as a backup pacemaker at a lower rate of 40 – 60 beats per minute.
The A-V node also delays the electrical impulses, slowing them down by about 120 ms to give the atria enough time to eject their blood into the ventricles before the ventricles contract. After the action potentials leave the A-V node, they move to an area known as the “His” bundle and down the left and right bundle branches. Conductive fibers known as Purkinje fibers depolarize the ventricles, starting from the left ventricle and swinging to the right. After the ventricles have been depolarized and contract, the cycle starts over with another impulse from the S-A node.
Figure 1-1: An ECG waveform is composed of action potentials originating in different areas of the heart. The ECG waveform recorded at the skin is the superposition of
these different action potentials. Source: [5]
The ECG represents the electrical activity of an ensemble of cells in the myocardium. It is a differential measurement across the surface of the body and can be thought of as a vector measurement of electrical potential. A typical ECG waveform
(as shown on the right side of Figure 1-1) consists of three primary features: the P
wave, the QRS complex, and the T wave. Each wave corresponds to the electrical
18
activity in specific parts of the heart. The P wave comes first and represents the depolarization of the atria. The delay between the P wave and the QRS complex is known as the PR interval. This interval signifies the delay that occurs in the A-V node that gives the atria time to contract before depolarizing the ventricles. The
QRS complex represents depolarization of the ventricles, and is usually the strongest wave in an ECG. The last wave is the T wave and it represents the repolarization of the ventricles. The repolarization of the atria is usually hidden in the QRS complex.
Taking a differential measurement of the electrical potential on the body surface at different locations generates different ECG vectors. A standard clinical ECG consists of 12 different vectors known as “leads.” Six of these leads are in the plane parallel to the body and are known as “frontal” ECG leads. The other six ECG leads are views of the heart in the plane perpendicular to the body and are known as “pre-cordial”
leads. A view of all 12 ECG leads can be seen in Figure 1-2.
Figure 1-2: A vector view of the standard 12 Lead ECG. The frontal leads are light
blue and the pre-cordial leads are dark blue. Source: [6]
The frontal ECG leads are formed from three different electrodes placed on the body, with an optional fourth electrode used as a reference. These electrodes are usually placed on the right arm (RA), left arm (LA), and left leg (LL). The optional reference electrode is usually placed on the right leg (RL). The three sensing electrodes
(RA, LA, and LL) form what is known as Einthoven’s triangle (see Figure 1-3). The
19
six frontal leads are derived from this triangle. Leads I, II and III are formed directly from the triangle itself. An additional three leads, known as the augmented limb leads (augmented foot, augmented right, augmented left) are formed by subtracting the average of two electrode potentials from the value of the third. These leads are known as augmented leads because their amplitude is 1.5 times the amplitude of the frontal leads. Each lead is derived from one of the three sensing electrodes using the
equations provided in Equation Array 1.1. The vector representation of each frontal
Figure 1-3: Einthoven’s triangle, formed by the right arm, left arm and left leg
electrodes. These three electrodes form the basis for the frontal axis. Source: [7]
I = LA − RA
II = LL − RA
III = LL − LA aV F aV R = RA − aV L
=
=
LL
LA
−
−
1
2
1
( LA + RA )
( LA + LL )
2
1
2
( RA + LL )
The remaining six leads of the 12 lead system are the pre-cordial leads. The
electrodes V1–V6 are placed on the chest as demonstrated by Figure 1-5. The lead is
20
(1.1)
(1.2)
(1.3)
(1.4)
(1.5)
(1.6)
Figure 1-4: The frontal axis of the ECG consists of six vectors derived from three
electrodes: left arm (L), right arm (R), and left leg (F). Source: [7]
the difference between the potential at the electrode site and the average potential of
RA, LA and LL. This can be thought of as the difference between the center of the body and the pre-cordial electrode, which creates a vector perpendicular to the body.
Figure 1-5: The electrodes V1–V6 are placed on the chest roughly in a line. Source:
21
1.4
Using the Electrocardiogram
When using the ECG to assess heart health, the 12 lead ECG is the clinical standard.
The combination of 12 different leads gives the physician a view of the heart’s electrical activity from a variety of different views. Although 12 lead ECG is standard, it is not always required. Detecting an arrhythmia, for example, only requires one ECG lead, so long as that lead has a good view of the different ECG waves. When analyzing
an ECG lead for arrhythmias, there are three important steps [4]. The first step is
to find the QRS complexes and examine their rate, regularity and shape. The next step is to find the P waves, check if each P wave corresponds to a QRS complex, examine their shape and check for anything else irregular about their pattern. Most arrhythmias can be identified using this information.
The risk stratification method known as Morphology Variability can also be per-
formed using a single ECG lead [2] [3]. This method uses the ECG and compares
adjacent beats. It is interested in small differences between the two beats that are often undetectable by the naked eye. These small differences can change over time.
Because two neighboring beats are not identical, dynamic time warping is used to
compare the correct parts of each beat (see Figure 1-6). Morphology Variability was
shown to be higher in those who died after cardiac events than in those who did not
It is useful to be able to record an ECG while an individual is carrying out his/her daily activities. To record ECG in this ambulatory setting, wearable cardiac monitors are used. When an individual is having heart problems or discomfort (especially if the origin of which cannot be determined from a routine checkup), a physician often has
the individual wear a device known as a Holter monitor for 24 – 48 hours [8] (see Figure
1-7). A typical Holter monitor measures one or more ECG leads using electrodes
placed on the chest. The electrodes are connected to wires that are connected to a central processing unit, usually worn on a belt or in a pocket. Although in wide use today, there are a number of shortcomings with a standard Holter monitor. First, the
22
Figure 1-6: Dynamic Time Warping as applied in [2] [3].
Vertical lines connect corresponding samples between the two beats. If samples taken at equally spaced intervals from the beginning, different parts of the beats could be compared. Dynamic
time warping forces similar parts of the two waveforms to be compared. Source: [2]
wires that attach to the electrodes can be a significant noise source, especially during higher levels of physical activity such as walking or running. They are cumbersome to wear and can be inadvertently detached if pulled. With many Holter monitor implementations, even if the device is worn continuously, only small subsets of the
every ten days to one month that are not recorded by the Holter monitor.
Figure 1-7: A patient wearing a Holter Monitor. Source: [10]
To capture events that occur less frequently, patients can be fitted with devices
known as event monitors, which can be worn for a month or longer [11]. However,
event monitors typically only save ECG data around the irregular cardiac episode,
23
which minimizes the amount of analysis that can be performed. Continuous long-term data allows the clinician to detect overall heart health trends and possibly see initial
markers before the cardiac irregularity occurs [12]. Longer term ECG recordings can
be used both to develop new risk biomarkers and stratify risk of individual patients
1.5
Previous Work
Several groups have worked on wearable monitors to improve the form factor of wear-
able monitors and increase patient compliance [13] [14]. In this section, a few note-
worthy examples will be discussed. The first such device is the Netguard, developed
is designed for in hospital use and has a wireless ECG monitor that weighs less than an ounce and communicates with a standard PC at a nurse’s station. The device can detect asystole (when the heart stops beating) and ventricular fibrillation (when the ventricles contract in a rapid and uncontrollable way). When a dangerous cardiac rhythm is detected, an alert is sounded and an ECG display with the dangerous rhythm is displayed. The nurse can confirm the alert and call an emergency as per hospital protocol.
Figure 1-8: The Netguard device developed by mindray. Source: [15]
One of the benefits of the Netguard is that it costs a fraction of that of conventional monitors. Having such a device on all patients in a ward can help reduce a hospital’s
24
potential exposure to liability from incidents that occur to unmonitored patients that could otherwise have been prevented.
However, a limitation of the work is that the Netguard only works so long as the individual is in range of its base station.
Additionally, once a patient has been admitted to the hospital, it is likely that a full
12 lead ECG would be taken, thus making the Netguard device redundant.
The V patch from Intelesens is similar to the Netguard, but can be used in an
ambulatory setting. The patient wears a device (shown in Figure 1-9) that connects to
electrodes worn at the chest. ECG is monitored continuously and events detected by the system are transmitted to a base station. From the base station, data is uploaded to a web-based interface that can be accessed by the physician. The V patch can automatically detect a number of the most commonly occurring arrhythmias, but will also respond to a patient alarm button.
Figure 1-9: The V patch device developed by Intelesens. Source [16]
A major benefit of the V patch device is that it can be worn for a week before recharging. Additionally, the patient wearing the device can go about their daily life while wearing the device. The patch is designed to minimize the use of wires and dramatically reduces the effects of motion artifacts. A downside of this device is that it requires a somewhat bulky base station worn in the patient’s pocket to connect to the internet.
IMEC (Belgium) has announced the release of a promising ECG device. This
patch will be worn at the chest and can measure three ECG leads (see Figure 1-10).
25
The patch measures heart rate on board and also has a 3 axis accelerometer. The system on a chip design and Bluetooth low energy radio enables the device to last up to one month with a 200 mAH battery.
Figure 1-10: The wireless ECG patch developed by IMEC. Source: [17]
One of the advantages of the IMEC patch is that it uses Bluetooth low energy to transmit its data. While requiring a base station is not ideal, it’s more likely that a user would already have an existing Bluetooth enabled device and would not have to carry an additional one with them. However, the IMEC device is limited in that it does not transmit raw ECG data. It extracts relevant information from the ECG waveform (heart rate, along with onset, peak and offset of the P, QRS, and T waves).
While this would allow adequate reconstruction of the basic features of the ECG wave form, this alone would not allow a physician to view the morphology of the waveform.
Specification
Battery Life
Netguard V patch
?
7 days
Leads
Data Transmitted
1
Events
Detectable Arrhythmias 2 types
3
Events
10 types
IMEC patch
1 month
3
Events, Heart Rate, Features
?
Table 1.1: Comparison of specifications of existing devices.
The monitors described above are all limited in similar ways. While they improve on the Holter monitor form factor by reducing the number of wires, they only transmit
26
information about specific cardiac events, or limited information such as heart rate.
Additionally, they all rely on transmitting data to a base station, which limits their effectiveness when a cell phone or computer is not nearby, and requires the user to have an additional device with them at all times. This thesis aims to develop a device that can save high quality ECG data continuously for up to one week, without requiring the use of a base station.
A first generation of the cardiac monitor was developed prior to the start of the device presented in this thesis. The first generation device, known as “the patch,” was fabricated on a flexible PCB and was designed to be completely free of wires (see
Figure 1-11). Different ECG leads (I, II, III or aVF) can be used by selecting three of
the appropriate electrodes as a +/- input or a ground drive. The patch was validated in a clinical study at the Clinical Research Center (CRC) at MIT. The performance of the patch was compared with that of a Criticare 504-US ECG recorder with electrodes placed adjacent to the active patch electrodes.
Figure 1-11: Board dimensions on the wearable ECG patch developed by Eric
Winokur of the Sodini Group. Photo credit: David He
Overall, the patch performed as well (if not better) than the Criticare monitor.
However, there were a few areas in which the patch could be improved. Specifically,
27
improvements in mechanical reliability and memory capacity can be made. Because the patch was designed on a single flexible PCB, the board was constrained to move as one piece, often resulting in broken solder connections that caused the board to malfunction. Because of limited memory capacity on the patch (128 Mb), the total possible test duration was less than one day.
In the next several chapters, implementation of a new wearable, long term cardiac
monitor will be presented. In Chapter 2, design requirements and considerations for
developing such a device will be discussed. Implementation of the final prototype
device is described in Chapter 3. Chapter 4 will describe a clinical test that was
conducted to validate the wearable cardiac monitor, along with some preliminary
results. Finally, Chapter 5 will discuss what has been learned from this thesis, along
with future work that will be carried out after this thesis has been submitted.
28
Chapter 2
Design
In the previous chapter, motivation for a wearable, long term cardiac monitor was presented. This chapter will discuss the design considerations for implementing such a system. It will outline considerations in three different categories: electrical, software, and mechanical. Relevant design decisions and background will be discussed.
2.1
Electrical Specifications and Challenges
Electrical considerations for the cardiac monitor are driven by its use model. It must perform at least as well as commercially available devices, with a small and comfortable form factor. It must be able to record ECG and acceleration data continuously for at least one week.
2.1.1
ECG Signal Requirements
A summary of the electrical specifications relating to ECG signal quality are presented
in Table 2.1. The minimum requirements for the system, along with the desired target
specifications are listed. In the paragraphs below, each specification will be discussed in detail.
