Can biodiversity hotspots protect more than tropical forest plants and vertebrates?
advertisement

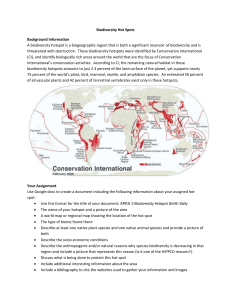
Journal of Biogeography (J. Biogeogr.) (2014) 41, 421–428 GUEST EDITORIAL Can biodiversity hotspots protect more than tropical forest plants and vertebrates? Nigel E. Stork1* and Jan Christian Habel2 1 Environmental Futures Centre, Griffith School of Environment, Griffith University, Nathan, QLD 4111, Australia, 2Department of Ecology and Ecosystem Management, Technische Universit€at M€ unchen, D-85350, Freising-Weihenstephan, Germany *Correspondence: Nigel E. Stork, Environmental Futures Centre, Griffith School of Environment, Griffith University, 170 Kessels Rd, Nathan, QLD 4111, Australia. E-mail: nigel.stork@griffith.edu.au ABSTRACT Conservation International’s biodiversity hotspots are areas of high vascular plant endemism combined with high levels of habitat destruction and land use change. Although such hotspots have also been shown to be centres for terrestrial vertebrate endemism, much less is known about how well these areas function as hotspots for other less well-studied groups, including the hyperdiverse arthropods, other invertebrates and fungi. Because there is a close evolutionary and ecological relationship between insects and plants, we suggest that the potential role of plants as umbrella species for herbivorous insects, potentially herbivorous fungi and nematodes, and parasitic insects should be explored. Finally, we reflect on the increasing social, economic, human conflict and governance issues and the impacts of increasing land use change and global climate change that threaten the biodiversity hotspot system. Keywords Conservation biogeography, Conservation International’s hotspots, endemic species, global change, invertebrate diversity, plant diversity, species richness, strategic conservation planning, umbrella species. THE CONCEPT Why biodiversity is not homogenously distributed across the globe, but is concentrated in specific geographical areas, has fascinated biologists for centuries and has been the inspiration for key ecological and evolutionary theories (Darwin, 1859; Wallace, 1860; Briggs, 1987; Gaston, 2000). In the early 1980s attention was drawn to the loss of biodiversity resulting from increasing deforestation of tropical forests (Lovejoy, 1980; Ehrlich & Ehrlich, 1981; Myers, 1988a; Wilson & Peter, 1988). The importance of such losses was heightened first by the recognition that some of the main centres for deforestation were also centres for high plant and animal species richness and endemism (Davies et al., 1986), and second by new estimates of global species richness which suggested that there were many more undescribed species than had previously been considered (Erwin, 1982). Myers (1988b), in first defining the hotspot concept, identified 10 tropical forest areas as key hotspots with high diversity, endemism and threat from deforestation. He suggested that concentrating resources to protect these areas would lead to the greatest benefit in conserving biodiversity. Myers et al. (2000) later refined the concept, defining a biodiversity hotspot as a restricted area that must contain 1500 endemic plant species ª 2013 John Wiley & Sons Ltd and has already lost at least 70% of its former natural vegetation cover through land use change. The hotspot list has now increased to 35, with these areas recognized as including 77% of all endemic plant species, 43% of vertebrates (including 60% of threatened mammals and birds), and 80% of all threatened amphibians (Mittermeier et al., 2004, 2007, 2011; Williams et al., 2011). The conservation of biodiversity hotspots sensu Mittermeier et al. (2004), also called ‘CI biodiversity hotspots’, is a key element of the baseline conservation strategy for Conservation International. Because it focuses on endemic species it inherently incorporates the key concept of complementarity (Ladle & Whittaker, 2011) and has also become a key conservation strategy for many other organizations (e.g. the MacArthur Foundation, the Gordon and Betty Moore Foundation, the Global Environmental Facility, and the World Bank), with more than US$1 billion being invested to support it. Some have warned that the biodiversity hotspots approach is not a cure-all strategy for the conservation of biodiversity (Jepson & Canney, 2001) and have placed it in the context of several other global strategies (Brooks et al., 2006; Jepson et al., 2011; Rodrigues, 2013). In this review we question how applicable the biodiversity hotspot concept may be for http://wileyonlinelibrary.com/journal/jbi