MatE271 ... (a) Metals and ceramics are all crystalline materials. Metals have...
advertisement
MatE271
[1]
Answer HW # 2
9/21/2001
(a) Metals and ceramics are all crystalline materials. Metals have simple
crystalline structures (bcc, fcc and hcp), which favor dislocation mobility
and make them ductile. Metals also have metallic bond, which provides high
electrical conductivity. On the contrary, ceramics have more complex
crystalline structure which prevent dislocation motion and thus rendering
them very brittle. Ceramics also have mix between ionic and covalent
bonding which make them good electrical insulators.
(b) Ceramics have crystalline structure, while glasses have very short order
structure with amorphous (random) arrangements.
[2] See page 73-74, 574
The existence of a central cation atom with a positive charge (Ti+4 ) within the unit cell of
BaTiO3, make it more responsive to the applied electric field and forming dipole by moving
up and down within the unit cell. Such movement manifests itself as expansion or contraction
in the direction of the applied electric field.
[3] Without the applied electric field, the Bravais lattice BaTiO3 crystal is simple cubic. Such
cubic structure is symmetric, and will present isotropic mechanical response to the applied
forces (i.e. will contract or expand with the same amount under the same applied force in a
three perpendicular directions However, once the electric field is applied, the crystal loses its
cubic symmetry and become tetragonal. A tetragonal structure has anisotropic (unequal)
response to the applied load. Therefore the displacement in x-dn is not equal to that in the
y-dn.
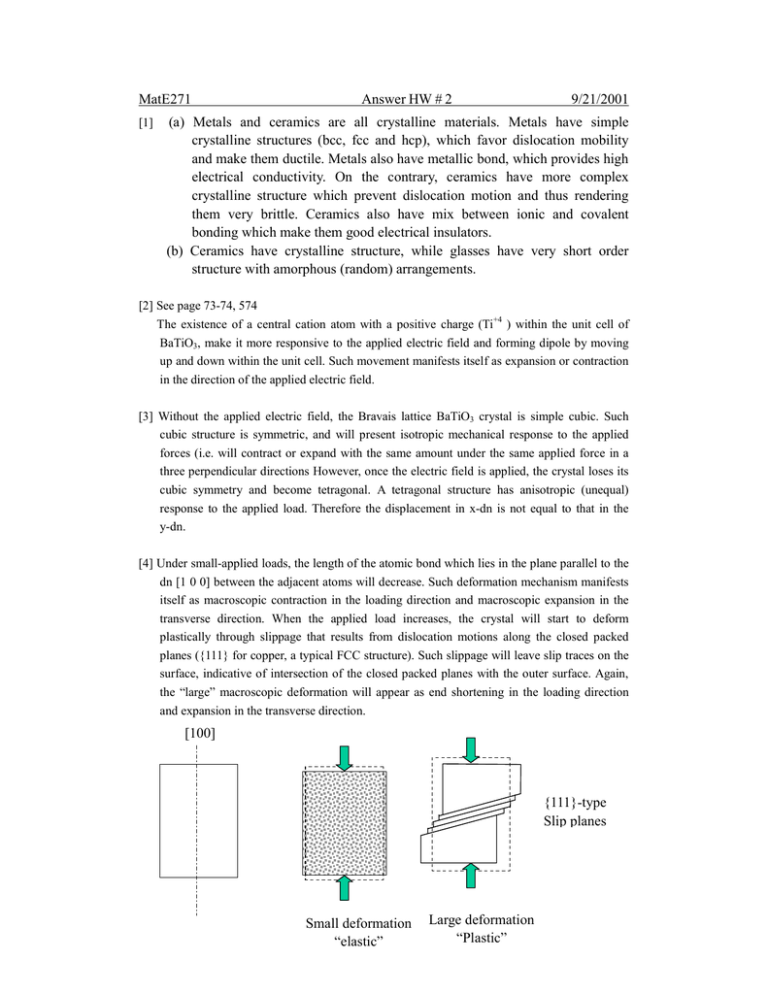
[4] Under small-applied loads, the length of the atomic bond which lies in the plane parallel to the
dn [1 0 0] between the adjacent atoms will decrease. Such deformation mechanism manifests
itself as macroscopic contraction in the loading direction and macroscopic expansion in the
transverse direction. When the applied load increases, the crystal will start to deform
plastically through slippage that results from dislocation motions along the closed packed
planes ({111} for copper, a typical FCC structure). Such slippage will leave slip traces on the
surface, indicative of intersection of the closed packed planes with the outer surface. Again,
the “large” macroscopic deformation will appear as end shortening in the loading direction
and expansion in the transverse direction.
[100]
{111}-type
Slip planes
Small deformation
“elastic”
Large deformation
“Plastic”
z
5) See page 97
The structure of Cr is a typical of BCC, (p. 65 fig 3-4).
From the atomic radii tables (App. 2, P 796)
(111)
a
rCr = 0.125 nm;
4r
a Cr =
= 0.2887 nm;
3
for (111) plane, h = 1; k = 1;l = 1;
a
d 111 =
= 0.0962nm;
2
h + k 2 + l2
x
The planar density canbe either given as percentage fill or as atoms / nm2
h = 2^0.5* a = 0.4082nm;
b = 3^0.5 / 2 * H = 0.3536nm;
1
1
bh =
2 a 3 2 a = 0.0722 nm2 ;
2
2
There is a 1 6 atom at each of the (111) plane corner ,
A 111 =
e je
j
3 x1 6
= 6.93 atom / nm2 ;
A 111
Linear density [1 0 0] = 1atom / a = 3.4641atom / nm;
Planar density =
Linear density [1 0 1] = 1atom / b = 2.4495 atom / nm;
Linear density [1 1 1] = 2 atoms / ( 3 a) = 4.0 atom / nm;
b
y