29
Specification
Leads
Electrodes
Bandwidth
Input Referred Noise
Gain
ADC Resolution
CMRR (@ 60 Hz)
Minimum Target
1
2–3
1
2–3
0.5 – 40+ Hz 0.5 – 100 Hz
< 30
µ
V < 20
µ
V
100–1000+ 100–1700+
8+ bits
40+ dB
12 bits
90+ dB
Table 2.1: ECG Signal Quality Requirements
Leads
The cardiac monitor needs to be as unobtrusive as possible. It should have a small form factor with as few electrodes as possible while maintaining signal integrity. The device will be used for general monitoring and basic arrhythmia analysis. Individuals with existing heart conditions can wear the device to give their physician a better idea of their baseline physiology. Those suspected of having an arrhythmia or other problem can wear the monitor for longer periods of time (eg. 1 day to 1 week) to help their physician diagnose a condition that occurs infrequently. For this use model, a single lead with a good view of the cardiac axis will suffice. Leads I and II both satisfy this requirement and were selected as the two desired leads for the cardiac monitor.
Electrode Number and Placement
Although only 2 electrodes are required to take a single ECG lead, a third reference electrode is often used to set the body to a common potential. A 2 electrode implementation has the advantage that it minimizes the footprint on the user. However, a 3 electrode implementation has the advantage that it is more robust to noise and has more options for lead selection. Both possibilities are explored in this thesis.
To obtain a Lead I or Lead II, there are a few different options for electrode placement. In the three electrode configuration, any two electrodes can form a vector with the third electrode as the reference, so up to three unique vectors can be selected from the three electrodes. The three electrode monitor, if worn in the configuration
shown in 2-1a, for example, would allow a Lead I, Lead II or Lead III to be selected.
30
However, this configuration might not be comfortable for the user, as wires or electronics might have to cross over the pectoral muscles and breast tissue. The alternate
configuration in 2-1b provides configuration that should be more comfortable for the
user, but with a good view of Lead II only.
(a) Option One (b) Option Two
Figure 2-1: Two different options for electrode placement in the three electrode configuration.
In the two electrode configuration, the electrodes must be placed in the orientation desired for the lead and only one unique lead is possible. If the electrodes are placed
on the body to obtain a Lead II vector, they would look like that shown in Figure 2-
2a. However, similar to what happened in the three electrode case, this might result
in wires or electronics across the pectoral muscle and breast tissue. An alternate
possibility is that shown in Figure 2-2b. This provides a good view of Lead I only.
For both the three and two electrode implementations, two possibilities for electrode placement were presented. In the three electrode case, the most ideal configuration would provide the best view of Lead II, whereas in the two electrode case, the most ideal configuration would provide the best view of Lead I.
ECG Bandwidth
ECG signal bandwidth requirements vary depending on the application. For nondiagnostic ECG, a bandwidth of 0.5 to 40 Hz is typical. For diagnostic quality ECG,
31
(a) Option One (b) Option Two
Figure 2-2: Two different options for electrode placement in the two electrode configuration.
the bandwidth must be at least 0.05 to 150 Hz. A lower high-pass frequency is required in diagnostic quality ECG to allow accurate ST segments to be recorded.
The ST segment is not as relevant for arrhythmia analysis, so a non-diagnostic ECG bandwidth can be used. The higher high-pass frequency in non-diagnostic ECG helps reduce baseline drift and is preferred for use with monitoring devices. The high-pass frequency of the monitor will be set to 0.5 Hz, and the low-pass frequency will be set to 125 Hz.
Noise and Gain
In a typical ECG, the P wave is the smallest feature of interest. A typical P wave has an amplitude of 200 – 300
µ
V. The input referred noise should be much lower than 200 µV rms for detection of the P wave. It is typically specified to be less than
30 µV rms
. A typical QRS complex is the largest feature of interest and can have an amplitude of 2 mV or greater. This value sets the upper bound on the overall system gain. The amplitudes of these waves varies person to person. A system with variable gain is needed to account for potential differences between the amplitude of different individuals’ biosignals. Gains ranging from 100 to 1000 will provide adequate amplification for signals as large as tens of millivolts and as small as hundreds of microvolts.
32
ADC Resolution
The bandwidth, noise and gain specifications have now been set. The next consideration is the ADC resolution. As an example, consider a single supply system at
2.5 V with an 8 bit ADC. An ECG with a QRS complex of 2 mV, and a P-wave of
200
µ
V is the input to the system. A gain of 400 would result in a QRS complex of
800 mV and a P-wave of 80 mV. This results in about 3 bits of resolution for the
P-wave. However, from experience with a previous iteration of the cardiac monitor, an ADC resolution of 8 bits resulted in a small but noticeable amount of quantization error. Greater than 8 bits of resolution (with sufficient gain) will meet the signal requirements. The target ADC resolution is set to 12 bits.
CMRR
The ECG is a differential measurement between two electrodes. Because the signal amplitude is on the order of millivolts or less, an amplifier with high differential gain is required. The most common topology used for ECG and other biopotentials is the
Instrumentation Amplifier (IA). The IA is a differential operational amplifier with
input buffers connected in a resistor totem pole (see Figure 2-3). The differential
gain of the circuit is a vd
= 1 +
2 R
1
R gain
R
3
R
2
. Connecting R gain in the method shown increases the differential gain of the first stage of the circuit while maintaining a common mode gain close to one. This gives the circuit a high CMRR. CMRRs of greater than 90 dB are common in IC implementations of the IA.
Reducing Interference in ECG
Although an IA has a high CMRR, large potential differences between the body and the system common can result in common mode voltages outside the IA’s input range. Additionally, 60 Hz interference from electronic devices can be converted into a differential signal that cannot be eliminated even with an infinitely high CMRR.
60 Hz interference is most often caused by an impedance mismatch between the two
sensing electrodes [19]. If powerline interference capacitively couples onto electrode
33
Figure 2-3: A block diagram view of an instrumentation amplifier. Source: [18]
Figure 2-4: Displacement currents when measuring ECG can cause a differential voltage difference at the IA if Z
1
I
1
= Z
2
I
2
. Either an impedance mismatch or a displacement current mismatch can cause this differential voltage.
leads, displacement currents are induced. A mismatch in electrode impedance can
result in differential voltages at the amplifier inputs (see Figure 2-4). Common mode
voltages can also be converted to differential voltages by the “potential divider effect”
There are three common ways to reduce the effect of large common mode voltages and 60 Hz interference. The first method uses a third electrode, while the second method uses capacitive coupling and filtering. The third method can be used in conjunction with either the first or the second method and involves actively buffering the biosignal at the electrode site.
The first method to reduce common mode voltages and 60 Hz interference involves
a circuit known as a driven right leg (DRL) circuit [21]. This circuit takes a standard
34
two electrode lead and ECG amplifier (usually an IA) and adds a third, driven elec-
trode (see Figure 2-5). The additional circuitry measures the common mode voltage
of the two electrodes and negatively amplifies the resulting signal. The amplifier is referenced to the circuit common and so the body is driven to that potential. Any
60 Hz interference present is also negatively amplified and driven back into the body, reducing its amplitude. This approach works well to drive the body within the circuit voltage rails and eliminate 60 Hz interference, but requires a third electrode.
Figure 2-5: An example of a right leg driven circuit. [22]
The second method to reduce large common mode voltages is to capacitively couple the biosignal into the circuit. A resistor connected to the circuit common combined with the DC blocking capacitor form a high pass filter at the electrode site and reference the resulting signal to the circuit common. This approach does not require an additional electrode, but also does not remove any 60 Hz interference.
Additional filtering is usually required. The 60 Hz interference can be eliminated by a notch filter, implemented in analog or digitally anc can be done before sampling the signal or in post-processing.
The third method to reduce common mode voltages specifically reduces 60 Hz interference. Because most 60 Hz interference is caused by an impedance mismatch between the electrodes, a strategy that reduces this impedance can reduce interference. It has been shown that actively buffering a biopotential can reduce signal
interference by up to 40 dB [19]. This is because a buffer has a low output impedance.
35
When placed right at the electrode site, the biopotential becomes a low impedance signal. With a lower impedance for each electrode, the difference between the two electrode impedances is also reduced. Actively buffering the electrode site can be used in combination with both the first and second methods described above.
2.1.2
Accelerometer Signal Considerations
Signal requirements for the accelerometer are not as constrained as that of the ECG.
The most important considerations are the acceleration range, resolution, and sample rate. To determine a reasonable acceleration range, a prototype board with an accelerometer was worn while an individual tried to generate large accelerations (such as by jumping up and down and running). Acceleration values were usually within
± 4g, so that should be sufficient for obtaining the full range of accelerations. However, in practice, the accelerometer on the cardiac monitor (ADXL346) has a range vs resolution scheme such that a ± 8g range can be obtained with the same LSB value as with ± 4g range. At ± 8g, the ADXL346 has a full resolution of 12 bits. In a previous iteration of the cardiac monitor, acceleration was recorded at both 2 Hz and 250 Hz. While 250 Hz was clearly oversampled, 2 Hz may have been too low. As a compromise, a sample rate of 12.5 Hz was selected.
Specification Minimum Target
Range ± 4g ± 8g
Resolution
Sample rate
8 bits
2 Hz
12 bits
12.5 Hz
Table 2.2: Accelerometer Signal Quality Requirements
2.1.3
System Level Requirements
A summary of the desired system specifications are listed in Table 2.3. Both minimum
and desired specifications are listed. Further details of each specification are provided below.
The signal requirements and use model for the cardiac monitor provide system
36
Specification Minimum Target
Test duration
Memory
24 hours
128 MB
1+ weeks
1+ GB
Power dissipation < 9.7 mW < 9.7 mW
Table 2.3: Overall system specifications level electrical requirements. To meet the bandwidth requirement, the ECG is sampled at 250 Hz. The accelerometer is sampled at 12.5 Hz. Allocating 2 bytes per sample, (250 + 3 ∗ 12 .
5) ∗ 2 = 575 bytes of data are generated each second, and about
2 MB in an hour. To record for a full week, over 300 MB of space is required (not including overhead). The data can either be saved on board or transmitted to a base station and/or cell phone. Because the device needs to be able to sample continuously and last at least one week, while maintaining a small footprint on the user, data was saved on board. Transmitting the data to a base station / cell phone is power intensive to do continuously and also requires the user to wear an additional device or be located near a base station at all times.
Because all data will be stored on board, high density memory must be used. A micro SD card, commonly used in devices such as cameras, is well suited to this task.
However, writing to a micro SD card requires high write currents and takes on the order of several hundred milliseconds. Most low power microcontrollers have low on board memory, so the system must have adequate space on the microcontroller or use an additional memory source for buffering the data. For a test duration of at least one week, a battery with several hundred milliamp hours is required. A 600 mAH battery, for example, could support an average current of 9.7 mW for up to one week at a supply voltage of 2.7 V.
2.2
Software Specifications and Challenges
The software requirements for the cardiac monitor are straightforward. The software must be able to sample the ECG and accelerometer waveforms continuously at 250 Hz and 12.5 Hz, respectively. Data must be sampled and saved continuously. Addition-
37
ally, the software should be able to control the system to keep the power consumption as low as possible. A low power microcontroller is best suited to this task. The microcontroller must have enough communication ports for all the peripheral devices
(the accelerometer and memory) and enough I/O ports for electrode configurability, gain configurability, chip selects, low power operation and debugging.
2.3
Mechanical Specifications and Challenges
The mechanical requirements for the cardiac monitor are not easily quantifiable. The most important mechanical requirement is that the device be robust, yet comfortable.
The board needs to last a week under ambulatory conditions, and be as unobtrusive as possible. The mechanical requirements for the device must be achieved while
maintaining the signal quality requirements discussed in Section 2.1.1.
Work on the first generation device first mentioned in Section 1.5 helped provide
feedback on a form factor that might achieve these goals. The first generation device was fabricated on a flexible “L” shaped PCB and was designed to be completely free of wires. The components of the board were distributed on the top surface of the PCB.
Although the device performed properly electrically, it was not as robust as the use model requires. Because the patch was all on a single flexible PCB, it was constrained to move as one piece. When worn, the device would bend and solder connections would break. If the board had already been coated in Paralene-C for water and sweat proofing, this rendered the affected board useless. Additionally, because the board was wide around the electrode sites, it did not always remain flush against
the electrodes when worn (see Figure 2-6). This caused loose electrode connections,
especially during heavy activity such as walking briskly or running. Finally, the board was not flexible or comfortable enough for use with women. The board is worn on the sternum and is too wide and not flexible enough with breast tissue present.