doi:10.1111/jbi.12223 421 N. E. Stork and J. C. Habel protecting global biodiversity in its widest sense when plants and vertebrates comprise only a small proportion of all species. We provide ideas on how this global conservation strategy may be an umbrella for a large part of the greater variety of life. We focus particularly on insects but suggest that the principles we use might apply to other terrestrial invertebrates and fungi. Finally, we examine how a range of anthropogenic factors are threatening the survival of biodiversity hotspots. TAXON BIAS The taxonomy of plants is comparatively well explored and therefore sensibly used as a basis for delineating biodiversity hotspots. However, they, along with vertebrates, comprise less than 6–8% of global diversity (Hamilton et al., 2010, 2011; Mora et al., 2011). Arguably, there are some 5 3 million species on Earth (Costello & Wilson, 2011; Hamilton et al., 2011, 2012; Costello et al., 2013), with arthropods comprising the majority of species. Other invertebrates, such as nematodes, and fungi also contribute an additional large proportion of the total but they, along with arthropods, are ignored in many comparative conservation analyses, largely because 80–95% of species are unknown to science or are taxonomically intractable. Plants have been used as the focal taxa for hotspots because their distribution is relatively easily measurable by comparison to many other taxa and not necessarily because they deserve protection and conservation more than other taxa. Here we ask whether a strategy based on a minority of taxa might also serve to conserve and protect the majority of the world’s biodiversity. We are not the first to pose this question. For example, Prendergast et al. (1993, p. 336) commented: ‘a strategy based … on a limited number of taxa, may fail to provide adequate protection for many other organisms’. Insects, other invertebrates and fungi form the majority of species on Earth and we argue that these deserve consideration and protection as much as other more charismatic taxa because of the unique contributions they make to the functioning of ecosystems, representation of the evolutionary past, and the future evolutionary potential of the world’s biota. Insects and other arthropods play essential roles in key processes such as herbivory, pollination, seed dispersal, predation and decomposition (e.g. Weisser & Siemann, 2007). Since the Cretaceous, the extraordinary co-evolution of insects and flowering plants has given rise to a large part of today’s biodiversity and the survival of many vascular plants depends on their pollinators and seed dispersers. Herbivory is suggested as one of the key mechanisms promoting plant coexistence and diversity, especially in tropical forests (Lubchenko, 1978; Viola et al., 2010), and the vast majority of tropical trees are pollinated by insects (Bawa, 1990). Despite their small size, many arthropods and fungi are recognized as ecosystem engineers and keystone species, and play key roles in communities (Smith et al., 1991; Lill & Marquis, 2003; Buse et al., 2008; Collier & Bidartondo, 2009). 422 These facts highlight the irreplaceability of these poorly studied taxa, but are these organisms as threatened with extinction as plants and vertebrates and therefore of concern for conservationists? The answer is that we are not sure. For the best-known biota in the world, that of the UK, the evidence is equivocal, with some suggesting that the decline of insects may be greater than or equal to that of vascular plants or birds (Thomas et al., 2004; Hambler et al., 2011) and others suggesting that birds and mammals are on average seven times more likely to be threatened with extinction than invertebrates (Mawdsley & Stork, 1995). McKinney (1999) concluded that in the USA some, but not all, insects and other invertebrates are more threatened than many vertebrates and plants. Of direct relevance to this essay, Fonseca (2009) estimated that roughly a third of insect species in biodiversity hotspots may be committed to extinction because of past reductions in the geographical range size of their endemic host plants. Fonseca argued that if the 150,371 endemic plant species in CI’s biodiversity hotspots had an average of roughly 5.3–10.6 monophagous insect species per plant species, this would mean that there were 795,971– 1,602,423 monophagous insect species in these biodiversity hotspots. In light of these studies, we should assume that at least some invertebrate groups, and probably some fungi, are as threatened as some vertebrates and plants. It would also be prudent to consider how well