These issues suggest that a single flexible PCB with circuit components distributed on the surface is not an ideal form factor. A PCB that is more rigid with circuitry concentrated in a smaller area might achieve better results. If this is done, connections
38
Figure 2-6: A view of the top of the ECG patch while worn.
from this central board to the electrodes must be made, either with flexible PCB, wires, or another interconnect. Additionally, because the biopotential will be buffered at the electrode site, circuitry is needed close to the electrode. One solution is to create an additional PCB that interfaces with only the electrodes at the electrode site. The
PCB would be very small and sit on top of the electrode itself. These small PCBs would connect to the larger, more central PCB with an interconnect.
39
40
Chapter 3
Implementation
This chapter will describe the final implementation of the cardiac monitor. First, an overview of the cardiac monitor’s form factor and how it is worn on the body will be described. Next, diagrams and explanations of each aspect of the system will be provided. Finally, measurements of the performance of the system versus the desired
specifications discussed in Chapter 2 will be presented. Validation of the cardiac
monitor in a clinical setting can be found in Chapter 4.
3.1
Final System Overview
The final implementation of the cardiac monitor consists of a central board, along with
either one or two electrode attachments (see Figure 3-1). The additional electrodes
attach to the central board via wires and solder pads on each board. Each electrode connects to a conventional 3M
2560 red dot wet electrode. In the three electrode implementation, the system is designed such that any electrode can act as an input or output. Details about electrode configuration and setup are provided in section
3.2.2. In the two electrode implementation, the electrode configuration circuitry is
eliminated to reduce the footprint on the user. Without a third electrode, the two electrode implementation requires capacitive coupling at the electrode inputs. The
input circuitry for both implementations is discussed in Section 3.1.2.
41
Figure 3-1: The wearable, long term cardiac monitor (three electrode implementation).
3.1.1
Central Board
Solder joints on a previous, more distributed implementation of the cardiac monitor occasionally broke when the PCB was flexed. Therefore, for this iteration of the cardiac monitor, the majority of circuitry is concentrated on the central board. It is 5.5cm wide by 4.2cm tall in the three electrode configuration and 5cm wide by
3.6cm tall in the two electrode configuration, only slightly larger than the 3M
2560 electrodes (4.1cm by 3.5cm). The boards are 4 layers and 0.066cm thick. They has been fabricated with Dupont FR material to allow them to bend slightly when worn.
All circuitry (other than that which must be at the additional electrode sites) is located on these boards. A close up view of the three electrode central board can be
42
(a) Top (b) Bottom
Figure 3-2: The Central Board
3.1.2
Electrode boards
In most ECG monitors, only an electrode exists at the electrode site. This electrode is then connected to the main system with long wires. However, in this cardiac monitor,
the biopotential at the electrode site is actively buffered. As discussed in section 2.1.1,
it has been shown that actively buffering the electrode signal can reduce power line
interference by up to 40 dB [19]. This requires an op-amp as close to the electrode
site as possible. Additionally, to accommodate both the two and three electrode implementations, either capacitive or resistive coupling of the ECG input can be used. Recall that the two electrode implementation requires capacitive coupling as it does not have a third electrode. Additionally, each electrode can act as an output
(i.e. driven electrode) if used in the three electrode implementation. This electrode would then act as the driven right leg electrode in the circuit discussed in section
2.1.1. The electrode board is 2cm wide and 2.4cm tall. A close up of the electrode
3.1.3
Cardiac Monitor Placement
The cardiac monitor is worn in one of two configurations, depending on the number of electrodes in use. In the three electrode case, the central board is placed just below the sternum. The first electrode board is placed above the central board on
43
Figure 3-3: The Electrode Board
Figure 3-4: The electrode board is configured as an input. The signal from the electrode is buffered by an op amp that is connected to the IA.
Figure 3-5: The electrode board is configured as an output. The signal from the driven right leg circuit is connected to the electrode. This electrode then drives the body.
44
the sternum, and the third electrode is placed just under the left pectoral near where
a V6 electrode would be placed (see Figure 3-6). As discussed in section 2.1.1, one of
three different ECG leads can be selected without moving the electrodes. In the two electrode configuration, only one lead can be used at a time. Although the device can be placed anywhere on the chest to obtain an ECG lead, it is placed in a lead
I configuration (see Figure 3-7) with one electrode on the upper sternum and the
other at least 10 cm away parallel to the first electrode. It was shown in [23] that the
maximum ECG signal amplitude was obtained at an interelectrode distance of 10 cm.
The cardiac monitor does not protrude significantly from the chest. As demonstrated
in Figure 3-8, it is very discrete when worn.
Figure 3-6: The three electrode implementation of the cardiac monitor worn at the chest.
3.2
Hardware
The final system implementation consists of three main subsystems: the analog front end, digital back end, and power management circuitry. A block diagram of the final
45
Figure 3-7: The two electrode implementation of the cardiac monitor worn at the chest.
(a) Cardiac monitor with shirt (Front) (b) Cardiac monitor with shirt (Side)
Figure 3-8: A user wearing the three electrode implementation of the cardiac monitor.
The monitor is very discrete and does not protrude significantly from the chest.
46
system can be found in Figure 3-9.
Figure 3-9: A block diagram of the overall system. A simplified version of the Analog
Front End is shown in orange. The Digital Back End consists of the red tinted blocks, and the inputs are shown as red (+), green (-), and black (gnd). Power Management and the battery are shown in blue.
3.2.1
Analog Front End
The analog front end (AFE) is responsible for acquiring the ECG signal from the electrodes and amplifying the signal. It also filters the signal before it is sampled by the ADC. The AFE consists of the electrodes themselves along with any analog circuitry used in the signal chain before the ADC. A circuit diagram of the analog
front end can be found in Figure 3-10. While the electrode configurability circuitry
influences the analog front end, it is included as part of the digital back end and is
presented in section 3.2.2. The output from each of the sensing electrode goes through
two different paths. The first path is used to amplify the ECG signal and filter it before it is sampled by the ADC (i.e. ECG signal conditioning). The second path will be used to generate the common mode feedback signal used to drive the body to a fixed potential.
47
Figure 3-10: The Analog Front End, beginning with the Instrumentation Amplifier.
The signal then goes through a gain selection stage and filter before being sampled by the MSP430.
ECG Signal Conditioning
The AFE’s forward path consists of an instrumentation amplifier and a variable gain
stage with anti-aliasing and smoothing filters (see Figure 3-10). The signal from the
two sensing electrodes provides the input to the instrumentation amplifier, which is an INA333 configured with a gain of about 20. The output of the INA333 is used as an input to an OPA333 with input resistance of 300kΩ and a feedback capacitance of
1 µ F. The output of this amplifier goes to the reference pin of the INA333, effectively acting as a high pass filter with a cutoff frequency of 0.5 Hz. This high pass filter AC couples the signal without placing large capacitors in the signal path.
The output of the IA is passed through a SN74CBTLV3125 quadruple FET bus switch that is connected to four binary weighted resistors. The four resistors act as the input resistor of an inverting amplifier that follows the IA. With a constant feedback resistance and 4 bits of selectable input resistance, gains ranging from about
120 to about 1800 can be achieved with a resolution of 120. Gain can be adjusted using the MSP430 to maximize dynamic range and account for variation in biosignal amplitude from user to user. The inverting amplifier also acts as a low pass filter.
48
It has a feedback resistance of 1MΩ and a feedback capacitance of 1.2 nF, which corresponds to a low pass cutoff frequency of approximately 125 Hz. Along with noise bandwidth reduction, this amplifier serves as an antialiasing filter for the system, which is sampled at 250 Hz. The output of this amplifier then goes through a singlepole low pass filter with a cutoff of 285 Hz and then is sampled by the ADC. This low-pass filter is used to reduce high frequency ADC sample clock feed-through.
Driven Right Leg Circuit
The AFE’s feedback path is the driven right leg circuit. This circuit takes the signal from the two sensing electrodes and sums them. The sum is then buffered by an
OPA333. The buffered output is then negatively amplified by another OPA333 by a factor of 39.5 with respect to the circuit midrail. A 120 pF capacitor is included on the second OPA333. This capacitor in combination with the feedback resistance creates a low pass frequency of the driven right leg circuit of 3.4 kHz. This ensures loop stability of the entire front end. Circuit stability in the driven right leg circuit is
discussed in depth in [21]. The driven right leg circuitry is included in the schematic
3.2.2
Digital Back End
The digital back end is controlled by an MSP430F247 microcontroller. This MSP430 was selected for its low active and standby power, and because it had enough serial peripheral interface (SPI) and I/O ports required for the system. The MSP430 interfaces with all peripheral chips. This includes all the electrode configuration circuitry), memory, accelerometer, and 3 LEDs for debugging. The MSP430’s internal
12 bit ADC is also used. In this section, the interface between the MSP430 and all peripheral devices will be described. Software implemented on the MSP430 to control each peripheral and implement the final algorithm for the cardiac monitor will
49
Accelerometer
The accelerometer (ADXL346), like the memory, communicates with the MSP430 via the SPI protocol. It has its own dedicated SPI port. The ADXL346 has multiple resolution modes that maintain 4 mg resolution. Based on the requirements outlined
in section 2.1.2, the range of the accelerometer was set to
± 8g with full resolution
(12 bits). It samples at 400 samples / second in a “first in first out” (FIFO) stream mode. The accelerometer has FIFO buffers for each axis that store samples. The
FIFO stream mode samples continuously and overwrites the FIFO buffers with the most recent samples. Each FIFO buffer can store up to 32 samples. Sampling at
400 samples / sec and saving up to 32 samples corresponds to the first sample in the buffer being refreshed 400 / 32 = 12.5 times per second, which is the desired sampling frequency.
Electrode Configurability
In the three electrode implementation, each electrode can be configured as either a positive input, negative input or output/driven electrode. When an electrode is configured as an input to the system, the signal at the electrode site is immediately buffered by an OPA333 low-offset op-amp. The output of each buffer is connected to two TS5A3359 SP3T switches. The two control lines on each switch are controlled by the MSP430 and are used to connect one of the three electrodes to either the positive or negative terminal of the INA333 instrumentation amplifier. Each electrode is also connected to a third TS5A3359 switch, also controlled by the MSP430. This switch is connected before the signal is buffered and is used to connect an electrode to the output of the drive right leg circuit. When an electrode is configured as an output, the MSP430 uses a TS5A3160 switch to turn off the OPA333 to save power.
Memory
The cardiac monitor has two different types of memory: serial flash memory (2
S25FL128P) and a micro SD card. Because a micro SD card is power intensive
50
and takes hundreds of milliseconds to write to, the serial flash memory is used as a buffer for the ECG and acceleration data as it is sampled. Once enough data has been saved to the serial flash memory, the data is written to the micro SD card and can be accessed by a computer. Two serial flash memories are used for usability reasons.
Bits in the serial flash cannot be changed from 0 to 1 without an erase operation, which requires that a page be erased before it can be written to again. A bulk erase of the serial flash memory takes on the order of 120 seconds, which is an inconvenient duration for the user to have to wait to use the device. By having two serial flash memories, one device can be used at a time so the user does not have to wait for the serial flash to erase.
Both the serial flash chips and micro SD card interface with the MSP430 using
SPI. The serial flash chips share an SPI port. Only one chip can be accessed at a time using the appropriate chip select. The micro SD card has its own dedicated SPI port.
Each serial flash chip has 128 Mbits of memory for a total capacity of 256 Mbits. The serial flash chips are organized in 256 byte pages that are accessed using three page address bytes. The lowest page address byte (page address 0) refers to a byte within the page, and page address 1 and page address 2 act as a 16 bit number used to select the appropriate page. A micro SD card has a capacity anywhere from 1 MB to 32
GB. In this thesis, testing was done primarily using a Kingston 4 GB micro SDHC card and a SanDisk 2 GB micro SD card. The micro SD card is broken down into blocks of 512 bytes. One block of micro SD can hold two pages of flash memory.
3.2.3
Power Management Circuitry
A Cameron Sino CS-EC003SL lithium ion battery is used to power the system. It has a capacity of 600 mAH at 3.7 V and is 4cm by 3.2cm. The battery is placed on top of the central board and can be secured with medical tape during testing (see Figure
3-11). Charging for the battery is provided on an external board using an ADP2291.
There are two voltage regulators on the central board. A TPS78227 2.7 V linear regulator is used to provide power for all analog circuitry. This includes all chips used in the analog front end, along with the AV
CC of the MSP430. A TPS62200 switching
51
Figure 3-11: The three electrode board with battery on top.
regular is used as the digital voltage supply. A switching regulator was used to boost efficiency during high loads, such as writing to memory.
An undervoltage circuit is used to prevent the battery from discharging below about 3.1 V. A comparator (TLV3401) connected as a high side voltage sense circuit is used to provide this undervoltage protection. The negative terminal of the comparator is connected to a REF3312 1.25 V reference. The battery voltage goes through a voltage divider and the result is compared to the 1.25 V reference. The output of the comparator controls a TS5A3166 SPST switch. If the output of the comparator is high, the battery voltage is high enough and the SPST switch connects the battery voltage to the voltage regulators. If the output of the comparator is low, the switch
52
is off and the system receives no power.
3.3
Software
The software for the system is implemented on the MSP430 in C. It initializes the system, samples the ECG and accelerometer, and saves the data to memory. In this section, the final algorithm will be presented. Details about each particular aspect of the software will be described, with small code snippets if relevant. Much of the software implementation was straightforward. However, the micro SD code was the most difficult to write, despite the open source code available. Code used to initialize
and write to the micro SD card is included in Appendix A.
3.3.1
Final Algorithm
The final algorithm is presented in flowchart form in Figure 3-12. The system first
starts up and confirms that a micro SD card is present before proceeding. It selects a flash memory and then activates a timer interrupt that overflows once every 4 ms.
When the interrupt is active, the ECG is sampled, and the Accel is sampled every
20 interrupts. After each interrupt, the system checks to see if it should write data to the serial flash or micro SD card. If needed, writes to these different systems are executed. Note that the system continues to sample the ECG and the Accelerometer even while writing to memory. Samples taken during this period are stored in a separate buffer that is transferred back to the signal buffer at the end of each write.
The different blocks will now be described in more detail.
3.3.2
Allocate space and structures
The first thing the MSP430 does is allocate space for all the buffers used for sampling and storage. All space is pre-allocated at start up. Each data sample is 12 bytes, but is treated as a 16 bit (2 byte) sample with the upper nibble of the high byte zero
padded. Each sample is saved as a SAMPLE structure (see Listing 3.1). While not as
53
Figure 3-12: The Final Algorithm run on the cardiac monitor presented in flow chart form. ACount is a counter that increases each time an ECG sample is taken and then is reset after an accelerometer sample is taken. PCount is a counter that increases each time a page is written to flash and then is reset after a micro SD block is written.
54
Listing 3.1: The SAMPLE structure typedef s tr u c t
{ char BYTE0 ; char BYTE1 ;
} SAMPLE;
// Low b y t e
// High b y t e
Listing 3.2: The STORE structure typedef s tr u c t STORE
{ char i n d e x ; char tempIndex ; char DATA[ 2 5 0 ] ; char TEMP[ 2 0 0 ] ;
// DATA b u f f e r i n d e x
// TEMP b u f f e r i n d e x
// DATA b u f f e r
// Temporary o v e r f l o w b u f f e r
} STORE; space efficient as possible, it is more simple when analyzing the data. Additionally, the upper nibble can be used in the future to encode additional information (see
Chapter 5). All samples are saved in STORE structures (see Listing 3.2). These
structures have two buffers with indices. The DATA buffer is the primary data buffer that is used until it is full and is 250 bytes. The TEMP buffer is 200 bytes. This size was selected based on write times to memory and should be large enough to prevent data loss. A large buffer called SPACE is pre-allocated for use as a buffer for the micro SD transfer.
A structure to keep track of access to the flash memory is also used. Because the flash memory will be accessed in two different ways, two structures are initiated. The flash will be written to when a signal buffer is full, and it will be read from when preparing to write to the micro SD. The FLASH structure keeps track of the flash in use and the current page address. It also knows how many flash devices are available in case it reaches the end of a flash device. The FLASH structure is shown in Listing
55
Listing 3.3: The FLASH structure typedef s tr u c t
{ char DeviceNum ; char D e v i c e S t a r t ; char DeviceEnd ; char PA2 ; char PA1 ;
} FLASH ;
// C u r r e n t d e v i c e number
// F i r s t d e v i c e number
// L a s t d e v i c e number
// Page A d d r e s s 2
// Page A d d r e s s 1
3.3.3
Initializing the System
The first thing the MSP430 does after power-on is initialize the clock setup. The master clock (MCLK) is generated from an internal oscillator and set to 1 MHz. The sub-main clock (SMCLK) is used as the clock for the memory and accelerometer and is also set to 1 MHz. After the clocks have been set up, the I/O pins are configured. A
full listing of the pins used on each port is provided in table 3.1. Detailed descriptions
of each pin used are summarized in table 3.2. The pins corresponding to the three
SPI ports in use are configured, along with all logic circuitry and chip selects. Chip selects for all peripherals start out high. By default, the system is set for a gain of about 250. In the three electrode implementation the electrodes are configured to give a Lead II projection. The I/O mapping in the two electrode configuration is similar to that of the three electrode, but is not shown for simplicity. A few I/Os changed position to optimize layout.
Port BIT7 BIT6 BIT5 BIT4 BIT3 BIT2 BIT1 BIT0
P1 INS11 INS22 INS21 BUF3 GREEN YELLOW RED
P2 NC SEL4 SEL3 SEL2 SEL1 BUF2 NC
NC
INS12
P3
P4
SO2
CS5
SI2
GS1
CS1
GS2
BUF1
NC
SCLK1
NC
SO1
NC
SI1
NC
CS6
CS2
P5 NC NC
P6 ADC0 NC
NC
NC
NC SCLK3
NC NC
SO3
NC
SI3 SCLK2
NC NC
Table 3.1: The GPIO configuration of the cardiac monitor.
After the I/Os are configured, the SPI ports for all the peripheral devices are set up (configuring the I/Os only puts the pins in SPI mode). The timer and ADC are
56
I/O Name BIT Description
ADC0
BUF1
BUF2
BUF3
CS
CS
CS
1
2
5
P6.7
Output of the AFE / Input to the internal ADC
P3.4
Set electrode 1 V
DD
P2.2
Set electrode 2 V
DD
P1.4
Set electrode 3 V
DD of buffer high of buffer high of buffer high
P3.5
Serial Flash 1 chip select
P4.0
Serial Flash 2 chip select
P4.7
Micro SD chip select
CS 6
INS11
INS12
INS21
INS22
GS1
GS2
P3.0
P1.7
P2.0
Accelerometer chip select
Control logic to select electrode input to IA+ (1)
Control logic to select electrode input to IA+ (2)
P1.5
Control logic to select electrode input to IA- (1)
P1.6
Control logic to select electrode input to IA- (2)
P4.6
Control logic to select electrode output to DRL (1)
P4.5
Control logic to select electrode output to DRL (2)
GREEN P1.3
Green LED
YELLOW P1.2
Yellow LED
RED
SEL 1
P1.1
P2.3
Red LED
Switch connected to 1x resistor in AFE
SEL 2
SEL 3
SEL 4
P2.4
P2.5
P2.6
Switch connected to 2x resistor in AFE
Switch connected to 4x resistor in AFE
Switch connected to 8x resistor in AFE
SI1
SCLK1
SO1
SI2
SCLK2
SO2
SI3
SCLK3
SO3
P3.1
P3.3
P3.2
P5.2
Slave data in for accelerometer
Slave clock for accelerometer
Slave data out for accelerometer
P3.6
Slave data in for serial flash
P5.0
Slave clock for serial flash
P3.7
Slave data out for serial flash
P5.1
Slave data in for micro SD
P5.3
Slave clock for micro SD
Slave data out for micro SD
Table 3.2: GPIO descriptions
57
also configured. The timer is set to use the SMCLK, divide by 8, and then count up to 500 (1 MHz / 8 = 125 kHz, 125 kHz / 500 = 250 Hz). The ADC is configured to sample on each timer interrupt.
3.3.4
Checking the micro SD card
A standard micro SD card is used to transfer data from the serial flash memory to a form readable by the user. When the system starts, the MSP430 tries to initialize the micro SD card. If no micro SD card is present, the main loop exits and a red LED indicates no card is present and that the test has failed. Micro SD card initialization
is described extensively elsewhere [24]. However, open source code available was not
as robust as was needed for this application, and did not always have proper error
checking. The code used for this system is included in Appendix A. Briefly, the
initialization process first powers on the device and waits a few milliseconds before sending 100 clock pulses with the card deselected (CS high). Next, a reset command is sent to the card. If this operation succeeds, another command to identify the type of card is sent. Some micro SD cards use block addressing, and this setting needs to be appropriately determined to properly address the card. The initialization process is relatively straightforward, but care must be taken to ensure each operation has time to complete before starting the next one.
3.3.5
Selecting the available flash memory
Once the system has verified that a micro SD card is present, it checks to see which
(if any) of the two flash memories have been written to previously. This is important because bits in the serial flash memory can only be changed from 1 to 0. Changing a bit from 0 to 1 requires an erase operation. Unfortunately, erasing the serial flash memory can take up to 128 seconds, which is a long delay for the user two wait before use. To avoid this long wait, the system checks to see if the first flash has been written to by checking if any of the bits on the first 8 pages are 0. If the flash has been written to, the MSP430 sends the bulk erase command to the device and tries
58
the second device. If this device is also in use, it sends the bulk erase command again and waits for the first device to finish. If the second device is not in use, it is used in place of the first device. Even if the first device is not in use, the system still checks the second device and issues the bulk erase command for future use.
3.3.6
Sampling Timer Interrupt
Timer A on the MSP430 is used to trigger an interrupt 250 times per second. The
ADC is sampled during each interrupt to provide the 250 Hz sampling rate required for the ECG. Each sample is appended to a buffer dedicated to ECG data only.
Because the accelerometer only needs to be sampled at 12.5 Hz, a counter within the interrupt ensures that the accelerometer is sampled only once every 20 interrupts, which results in an effective sample rate of 12.5 Hz. Each time the accelerometer is sampled, values from each axis are saved to their respective data buffers.
Because the cardiac monitor must be able to sample continuously and without interruption, the timer interrupt must continue to operate even when data is being written to memory. When possible, sampled data is saved directly to a primary data buffer. When the primary data buffer is full, any additional samples are saved to the
TEMP buffer and then are returned to the DATA buffer after it has been written to memory.
After a timer interrupt has been completed, the MSP430 enters low power mode
1, which disables the CPU and MCLK. SCLK, which is used for timer A and the other peripheral devices, remains active. When timer A overflows, it exists low power mode 1 and carries out the timer interrupt. Normally after such an interrupt the
MSP would return to low power mode 1. However, at this time the device is put into active mode. In active mode, the system has a while loop that checks if data should be written to the serial flash and/or micro SD. After data is written (if necessary), the system re-enters low power mode 1 until the next timer interrupt.
59
3.3.7
Saving Data to Serial Flash
When a STORE buffer is full, the MSP430 writes a page of data to the serial flash.
Each 256 byte flash page is broken down into a 3 byte header, 250 byte data block,
and 3 byte footer (see Figure 3-13). The header contains the 3 page address bytes
and the footer identifies the type of data block written, along with which of the two flashes it was written to. To write a flash page, the MSP430 first checks the status register of the flash to see if it is busy. Next, the write enable latch is set high and the chip select is set low. The write command is passed to the serial flash along with the 3 page address bytes (page address 0 is always 0). After the page address bytes are sent, the header, 250 data bytes, and footer are transmitted. The MSP430 checks the status register again until the write has completed, and then increments the page address pointer to the next page. If the page address pointer is at the end of the flash device, it moves to the first page of the next flash. Any data samples that were obtained while writing to the serial flash are restored to their proper buffers. Each type of data is written to its own page. With ECG samples of 2 bytes taken at 250
Hz, a page of ECG data will be written about twice per second. With Accel samples of 2 bytes each taken at 12.5 Hz, three pages of Accel data (X,Y,Z) will be written
about once every 10 seconds (see Figure 3-14).
Figure 3-13: An Example Flash Block
60
Figure 3-14: Data saved on flash memory.
3.3.8
Saving Data to micro SD
Once enough data has been buffered by the serial flash memory, it is written to the micro SD card. Each write to the micro SD card requires significantly more power than the serial flash memory, but improves with number of blocks written. The micro
SD card is written to when at least 8 pages of flash memory have saved but are not present on the micro SD. This occurs about once every 4 seconds and corresponds to a multi block write of at least 4 micro SD memory blocks. When the micro SD is ready to be written to, up to 8 pages of data are read from the serial flash and saved in the SPACE buffer. Once the SPACE buffer is full, this data is written to the micro SD in a single write. Next, any additional data is read from the serial flash, saved in the SPACE buffer, and written to the micro SD. If an odd number of serial flash pages need to be written to the micro SD, the second half of the last micro SD block is filled with a constant value that can be removed in post processing. After the micro SD write has completed, an end of file (EOF) marker is written. When the next write is initiated, the EOF marker is overwritten and a new one is appended to the card.