the biodiversity hotspots strategy protects them. ARE PLANTS SUITABLE SURROGATES FOR INSECTS AND OTHER BIODIVERSITY? Because CI’s biodiversity hotspots are in large part defined by endemic plant species richness, we should question whether endemic plants are good surrogates for endemic insects and other biodiversity and whether their protection will provide protection for other biodiversity. There is an extensive literature examining the close relationship between herbivorous insects and plants (e.g. Southwood, 1961; Ehrlich & Raven, 1964; Lewinsohn et al., 2005). Most species of herbivorous insects show a strong association with a group of closely related host plant species or single host plant species and these are reflected in close co-evolutionary phylogenies for the plants and insects (Novotny et al., 2002). For example, recent phylogenetic analysis of Australian chrysomelid beetles shows general conservation of host association with Australian plants, with host shifts between distant plant lineages occurring only rarely (Jurado-Rivera et al., 2009). This close co-evolutionary link between many insects and plants has led to the suggestion that the loss of some plant species will lead to the co-extinction of their host-dependent insect species (Stork & Lyal, 1993; Fonseca, 2009; Moir et al., 2010; Colwell et al., 2012). Implicit in Erwin’s (1982) estimation of 30 million species of insects on Earth was his assumption that 84% of all insects are host-specific herbivores. Recent recalculations of global species richness suggest much lower numbers of insect Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd Biodiversity hotspots under review species specific to trees and other plants and hence a lower proportion of perhaps 25–40% of all insects being herbivores (e.g. Hamilton et al., 2010, 2011). In addition, a large proportion of both nematodes and fungi are plant-feeding and tied closely to plant species but as yet their host-specificity is still largely unknown. As many parasitic insects are tightly bound to host insect species then this might also be generally true for a large component of this trophic group (Smith et al., 2008). If parasitic insects comprise perhaps 15–30% of global insects (LaSalle & Gauld, 1992) then, when combined with herbivores, this would suggest that roughly 40–70% of insect species are linked to particular plant species. The species richness of other trophic levels has been shown to be influenced by plant species richness, with this influence declining with increasing trophic level (Scherber et al., 2010). Hence, one would predict less tenuous links with plants for groups such as predators and for those organisms involved in decomposition. Current evidence would suggest that such links between plants and below-ground microbial diversity are more tenuous (De Deyn & Van der Putten, 2005). If such a large proportion of invertebrates and possibly fungi is linked to particular plant species how well might plants or even other taxa act as surrogates for these groups? There are few studies that directly examine the potential surrogacy of plants for insects, but recently Basset et al. (2012) found that models based on plant diversity fitted the accumulated species richness of both herbivore and non-herbivore insects in Panama tropical rain forest exceptionally well. Given that this study reported one of the most extensive insect surveys ever carried out, this finding is important. Elsewhere a weak but positive power of surrogacy was observed in a review of the effectiveness of cross-taxon surrogacy based on complementarity using data for a wide range of taxa from 27 studies from across the world (Rodrigues & Brooks, 2007). Two further studies, one from a tropical forest hotspot and one from Europe, are discussed here to demonstrate some of the confounding issues in examining surrogacy and in particular the use of non-herbivorous insect groups in preference to herbivores. Both studies also show the importance of past history in determining current distribution patterns. First, Moritz et al. (2001) found that endemism and species richness varies significantly between amphibians, birds, mammals and selected groups of invertebrates across the Wet Tropic rain forests in North Queensland, Australia, suggesting that these groups do not necessarily act as a surrogate for the others (Williams et al., 1996; Moritz et al., 2001). However, plants were not examined and the relatively small groups of insects they selected (330 endemic species, mostly flightless) included no herbivores, comprising mostly flightless predatory Carabidae, dung-feeding Scarabaeidae and fungus feeding aradid bugs. The distribution of the taxa studied, including vertebrates, appears to be the result of past Pleistocene climates with many of the endemic vertebrates and invertebrates being cool-adapted species trapped on the tops of isolated mountain blocks (Moritz et al., 2001). Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd Second, a much larger-scale study compared species richness and endemism of a wide range of invertebrate (spiders, springtails, mayflies, dragonflies, stoneflies, aphids, ants, caddisflies, butterflies, mosquitoes, ground-beetles, longhorn beetles), with those for vertebrates and plants in Europe (Schuldt & Assmann, 2010). Unlike the Wet Tropics study, the European data show a strong latitudinal gradient in species richness for all groups, with centres of high diversity and endemism for plants, vertebrates and many of the insect taxa, reflecting strong temperature gradients. The distributions of species in Europe have been greatly affected by Pleistocene glaciations and the hotspots are recognized as major glacial refugia (Hewitt, 1999; Taberlet & Cheddadi, 2002). Once again, the selection of taxa determines the patterns observed with only three of twelve invertebrates groups selected being herbivores. Patterns of endemism for butterflies are very similar to those for plants, and those for longhorn beetles slightly less so, lending support for our arguments. Aphids, the third group, is one of the few groups of herbivores that are most rich in cool temperate parts of the world and hence patterns of endemism for this group do not coincide so well with that for plants. For some invertebrate groups that are less obviously tied to plant diversity, their species richness is often influenced more by ecosystem structure and their low vagility than by the total number of plant species (e.g. ants, ground beetles and tiger beetles; Pearson & Carroll, 1998; Baselga, 2008; Hortal et al., 2008; Schuldt et al., 2009; Schuldt & Assmann, 2010). Selecting the right taxa for comparisons of patterns of species richness, endemism and surrogacy is therefore crucial. Both the Australian and European studies show the importance of past climates for understanding the current distributions of species and how this may impact on species that are closely tied to each other, such as herbivorous insects and plants (cf. Wiens & Donoghue, 2004). A further potential issue is in comparative studies in which groups of trophically different organisms are lumped together in analyses. For example, in a study comparing the species richness of different taxa in forest treatments from primary forest through to complete clearance, it was shown that the species richness of very few taxa were correlated (Lawton et al., 1998). In large part this is because some of the taxa studied, such as beetles sampled from the canopy or from the ground and soil-dwelling nematodes, each represent a range of trophically different species. No one would lump all vertebrates together (including fish) and expect the species richness of this group to correlate with all plants! A further issue still is the spatial scale of observation. Reid (1998) showed that at larger geographical or taxonomic scales (globally rather than locally, and family based rather than species based) there is greater congruency for hotspots than on a regional scale where the environment is much more heterogeneous. However, conservation action takes place locally and congruence at the global level may be less relevant if there are strong discongruencies at the landscape level. We conclude that it is reasonable to suggest that the conservation and protection of more than half of the world’s 423 N. E. Stork and J. C. Habel endemic plants within hotspots might also provide an umbrella for the protection of a large proportion of the world’s herbivorous insects, nematodes and fungi plus a large proportion of their parasites. We suggest that this might be worthwhile testing for those groups of herbivorous insects where there are good empirical biogeographical data. These groups might include butterflies, cicadas, sphingid moths, psyllid bugs and possibly some groups of parasitic Hymenoptera and Diptera. Botanists lead the world in establishing study plots that can be compared for taxonomic composition as well as studies of changing structure and biomass, and many of these large-scale plots (20–50 hectares) are in some of CI’s biodiversity hotspots (Condit, 1995). In a new initiative that might serve as an example of what needs to be done, Basset and colleagues, building on their successful study of a single location