3.3.9
Importing the data
Once the data has been saved to the micro SD card and testing is complete, it can be
read directly by a computer. The hex editor Hex Workshop [25] is used to search for
the EOF marker on the micro SD card and then all the data is saved as a binary file.
This binary file is read by a matlab script that generates data vectors corresponding
61
to each signal. The matlab script iterates through the binary file 256 bytes at a time
(i.e. one page of flash memory at a time). First, the script determines if the page of interest is a valid page of data. If not, it either skips to the next page or terminates depending on the contents of the header and footer. If the page is a valid data page, the script converts the 250 data bytes into their corresponding 125 data samples. In the case of the ECG, this involves converting the high byte and low byte into a 16 bit number and changing from ADC code to volts. In the case of the accelerometer, the high byte and low byte are combined, and the resulting number is converted from a two’s complement number to a signed integer. This signed integer is then converted into gs. When the script is finished, MATLAB automatically plots the ECG data in one window and the 3-axis acceleration data in another.
3.4
Cardiac Monitor Specifications
This section will discuss how the cardiac monitor meets the specifications outlined in
Chapter 2. The ECG specifications will be presented first, followed by the accelerom-
eter specifications and finishing up with the system specifications.
3.4.1
ECG Specifications
Specification
Bandwidth
Target Calculated Measured
0.5 – 125 Hz 0.5 – 133 Hz 0.5 – 112 Hz
Input Referred Noise < 20 µV rms
Gain 100–1000+
CMRR (@ 60 Hz) 90+ dB
3.3
µV rms
118–1770
100 dB
3.6
µV rms
130–1500
99 dB
Table 3.3: ECG Signal Quality Requirements and Measurements
Specification Value
Leads 1
Electrodes 2–3
ADC resolution 12 bits
Table 3.4: ECG Signal Quality Specifications by Design
62
The cardiac monitor was designed to take a single ECG lead with 2 or 3 electrodes and is sampled at 12 bits. The high pass frequency of the cardiac monitor is set by an op amp fed into the reference pin of the instrumentation amplifier. The time constant set by the input resistor and feedback capacitance results in a high pass corner of 0.5
Hz. The low pass corner frequency of the system is set by the inverting amplifier in the analog front end. The actual low pass corner frequency is 112 Hz.
The input referred noise of the analog front end is calculated by considering the following noise sources: two OPA333 input buffers, one INA333, another OPA333 acting as an inverting amplifier, and resistors connected to this inverting amplifier.
The noise from the OPA333 and associated resistors is divided by the first stage gain.
The noise of the OPA333 is 53 √
Hz and the noise of the INA333 is 50 √
Hz
. The total noise integrated over the bandwidth of 0.5 to 125 Hz (assuming a single pole roll off) is 3.3
µV rms
. The power spectral density of the output referred noise was determined using the psd function in Matlab. Noise power was divided by the square of the amplifier transfer function and integrated over the range of 0 Hz to 112 Hz * π/ 2 (to account for single pole roll off) to determine the total RMS input referred noise of
3 .
6 µV rms
.
The gain of the system is set by 4 binary weighted resistors and, from calculations, should range between 118 and 1770. In practice, the gain appears to be slightly less than the calculated values. The CMRR is 99 dB, which is around what was specified by the Instrumentation Amplifier data sheet.
3.4.2
Accelerometer Specifications
Specification Value
Range ± 8g
Resolution 12 bits
Sample rate 12.5 Hz
Table 3.5: Accelerometer Signal Quality Requirements
The accelerometer specifications are easily configured using the ADXL346. It was configured to have a range of ± 8g, with a resolution of 12 bits and a sample rate of
63
12.5 Hz.
3.4.3
System Specifications
Specification Minimum Target
Test duration 24 hours 1+ weeks
Memory 128 MB 1+ GB
Actual
9 – 10 days
1+ GB
Power dissipation < 9.7 mW < 9.7 mW < 6.6 – 7.3 mW
Table 3.6: Overall system specifications
The test duration specification is set by the minimum of the memory and the power dissipation. It will either be memory limited or power dissipation limited. 20
ECG pages and 3 accelerometer pages are written every 10 seconds. This corresponds to 23 pages
10 sec
∗ 256 bytes page
= 5888 bytes
10 sec
.
For a test duration of one week, 0.33 GB of memory is required. A micro SD card can have higher capacity than this, so the device will be limited by its power dissipation.
To determine power dissipation, the relative contributions of the different parts of the final algorithm need to be considered. First, the standby current of the system was measured to be about 0.6 mA. Next, the average current was measured with the timer interrupt enabled. This current was about 0.8 mA. The average current drawn when only writing to the serial flash was between 1.5 mA and 3 mA. Finally, the average current drawn when writing to the micro SD (and getting the corresponding data from the serial flash) was between 9 mA and 10 mA.
Operation
Standby
Current
0.6 mA
Timer Interrupt 0.8 mA
Serial Flash Write 1.5 mA – 3 mA
Micro SD Write 9 mA – 10 mA
Table 3.7: Currents drawn for different operations of the cardiac monitor.
The current drawn with the timer interrupt enabled is considered an “always on” contribution to the overall power dissipation. To determine the contribution of writing to the flash and writing to the micro SD, the duty cycle of such contributions
64
must be determined. Writing a single page of serial flash data (with all overhead) takes 32 ms. Each write to the micro SD card (4+ blocks, including getting data
from the micro SD and writing the EOF marker) takes 680 ms total (see Table 3.8
for the breakdown).
Operation
Serial Flash write
Serial Flash read (8 pages)
Micro SD write (4 blocks)
Micro SD write (1 block)
Duration (ms)
32 ms
408 ms
208 ms
64 ms
Table 3.8: Write / Read times for different operations of the cardiac monitor.
The micro SD card is written to approximately once every 4 seconds. The worst case contribution of the flash is then:
3 mA ∗
23 pages
10 sec
∗
32 ms page
= 3 mA ∗
73 .
6 ms sec
= 0 .
22 mA (3.1)
And the worst case contribution of the micro SD card is 10 mA ∗ 680 ms
4 sec
= 1 .
7 mA .
The total average current drawn is then in the worst case 2.72 mA or 7.3 mW.
Repeating the same analysis with a current of 1.5 mA for the serial flash and 9 mA for the micro SD results in a power dissipation of 6.6 mW. The system should be able to run for 9 – 10 days before recharging.
65
66
Chapter 4
Clinical Test
After testing basic device functionality, the next step in validating the wearable cardiac monitor is with a clinical test. In this chapter, a clinical test conducted at the
Clinical Research Center (CRC) 1
at MIT will be presented. Motivation for the study, the study protocol, analysis and preliminary results will be discussed.
4.1
Motivation
The motivation for the study can be broken down into four distinct goals, summarized below.
To evaluate ECG signal quality of the cardiac monitor
To determine the comfort level of the cardiac monitor
To examine the relationship between activity levels and heart rate
To obtain a comprehensive suite of physiological signals in one data set for future algorithm development
1 This work was conducted with support from Harvard Catalyst — The Harvard Clinical and
Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the
National Institutes of Health.
67
4.1.1
ECG Signal Quality
The first goal is to evaluate the signal quality of the ECG on the cardiac monitor. The
ECG should have a clear view of the P wave, QRS complex and T wave. Modulation of the QRS amplitude associated with respiration should be readily visible. The ECG should be clean even during activities of high movement such as running, and should be of at least as high quality as a currently available ECG monitor.
4.1.2
Comfort Level
The second goal of the clinical test is to evaluate the comfort level of the device. The participants will be wearing the device while they carry out different activities. The board should be as minimally obtrusive to the performance of the tasks. Ideally, the participants would not even notice that they are wearing a device.
4.1.3
Activity Level
The third goal of the clinical test is to examine the relationship between activity level
(as measured by the 3-axis accelerometer) and heart rate.
4.1.4
Creating a Suite of Physiological Signals
The fourth goal of the clinical test is to obtain a comprehensive suite of physiological signals. This suite can be used to better understand the cardiovascular system as important signals will all be in the same, concurrent data record. Future algorithm development could benefit from such a comprehensive suite.
4.2
Protocol
Four healthy participants ages 22 to 40 with an average age of 28.5 years were tested.
Data from all four were deemed acceptable for analysis. All subjects wore the three electrode model in a lead II configuration (the two electrode model will be tested in
future work). A Criticare 504-US ECG recorder [26], with electrodes placed adjacent
68
to those on the cardiac monitor, was used as a comparison. Participants also wore
signals during the test. After all the devices are positioned on the participant, the participant’s left hand is placed in a sling. This sling keeps the Portapres at the level of the heart and helps keep some of the wires out of the way throughout the interventions.
Figure 4-1: Participant with all the devices attached and ready to begin the clinical test.
The test was broken down into three different activity levels: rest, moderate, and heavy. When possible, the participant was given a minute of stationary rest between interventions to allow the body to return to a baseline state and re-calibrate the blood pressure monitor. A button was pressed at the beginning and end of each intervention
(once to signal a start, twice to signal and end). Times of the corresponding button
described above is conducted twice in a row (time permitting) for each participant.
Two researchers on the IRB and a nurse were required to be present for the duration of the test. Before the test, vital statistics such as age, height, weight and blood pressure were recorded. After the test, participants rated the comfort of the device.
Oversight for research participants was provided by Catherine Ricciardi RN, BSN and Ravi Thadhani MD, PhD.
69
Figure 4-2: Sequence of interventions in the clinical test (run twice per partcipant).
Figure 4-3: Researcher recording the times associated with each interval.
The participant begins the test in the supine position by lying down on a hospital bed for 5 minutes. After time has elapsed, the participant sits up quickly, rests for 1 minute, and then performs the action of sitting for 5 minutes. Finally, the participant stands up, rests for 1 minute, and then performs the action of standing for 5 minutes.
After the participant has stood for the appropriate duration, the moderate activity tests begin. The moderate movement phase begins with light hopping, followed by left arm movement, and finishes with a valsalva maneuver. A valsalva maneuver is performed by attempting to exhale against a closed airway and is used to evaluate the autonomic nervous control of the heart. The light hopping and left arm movement are each 1 minute in duration, with 1 minute between interventions for calibration.
The valsalva maneuever takes a total duration of 10 seconds.
The heavy activity portion of the clinical test begins with 5 minutes of walking
70
on a treadmill, at a pace of no more than 4 mph. Without stopping, the speed of the treadmill is then increased to a light jog / run at a pace of no more than 7 mph. The participant is given 2 minutes to rest after stepping down from the treadmill. At this point in time the participant is also offered water and given any additional time if necessary. The last intervention is stair stepping. The participant steps up and down off the back of the treadmill for 1 minute. After the stair stepping is complete, final calibration of the blood pressure monitor and the protocol is complete.
Figure 4-4: Participant walking on the treadmill while the data is being monitored on the PC.
4.3
Analysis
4.3.1
Cardiac Monitor Signal Quality
The first objective of the clinical test was to evaluate the performance of the cardiac monitor described above. This was achieved by simultaneously recording an ECG trace from the cardiac monitor and a device already accepted in the literature (i.e.
the Criticare recorder). We chose to compare the two ECG traces based on how each trace performed when analyzed with a QRS detection algorithm (in this case, the
71
The first step to compare the two ECG traces is to align the two signals in time.
The start and stop times of both traces were recorded. First, the clinical recorder data was imported into the WaveForm Database (WFDB) format for use in Physionet’s
WAVE viewer [30]. The clinical recorder samples at 12 kHz, so the data was down-
sampled to 250 Hz. The cardiac monitor data was imported into a separate WFDB file. While this trace is ideally sampled at the same frequency as the downsampled clinical recorder trace, the effective sampling frequency of the cardiac monitor can vary by about ± 1 Hz. To determine the effective sampling rate, each ECG trace’s R waves were annotated. An R-R interval series was extracted for each trace. The two series were plotted in matlab and aligned in time. Once the series were aligned in time, the relative sampling rate between the two traces can be measured. For example, if for the same beat, one trace records an R-R interval of 126 samples whereas the other records an R-R interval of 125 samples, the effective sampling frequency can be determined by considering that:
126 x
=
125
250 or x =
126
125
250. With the effective sampling frequency and relative timings between the two traces in hand, the two traces can be aligned. In practice, dropped data from the clinical recorder prevented perfect realignment of the two traces for the entire duration of the clinical test.
Now that the traces are time aligned, a “gold standard” set of annotations is needed. These annotations should be the best possible representation of when a heart beat occurred during the clinical test. The “gold standard” annotations were created by first running the wqrs algorithm (a widely available QRS detection algorithm), then manually reviewing the annotations and making corrections as needed.
The “gold standard” set of annotations were then compared with the annotations generated by the wqrs algorithm (without any human intervention) for each trace. If a given ECG trace is of sufficient signal quality, the QRS detection algorithm should produce annotations in close proximity to the “gold standard” annotations. However, if sections of the given ECG trace have inadequate signal quality, additional annotations may be added, or skipped completely. An annotation from the test ECG trace and a “gold standard” annotation are considered a match if they are found within
72
150 ms of each other.
Comparisons were made using the bxb algorithm (a beat by beat comparison algorithm), which is readily available in the PhysioNet Toolkit. The bxb algorithm takes two different sets of annotations as inputs and returns two outputs: QRS sensitivity and QRS positive predictivity. QRS sensitivity is defined as the percentage of QRS matches the wqrs algorithm found of the total number of “gold standard” annotated beats. This is a representation of how capable the given ECG trace was of identifying a real beat (as indicated by the “gold standard” annotation). Missing a beat lowers this percentage. QRS positive predictivity is defined as the percentage of correct QRS detections compared with the total number of QRS detections. This is a representation of how reliable the given ECG trace was at identifying a real beat.
False positives lower this percentage.
4.3.2
Cardiac Monitor Comfort Level
The second objective of the clinical test was to determine how comfortable the cardiac monitor was to wear. Each participant was asked to complete an exit survey after testing. The survey asked how comfortable the cardiac monitor was, and asked for written feedback about any improvements that could be made to the device.
4.4
Results
4.4.1
Cardiac Monitor Signal Quality
The QRS sensitivity and positive predictivity of the two ECG traces are summarized
in Table 4.1. Both devices performed well in almost all the interventions. The biggest
differences between the two occurred during interventions with heavier movement, particularly running. When there was a lot of movement, the clinical recorder ECG trace had a lot of baseline drift and a lot of noise (possibly due to motion artifacts or the electrodes coming loose). Care was taken to ensure that the wires were as secure as possible, but the clinical recorder appeared to be generally less reliable than
73
the cardiac monitor. In two of the four subjects, the running section on the clinical recorder could not be properly annotated because there was so much noise. In the two subjects where running could be annotated, the QRS sensitivity was high (95.3%).
However, the predictivity was low (24.6%)
In the first two subjects, the analog front end appears to have saturated. While the ADC is not completely railed, a comparison of the relative amplitudes of the
P and T waves versus the QRS complex and a lack of respiration in much of the recording suggests that the amplifier is saturated. Data comparing the two ECG
traces during running and standing can be seen in Figure 4-5 and 4-6. A trace from
the third subject that demonstrates a more appropriate scaling between the QRS
complex and the P and T waves is presented in Figure 4-7.
Interventions
Supine
Sit
Stand
Hop
Move
Valsalva
Walk
Run
Step
QRS Sens. (mean,std) QRS Pos. Predict. (mean,std)
Cardiac Clinical Cardiac Clinical
100 (0) 99.89 (0.25) 100 (0) 100 (0)
99.94 (0.13) 100 (0)
100 (0) 99.87 (0.30)
100 (0)
100 (0)
100 (0)
100 (0)
100 (0) 95.57 (4.65) 100 (0) 69.71 (41.82)
99.28 (1.61) 97.26 (5.48) 99.05 (2.13) 85.41 (31.97)
100 (0)
100 (0)
100 (0) 100 (0)
96.31 (4.59) 100 (0)
100 (0)
50.19 (45.04)
99.18 (1.06) 54.73 (50.3) 98.93 (1.26) 26.98 (35.18)
100 (0) 97.18 (1.55) 100 (0) 100 (0)
Table 4.1: QRS Sensitivity and Positive Predictivity for both the Cardiac Monitor and the Clinical Recorder. All values reported are in percentages (n = 4).
4.4.2
Cardiac Monitor Comfort Level
Participants rated the cardiac monitor comfort level on a 5 point scale ranging from very uncomfortable to very comfortable. All participants rated the cardiac monitor either somewhat comfortable or very comfortable. In the comments, the most common complaint was about the portapres, which can be very constricting when worn for the duration of the clinical test.
74
4.4.3
Summary
Overall, both the cardiac monitor developed in this thesis and the clinical recorder performed well in low to moderate activity levels. However, the cardiac monitor substantially outperformed the clinical recorder under heavy activity levels, particularly running. The clinical recorder could not reliably acquire a clean ECG while a participant was running. When the clinical recorder was able to record a reasonably clean
ECG, the QRS sensitivity was high but the QRS positive predictivity was very low, suggesting that there is still a lot of noise in the signal that caused false positives to occur. This difference in reliability may have to do with the shorter wires used with the cardiac monitor, and/or the active buffering of the electrode sites on the cardiac monitor. Participants all rated the cardiac monitor comfort level as either somewhat comfortable or very comfortable (4 or 5 on a scale of 5, 5 being very comfortable).
Figure 4-5: ECG of a participant while running. The output is likely saturated because the P and T waves should not be that large compared to the QRS complex.
75
Figure 4-6: ECG of a participant while standing. The output is likely saturated because the P and T waves should not be that large compared to the QRS complex.
Figure 4-7: ECG of a participant while supine. The QRS complex is larger relative to the P and T waves than in previous images, suggesting that this participant had a smaller amplitude ECG.
76
Chapter 5
Conclusions
A wearable cardiac monitor has been motivated, designed and implemented. Clinical testing on the device is underway. In this last Chapter, a discussion of the cardiac monitor’s performance, along with ideas for future work will be presented. The thesis will be summarized after the future work.
5.1
Discussion
While preliminary results suggest that the cardiac monitor will be a viable alternative to existing clinical options, there are a number of things that the author has learned during the design process to improve future revisions of the board. First, it has been noted that one of the most sensitive parts of the system is the electrode connections.
In the first revision of the electrode board, the ring to which the electrode button was soldered is too thin in its outer diameter and was only a single layer of PCB. The
ring pops off the PCB very easily (see Figure 5-1). This issue was resolved in the
second revision of the electrode board. While the electrode ring no longer pops off the PCB, it has been noted that the electrode connection is very sensitive on both the electrode and central boards. Care must be taken to ensure that the buttons are firmly secured to the PCBs. The electrode used as a driven electrode appears to be most sensitive. This electrode is responsible for maintaining the common mode voltage of the system and rejecting 60 Hz interference. Without a good electrode connection,
77
motion artifacts and 60 Hz noise cannot be properly reduced by the analog front end.
Figure 5-1: A picture of the first revision of the electrode board. Note how the metal ring has detached from the PCB.
Because the electrode boards were small compared with the size of the electrodes, they were not fabricated on flexible PCBs. This proved to be a reasonable design decision. The central boards, on the other hand, were fabricated on flexible PCBs such that the boards could bend as the individual moved. However, after the PCBs were populated with all the required circuit components, the boards were not capable of bending much. It may be possible to implement future revisions of the board on thin, but rigid PCBs to save on cost.
The memory on the cardiac monitor takes up a considerable amount of space on the central board. While both serial flash and a micro SD card were used for buffering and redundancy, it may be possible to eliminate the serial flash devices. The MSP430 used in the cardiac monitor does not have enough RAM to guarantee that all data will be saved before a write, but an MSP430 with more RAM could allow the serial flash to be eliminated. This new device would need to have at least 6 KB of RAM available.
There were two primary usability issues with the device. The first issue is that because the device does not transmit to a base station, there is no live feedback about the ECG or accelerometer signal quality. The board has to be placed on the body, run for a few seconds and stopped. Then the micro SD from the board is removed and the data can be analyzed on the computer. A live feature with radio transmission meant for debugging could help confirm that good signal quality will be achieved for
78
the test.
Another usability issue with the device is that a hex editor must be used to save the data from the micro SD card. This requires additional proprietary software, and is an extra step before the data can be imported via Matlab. The biggest advantage of the micro SD card is that it can be read directly by a computer. Either software should be developed to eliminate the hex editor as the middle man, or the data should be written to the micro SD card in a computer friendly file format, such as FAT32.
Implementing FAT32, however, would require additional power, as additional micro
SD reads and writes would need to be made each time data was written to a file.
Implementing software to import the data directly into Matlab would be the lowest power solution, with minimal overhead.
5.2
Future Work
While basic proof of concept functionality with the cardiac monitor has been shown, further clinical testing is necessary to properly validate the device. Additional sub-
jects will be recruited for the clinical test protocol described in Chapter 4. The clinical
test will be run with at least one more participant wearing the three electrode device, and at least 5 wearing the two electrode board. Current analysis of the two ECG traces from the clinical test only assesses the detection of the QRS complex. Further analysis using these traces should also analyze information from the P and T waves.
Additionally, acceleration data should be considered to relate activity level to heart rate.
The analog front end was saturated in some of the participants of the clinical test.
The cardiac monitor was developed to have 4 bits of variable gain. In the future, an autocalibration routine will be implemented to use this functionality to set the gain for an individual. This autocalibration routine will be run when the device starts. It will check to see if the signal is above a defined threshold, and if necessary, decrease the gain. The routine should be optimized to always fill the ADC as much as possible while preventing saturation of the amplifier.
79
The form factor of the cardiac monitor has proven to be comfortable to wear, primarily limited by the use of the wet electrodes. Future work should investigate the use of more comfortable electrodes, such as dry electrodes. Future work should also evaluate the possibility of implementing a flexible PCB connector between the central and electrode boards. Additionally, a molded cover for the PCBs could help increase patient acceptance.
5.3
Summary
Cardiovascular disease is the leading cause of death in the United States and will become even more of an issue as the population of the United States ages. Wearable cardiac monitors can inform physicians about heart health trends. They provide the physician with additional information over a standard clinical examination. In this thesis, a wearable long term electrocardiogram (ECG) monitor was developed. This monitor can be worn by patients recovering from cardiac events to give a physician a better understanding of their baseline physiology, and by those at risk for cardiovascular disease. It can also be worn by those suspected of having an arrhythmia, particularly one that occurs infrequently. Previous work in wearable long term monitoring relied on use of a base station to transmit data (either continuous ECG or processed data). The implementation of the cardiac monitor in this thesis is able to record data for over one week without use of a base station. Preliminary clinical results from this device suggest that it is a viable alternative to existing clinical options.
80
Appendix A
Micro SD Code
In this Chapter, code for interfacing with the micro SD card is provided. For other code, contact maggied at mit.edu.
A.1
Micro SD Card Header File
/ * −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
/ Low l e v e l d i s k i n t e r f a c e modlue i n c l u d e f i l e
/ −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
#i f n d e f DISKIO
#define READONLY 0 /
*
1 : Remove w r i t e f u n c t i o n s
*
/
#define USE IOCTL 1 /
*
1 : Use d i s k i o c t l f u c n t i o n
*
/
#include ” i n t e g e r . h”
/
*
S t a t u s o f D i s k F u n c t i o n s
*
/ typedef BYTE DSTATUS;
/
*
R e s u l t s o f D i s k F u n c t i o n s
*
/ typedef enum {
RES OK = 0 , /
*
0 : S u c c e s s f u l
*
/
81
RES ERROR,
RES WRPRT,
RES NOTRDY,
RES PARERR
} DRESULT;
/ * 1 : R/W E r r o r * /
/ * 2 : Write P r o t e c t e d * /
/
*
3 : Not Ready
*
/
/
*
4 : I n v a l i d Parameter
*
/
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
/
*
P r o t o t y p e s f o r d i s k c o n t r o l f u n c t i o n s
*
/ i n t a s s i g n d r i v e s ( int , i n t ) ;
DSTATUS d i s k i n i t i a l i z e (BYTE) ;
DSTATUS d i s k s t a t u s (BYTE) ;
DRESULT d i s k r e a d (BYTE, BYTE
*
, DWORD, BYTE) ;
#i f READONLY == 0
DRESULT d i s k w r i t e (BYTE, char
*
, DWORD, BYTE) ;
#endif
DRESULT d i s k i o c t l (BYTE, BYTE, void
*
) ;
// p r i v a t e f u n c t i o n s void m i c r o S D s p i I n i t ( void ) ; void microSDTx ( char o u t g o i n g ) ; char microSDRx ( void ) ; char microSDTxRx ( char o u t g o i n g ) ; void microSDCSLow ( void ) ; void microSDCSHigh ( void ) ; char microSDSndCmd ( unsigned char cmd , unsigned long a r g ) ;
/ * D i s k S t a t u s B i t s (DSTATUS) * /
#define
#define
STA NOINIT
STA NODISK
0 x01 /
*
D r i v e n o t i n i t i a l i z e d
*
/
0 x02 /
*
No medium i n t h e d r i v e
*
/
#define STA PROTECT 0 x04 /
*
Write p r o t e c t e d
*
/
82
/
*
Command c o d e f o r d i s k i o c t r l f u c n t i o n
*
/
/
*
G e n e r i c command ( d e f i n e d f o r FatFs )
*
/
#define CTRL SYNC 0 /
*
F l u s h d i s k c a c h e ( f o r w r i t e f u n c t i o n s )
*
/
#define GET SECTOR COUNT 1 /
*
Get media s i z e ( f o r o n l y f m k f s ( ) )
*
/
#define GET SECTOR SIZE 2 /
*
Get s e c t o r s i z e ( f o r m u l t i p l e s e c t o r s i z e
( MAX SS > = 1 0 2 4 ) )
*
/
#define GET BLOCK SIZE 3 /
*
Get e r a s e b l o c k s i z e ( f o r o n l y f m k f s ( ) )
* /
#define CTRL ERASE SECTOR 4 / * Force e r a s e d a b l o c k o f s e c t o r s ( f o r o n l y
USE ERASE)
*
/
/
*
G e n e r i c command
*
/
#define CTRL POWER
#define
#define
CTRL LOCK
CTRL EJECT
5 /
*
Get / S e t power s t a t u s
*
/
6 /
*
Lock / Unlock media r e m o v a l
*
/
7 /
*
E j e c t media
*
/
/
*
MMC/SDC s p e c i f i c i o c t l command
*
/
#define MMC GET TYPE 10 / * Get c a r d t y p e * /
#define
#define
MMC GET CSD
MMC GET CID
11 / * Get CSD * /
12 /
*
Get CID
*
/
#define
#define
MMC GET OCR
MMC GET SDSTAT
13 /
*
Get OCR
*
/
14 /
*
Get SD s t a t u s
*
/
/
*
ATA/CF s p e c i f i c i o c t l command
*
/
#define ATA GET REV 20 /
*
Get F/W r e v i s i o n
*
/
#define ATA GET MODEL 21 /
*
Get model name
*
/
#define ATA GET SN 22 /
*
Get s e r i a l number
*
/
/ * NAND s p e c i f i c i o c t l command * /
#define NAND FORMAT 30 /
*
C r e a t e p h y s i c a l f o r m a t
*
/
#define DISKIO
83
#endif
A.2
Micro SD .C File
#include ” msp430f247 . h” // IMPORT YOUR OWN DEVICE SPECIFIC HEADER FILE
HERE
#include ” d i s k i o . h”
#include ” f l a s h . h”
#include ” l e d . h”
/
*
MMC/SD command ( SPI mode )
*
/
#define CMD0 ( 0 ) /
*
GO IDLE STATE
*
/
#define
#define
CMD1
CMD8
( 1 )
( 8 )
/
*
SEND OP COND
*
/
/
*
SEND IF COND
*
/
#define
#define
CMD9 ( 9 )
CMD10 ( 1 0 )
/
*
SEND CSD
*
/
/
*
SEND CID
*
/
#define CMD12 ( 1 2 ) / * STOP TRANSMISSION * /
#define CMD13 ( 1 3 ) / * SD STATUS (SDC) * /
#define CMD16 ( 1 6 ) /
*
SET BLOCKLEN
*
/
#define
#define
#define
#define
#define
#define
#define
#define
CMD17 ( 1 7 )
CMD18 ( 1 8 )
CMD23 ( 2 3 )
CMD24 ( 2 4 )
CMD25 ( 2 5 )
CMD41 ( 4 1 )
CMD55 ( 5 5 )
CMD58 ( 5 8 )
/
*
READ SINGLE BLOCK
*
/
/
*
READ MULTIPLE BLOCK
*
/
/
*
SET BLOCK COUNT
*
/
/
*
WRITE BLOCK
*
/
/
*
WRITE MULTIPLE BLOCK
*
/
/
*
SEND OP COND (ACMD)
*
/
/
*
APP CMD
*
/
/ * READ OCR * /
#define ACMD41 ( 0 x80 + 4 1 )
#define ACMD23 ( 0 x80 +23)
/
*
Card t y p e f l a g s ( CardType )
*
/
#define CT MMC 0 x01 /
*
MMC v e r 3
*
/
#define
#define
#define
CT SD1
CT SD2
CT SDC
0 x02
0 x04
/
*
SD v e r 1
*
/
/
*
SD v e r 2
*
/
( CT SD1 | CT SD2 ) /
*
SD
*
/
84
#define CT BLOCK 0 x08 / * B l o c k a d d r e s s i n g * / s t a t i c
DSTATUS S t a t = STA NOINIT ; /
*
D i s k s t a t u s
*
/ s t a t i c
BYTE CardType ; /
* b0 :MMC, b1 : SDv1 , b2 : SDv2 , b3 : B l o c k a d d r e s s i n g
*
/ unsigned i n t i =0; // u s e d d u r i n g f o r l o o p s , e t c .
unsigned char r e s p o n s e =0; // SPI r e s p o n s e p l a c e h o l d e r
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
Module P r i v a t e F u n c t i o n s − DEVICE DEPENDENT ! ! !
−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
// CS i s P4 . 7
// s e t c h i p s e l e c t l o w
// ( Be s u r e t o c o n f i g u r e GPIO e l s e w h e r e ; I i n i t i a l i z e a l l CS f o r s y s t e m i n
// main ) void microSDCSLow ( void )
{
P4OUT &= ˜BIT7 ;
}
// s e t c h i p s e l e c t h i g h void microSDCSHigh ( void )
{
P4OUT | = BIT7 ;
}
85
// i n i t i a l i z e SPI communication w i t h t h e micro SD void m i c r o S D s p i I n i t ( void )
{
// Begin i n i t i a l i z a t i o n p r o c e s s by s e t t i n g UCSWRST
UCB1CTL1 = UCSWRST;
// I n i t i a l i z e a l l r e g i s t e r s w h i l e UCSWRST = 1
// Send d a t a on r i s i n g edge , C l o c k i n a c t i v e h i g h ,
// 8 B i t d a t a , m a s t e r mode , s y n c h r o n o u s
UCB1CTL0 = UCCKPL | UCMSB | UCMST | UCSYNC;
UCB1CTL1 | = UCSSEL1 ; // SMCLK i s s o u r c e
UCB1BR0 = 3 ; // SMCLK/3 i s t h e c l o c k r a t e ( 3 3 3 kHz )
UCB1BR1 = 0 ;
// A f t e r r e g i s t e r s a r e s e t , c o n f i g u r e t h e p o r t s
P5DIR | = BIT3 + BIT2 ;
P5SEL | = BIT3 + BIT2 + BIT1 ; // E n a b l e P5 . 3 a s c l o c k , P5 . 2 a s MI , P5 . 1 a s MO
}
// C l e a r UCSWRST
UCB1CTL1 &= ˜UCSWRST;
// SPI t r a n s m i t ONLY void microSDTx ( char o u t g o i n g )
{
// Wait f o r t h e TX b u f f e r t o c l e a r while ( ( UC1IFG & UCB1TXIFG) == 0 ) ;
}
// Transmit d a t a o v e r USCI B1
UCB1TXBUF = o u t g o i n g ;
// SPI r e c e i v e ONLY char microSDRx ( void )
{
86
} char d a t a = 0 ;
// R e c e i v e d a t a o v e r USCI B1 while ( ! ( UC1IFG & UCB1RXIFG) ) ; d a t a = UCB1RXBUF; return d a t a ;
// BOTH SPI t r a n s m i t and r e c e i v e char microSDTxRx ( char o u t g o i n g )
{ char d a t a = 0 ;
// Wait f o r t h e TX b u f f e r t o c l e a r while ( ( UC1IFG & UCB1TXIFG) == 0 ) ;
// Transmit d a t a o v e r USCI B1
UCB1TXBUF = o u t g o i n g ;
}
// R e c e i v e d a t a o v e r USCI B1 while ( ! ( UC1IFG & UCB1RXIFG) ) ; d a t a = UCB1RXBUF; return d a t a ; i n t waitReady ( void )
{ i n t r e t r y = 0xFFFF ;
BYTE r e s p o n s e ; r e s p o n s e = microSDTxRx ( 0 xFF ) ; while ( r e s p o n s e != 0xFF )
{ r e s p o n s e = microSDTxRx ( 0 xFF ) ; r e t r y −− ; i f ( r e t r y == 0 )
87
}
}
{ s e t A l l ( ) ; return 0 ;
} return 1 ; i n t microSDRxData (BYTE
* b u f f , UINT b t r )
{
BYTE t o k e n ; do
{ t o k e n = microSDTxRx ( 0 xFF ) ;
} while ( t o k e n != 0xFE ) ; do
{
* b u f f++ = microSDTxRx ( 0 xFF ) ;
* b u f f++ = microSDTxRx ( 0 xFF ) ;
* b u f f++ = microSDTxRx ( 0 xFF ) ;
* b u f f++ = microSDTxRx ( 0 xFF ) ;
} while ( b t r − = 4 ) ;
// dummy c r c r e s p o n s e = microSDTxRx ( 0 xFF ) ; r e s p o n s e = microSDTxRx ( 0 xFF ) ;
} return 1 ; i n t microSDTxData ( char
* b u f f , BYTE t o k e n )
{ i n t wc ;
88
// s e t Y e l l o w ( ) ; i f ( ! waitReady ( ) ) return 0 ; microSDTx ( t o k e n ) ; i f ( t o k e n != 0xFD)
{ f o r ( wc = 0 ; wc < 5 1 2 ; wc++)
{ microSDTx ( * b u f f ++) ;
}
// dummy c r c r e s p o n s e = microSDTxRx ( 0 xFF ) ; r e s p o n s e = microSDTxRx ( 0 xFF ) ; r e s p o n s e = microSDTxRx ( 0 xFF ) ; r e s p o n s e = microSDTxRx ( 0 xFF ) ; i f ( ( r e s p o n s e & 0x1F ) != 0 x05 )
{ s e t R e d ( ) ; return 1 ;
}
} i f ( ! waitReady ( ) ) return 0 ;
// c l r Y e l l o w ( ) ;
} return 1 ;
89
// Send an SD command t o t h e d e v i c e
// SHOULD BE PLATFORM INDEPENDENT char microSDSndCmd ( unsigned char cmd , unsigned long a r g )
{ unsigned char r e t r y = 0 ; i f ( ! waitReady ( ) ) return RES ERROR ; microSDTx ( 0 xFF ) ; // e n s u r e i d l e f o r a t l e a s t 8 c l o c k c y c l e s
// 6 b y t e command microSDTx ( cmd | 0 x40 ) ; // s e n d command , f i r s t two b y t e s a l w a y s 01 microSDTx ( a r g >> 2 4 ) ; // f o u r b y t e l o n g argument microSDTx ( a r g >> 1 6 ) ; microSDTx ( a r g >> 8 ) ; microSDTx ( a r g ) ;
// s e n d CRC
// CRC o n l y n e e d s t o b e c o r r e c t f o r CMD8 and CMD0 i f ( cmd == CMD8)
{ microSDTx ( 0 x87 ) ;
} e l s e
{ microSDTx ( 0 x95 ) ; // CMD0 CRC
} microSDTx ( 0 xFF ) ; // e n s u r e i d l e f o r a t l e a s t 8 c l o c k c y c l e s while ( ( r e s p o n s e = microSDTxRx ( 0 xFF ) ) == 0xFF ) // w a i t r e s p o n s e i f ( r e t r y++ > 0 x f e ) break ; // t i m e o u t e r r o r
90
} return r e s p o n s e ;
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
P u b l i c F u n c t i o n s
−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
/ * −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− * /
/ * Get D i s k S t a t u s * /
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
DSTATUS d i s k s t a t u s (
BYTE drv /
*
D r i v e number ( 0 )
*
/
)
{
S t a t &= ˜STA PROTECT ;
} return S t a t ;
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
/
*
I n i t i a l i z e D i s k D r i v e
*
/
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
// NOTE: d e l a y c y c l e s command s h o u l d work f o r most MSP430
// Can comment o u t i f t h e r e i s a t l e a s t 1 ms a f t e r s t a r t up b e f o r e
// d i s k i n i t i a l i z a t i o n
// T h i s f u n c t i o n a l s o i n i t i a l i z e s SPI
DSTATUS d i s k i n i t i a l i z e (
BYTE drv /
*
P h y s i c a l d r i v e number ( 0 )
*
/
)
{
BYTE cmd , r e s p x [ 4 ] ;
91
m i c r o S D s p i I n i t ( ) ; // i n i t i a l i z e SPI i f ( drv ) return STA NOINIT ; /
*
S u p p o r t s o n l y s i n g l e d r i v e
*
/ d e l a y c y c l e s ( 1 0 0 0 ) ; // w a i t 1 ms ( assumes 1 MHz c l o c k )
/
*
Send 10 s p i commands w i t h c a r d n o t s e l e c t e d
*
/ f o r ( i =0; i < 10; i ++)
{ microSDTx ( 0 xFF ) ;
}
CardType = 0 ; microSDCSLow ( ) ; i = 1 0 0 ; do
{
// s e n d r e s e t and go i d l e command r e s p o n s e = microSDSndCmd (CMD0, 0 ) ;
} while ( r e s p o n s e !=1 && i −− ) ; i f ( r e s p o n s e != 1 )
{ microSDCSHigh ( ) ; return r e s p o n s e ; // CMD0 f a i l s ; r e t u r n r e s p o n s e and c h e c k e r r o r c o d e
} microSDCSHigh ( ) ;
// Try CMD8 t o d e t e r m i n e t y p e o f c a r d microSDCSLow ( ) ; r e s p o n s e = microSDSndCmd (CMD8, 0 x1AA) ;
92
i f ( r e s p o n s e ==1)
{
// SD v e r s i o n 2
// Get r e t u r n v a l u e s o f r e s p o n s e R7 r e s p x [ 0 ] = microSDTxRx ( 0 xFF ) ; r e s p x [ 1 ] = microSDTxRx ( 0 xFF ) ; r e s p x [ 2 ] = microSDTxRx ( 0 xFF ) ; r e s p x [ 3 ] = microSDTxRx ( 0 xFF ) ; microSDCSHigh ( ) ; i f ( r e s p x [ 2 ] == 1 && r e s p x [ 3 ] ==0xAA) // c a r d w o r k s f o r 2.7
− 3.6V
{
// Send ACMD41 w i t h HCS b i t
// f i r s t s e n d CMD55 t h e n ACMD41 f o r ( i = 0 ; i < 1 0 0 0 ; ++i )
{ microSDCSLow ( ) ; r e s p o n s e = microSDSndCmd (CMD55, 0 ) ; microSDCSHigh ( ) ; i f ( r e s p o n s e < = 1 )
{ microSDCSLow ( ) ; r e s p o n s e = microSDSndCmd (CMD41, 1UL << 3 0 ) ; microSDCSHigh ( ) ;
}
} i f ( r e s p o n s e == 0 )
{ break ;
} d e l a y c y c l e s ( 1 0 0 0 ) ;
93
}
}
} i f ( i < 1 0 0 0 )
{ microSDCSLow ( ) ; r e s p o n s e = microSDSndCmd (CMD58, 0 ) ; r e s p x [ 0 ] = microSDTxRx ( 0 xFF ) ; r e s p x [ 1 ] = microSDTxRx ( 0 xFF ) ; r e s p x [ 2 ] = microSDTxRx ( 0 xFF ) ; r e s p x [ 3 ] = microSDTxRx ( 0 xFF ) ; microSDCSHigh ( ) ;
CardType = ( r e s p x [ 0 ] & 0 x40 ) ? CT SD2 | CT BLOCK : CT SD2 ; //
SDv2 e l s e // SDv1 o r MMCv3
{ i = 1 0 0 0 ; microSDCSLow ( ) ; i f ( microSDSndCmd (ACMD41, 0 ) < = 1 )
{
CardType = CT SD1 ; cmd = ACMD41; // SDv1
} e l s e
{
CardType = CT MMC; cmd = CMD1; // MMCv3
} microSDCSHigh ( ) ;
// w a i t f o r c a r d t o l e a v e i d l e s t a t e do
94
{
} microSDCSLow ( ) ; r e s p o n s e = microSDSndCmd ( cmd , 0 ) ; microSDCSHigh ( ) ; while ( r e s p o n s e ==1 && i −− ) ;
} i f ( CardType )
{
// INSERT CHANGE TO FAST MODE HERE
UCB1CTL1 | = UCSWRST;
UCB1BR0 = 1 ; // SMCLK i s t h e c l o c k r a t e ( 1 MHz)
// C l e a r UCSWRST
UCB1CTL1 &= ˜UCSWRST;
}
S t a t &= ˜STA NOINIT ; e l s e
{
S t a t | = STA NOINIT ;
}
} return S t a t ;
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
/ * Read S e c t o r ( s ) * /
/ * −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− * /
DRESULT d i s k r e a d (
BYTE drv ,
BYTE
* b u f f ,
/
*
P h y s i c a l d r i v e nmuber ( 0 )
*
/
/
*
P o i n t e r t o t h e d a t a b u f f e r t o s t o r e r e a d d a t a
*
/
95
)
{
DWORD s e c t o r ,
BYTE c o u n t
DSTATUS s ;
/ * S t a r t s e c t o r number (LBA) * /
/ * S e c t o r c o u n t ( 1 . . 1 2 8 ) * / s = d i s k s t a t u s ( drv ) ; i f ( s & STA NOINIT ) return RES NOTRDY; i f ( ! c o u n t ) return RES PARERR ; i f ( ! ( CardType & CT BLOCK) ) s e c t o r
*
= 5 1 2 ; // c o n v e r t t o b y t e a d d r e s s e s microSDCSLow ( ) ; i f ( c o u n t == 1 )
{ i f ( ( microSDSndCmd (CMD17, s e c t o r ) == 0 ) && microSDRxData ( b u f f , 5 1 2 ) ) c o u n t = 0 ;
} e l s e
{ i f ( microSDSndCmd (CMD18, s e c t o r ) == 0 )
{ do
{ i f ( ! microSDRxData ( b u f f , 5 1 2 ) ) break ; b u f f += 5 1 2 ;
} while ( −− c o u n t ) ; microSDSndCmd (CMD12, 0 ) ;
}
} microSDTx ( 0 xFF ) ; // e x t r a c l o c k c y c l e microSDCSHigh ( ) ;
96
} return RES OK ;
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
/
*
Write S e c t o r ( s )
*
/
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
DRESULT d i s k w r i t e (
BYTE drv , /
*
P h y s i c a l d r i v e number ( 0 )
*
/ char
* b u f f , /
*
P o i n t e r t o t h e d a t a t o b e w r i t t e n
*
/
DWORD s e c t o r ,
BYTE c o u n t
/ * S t a r t s e c t o r number (LBA) * /
/ * S e c t o r c o u n t ( 1 . . 1 2 8 ) * /
)
{
DSTATUS s ; s = d i s k s t a t u s ( drv ) ; i f ( s & STA NOINIT ) return RES NOTRDY; i f ( s & STA PROTECT) return RES WRPRT; i f ( ! c o u n t ) return RES PARERR ; i f ( ! ( CardType & CT BLOCK) ) s e c t o r
*
= 5 1 2 ; // c o n v e r t t o b y t e a d d r e s s i n g microSDCSLow ( ) ; microSDTx ( 0 xFF ) ; i f ( ! waitReady ( ) ) return RES ERROR ; i f ( c o u n t == 1 )
{ i f ( ( microSDSndCmd (CMD24, s e c t o r ) == 0 ) && microSDTxData ( b u f f , 0xFE )
) c o u n t = 0 ;
} e l s e s e t R e d ( ) ;
97
e l s e
{ i f ( CardType & CT SDC) microSDSndCmd (ACMD23, c o u n t ) ; i f ( microSDSndCmd (CMD25, s e c t o r ) == 0 )
{ do
{ i f ( ! microSDTxData ( b u f f , 0xFC) )
{ s e t R e d ( ) ; break ;
} b u f f += 5 1 2 ;
} while ( −− c o u n t ) ;
}
} i f ( ! microSDTxData ( 0 , 0xFD) ) c o u n t = 1 ; i f ( ! waitReady ( ) ) return RES ERROR ; microSDCSHigh ( ) ; microSDTx ( 0 xFF ) ;
} return c o u n t ? RES ERROR : RES OK ;
/
* −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− *
/
/ * M i s c e l l a n e o u s F u n c t i o n s * /
/ * −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−− * /
DRESULT d i s k i o c t l (
BYTE drv ,
BYTE c t r l ,
/
*
P h y s i c a l d r i v e nmuber ( 0 )
*
/
/
*
C o n t r o l c o d e
*
/
98
)
{ void * b u f f
DRESULT r e s ;
/ * B u f f e r t o s e n d / r e c e i v e c o n t r o l d a t a * / switch ( c t r l ) { case CTRL SYNC : r e s = RES OK ; break ;
/
*
Make s u r e t h a t no p e n d i n g w r i t e p r o c e s s
*
/
} d e f a u l t : r e s = RES PARERR ; r e s = RES OK ;
} return r e s ;
99
100
Bibliography
[1] V. L. Roger et al. Heart Disease and Stroke Statistics – 2012 Update. A Report from the American Heart Association.
Circulation , 125:E2–E220, 2012.
[2] Z Syed, et al. Relation of Death Within 90 Days of Non-ST-Elevation Acute
Coronary Syndromes to Variability in Electrocardiographic Morphology.
Am J
Cardiol , 103:307–311, 2009.
[3] Z Syed, et al. Computationally Generated Cardiac Biomarkers for Risk Stratification After Acute Coronary Syndrome.
Science Translational Medicine , 3,
2011.
[4] Roger G. Mark. Clinical Electrocardiography and Arrhythmias. 2004.
[5] MIT Course 6.522 Lecture Notes.
[6] P. G. Morton.
Critical Care Nursing: A Holistic Approach , chapter 17. Lippincott Williams & Wilkins, 2005.
[7] P.W. Macfarlane.
Comprehensive Electrocardiology , chapter 11. Springer, 2010.
[8] H. Sugano et al. Continuous ECG Data Gathering by a Wireless Vital Sensor –
Evaluation of its Sensing and Transmission Capabilities. In IEEE ISSSTA , pages
98–102, Taichung, Taiwan, 2010.
[9] R. Goya-Esteban et al.
Heart Rate Variability on 7-Day Holter Monitoring
Using a Bootstrap Rhythmometric Procedure.
IEEE Transactions on Biomedical
Engineering , 57:1366–1376, 2010.
[10] http://en.wikipedia.org/wiki/File:Holter_monitor.JPG
.
[11] G. Hindricks et al. Atrial Fibrillation Detection by a Subcutaneous Monitoring
Device. In IEEE Computers in Cardiology Conference , pages 413–416, Bologna,
Italy, 2008.
[12] A. Singh, J. Lui, and J. V. Guttag. Discretization of Continuous ECG Based
Risk Metrics Using Asymmetric and Warped Entropy Measures.
Computers in
Cardiology , 37:473–476, 2010.
101
[13] J. Yoo et al.
An Attachable ECG Sensor Bandage with Planar-Fashionable
Circuit Board. In IEEE Symposium on Wearable Computers , pages 145–146,
2009.
[14] R. Harper et al. Evaluation of a CE Approved Ambulatory Patient Monitoring
Device in a General Medical Ward. In IEEE EMBC , pages 94–97, Buenos Aires,
Argentina, 2010.
[15] http://www.mindray.com/na/products/netguard.html?backUrl=pro_ line1_pre.html
.
[16] http://www.vpatchmedical.com/pages/vpms-components.php
.
[17] http://www.eebeat.com/?p=4204 .
[18] http://en.wikipedia.org/wiki/File:Op-Amp_Instrumentation_
Amplifier.svg
.
[19] A. Searle and L. Kirkup. A Direct Comparison of Wet, Dry and Insulating
Bioelectric Recording Electrodes.
Physiological Measurements , 21:271–283, 2000.
[20] James C. Huhta and John G. Webster. 60-hz interference in electrocardiography.
Biomedical Engineering, IEEE Transactions on , BME-20(2):91 –101, March
1973.
[21] Bruce B. Winter and John G. Webster. Driven-right-leg circuit design.
Biomedical Engineering, IEEE Transactions on , BME-30(1):62 –66, Jan. 1983.
[22] P.W. Macfarlane.
Comprehensive Electrocardiology , chapter 12. Springer, 2010.
[23] http://www.springerimages.com/Images/Engineering/1-10.1007_
978-1-4419-6597-4_10-19 .
[24] https://www.sdcard.org/downloads/pls/ .
[25] http://www.hexworkshop.com/ .
[26] CSI Criticare 504-US product pages.
http://www.dhbiomedical.com/product%
20pages/CSI-504.pdf
.
[27] http://www.ambulatory-monitoring.com/inductotrace.html
.
[28] http://www.finapres.com/site/page/2/9/Portapres/ .
[29] http://www.physionet.org/physiotools/wag/wqrs-1.htm
.
[30] G. Moody. Wave User’s Guide.
http://www.physionet.org/physiotools/ wug/wug.pdf
, 2011.
102