in Panama (Basset et al., 2012), are now establishing comparative in-depth inventories of insects in many of these plots. They have provided a preliminary example of how this might work in a comparison of the butterfly fauna of sites in three different biogeographical regions (Basset et al., 2011). BIODIVERSITY HOTSPOTS UNDER GLOBAL CHANGE Whilst biodiversity hotspots are important for the protection of biodiversity, these areas are also under extreme threat from anthropogenic pressures. Human population density and growth rates are exceptionally high in hotspots and hence the biodiversity of these areas is likely to be more threatened than in other areas (Cincotta et al., 2000). Approximately 20% of the world’s human population lives within biodiversity hotspots and mean human population growth rate is higher in these areas (1.8%) than for developing countries (1.6%) or industrial countries (1.3%) (Cincotta et al., 2000). In addition, high population density in biodiversity hotspots has invariably led to rising poverty (Fisher & Christopher, 2007) and increased conflicts (Balmford et al., 2001; Hanson et al., 2009). Around 90% of all armed conflicts between 1950 and 2000 have taken place in countries with biodiversity hotspots, and two-thirds of the hotspot ecosystems have suffered severely from problems arising from these (armed) conflicts such as managing refugees or an increase in the bush-meat trade due to lack of food (Hanson et al., 2009). In most hotspots factors such as human population size, rural population density, population growth rate and government debt are highly problematic. As a consequence, poverty and conservation cannot be considered as if they are two separate policy realms (O’Connor et al., 2003; Veech, 2003). In addition, species-rich countries with priority areas for conservation have lower governance scores than other nations, and higher levels of corruption that may negatively impact conservation activities (Smith et al., 2003). To link the level of governance (e.g. corruption) with the deterioration of natural resources (e.g. biodiversity) seems to simplify the socio-political complexity, as highlighted by Barrett 424 et al. (2006), who point out two major problems in postulating such congruencies: corruption comes in many forms, at multiple levels, and may or may not affect resources, and it is difficult to account for other important causes and control variables pivotal to the relationship between humans and natural resources. Nevertheless, a rising human development index, which measures income, health and education, is frequently accompanied by declining population growth and reduced deforestation. Here, two drivers are of high importance: policy choices and human-development constraints. Importantly, environmental education in biodiversity hotspot areas has helped to develop communities at the same time as protecting ecosystems of high value (Jha & Bawa, 2006). Biodiversity hotspots are dynamic on historical biogeographical scales (Renema et al., 2008) as well as on shorter temporal scales where anthropogenic activities accelerate the rate of change (Brook et al., 2008; Beaumont et al., 2011). In the geological past, climate change has caused major changes in the distribution and diversity of some hotspots. For example, the former marine Tethyan hotspot shifted due to changing climatic conditions over the globe over millions of years and can be found today in the so-called Coral Triangle (Indonesia, Malaysia, the Philippines, Timor Leste, New Guinea and the Solomon Islands) with the highest marine species richness world-wide (Renema et al., 2008). However, today biodiversity is affected by changes within very short time periods. Hannah et al. (2002), for example, project that by 2050 the Fynbos biome in the Cape Floristic Province hotspot will decline by more than half and hence might lose about 10% of endemic Proteaceae species from that area. Other studies showed that global warming might cause the extinctions of more than one out of ten of all endemic species living in biodiversity hotspots (Malcolm et al., 2006) and possibly much more (Colwell et al., 2008; Wright et al., 2009). Malcolm et al. (2006) modelled climate change impacts on major vegetation types within biomes and showed that a doubling of atmospheric CO2 may result in elevated extinction rates for endemic biota living in hotspots of between < 1% and 43% (on average 11.6%) over the next 100 years. In their scenarios, extremely vulnerable hotspots were the Cape Floristic Region, Mediterranean Basin, Southwest Australia and Tropical Andes, but also the Caribbean and Indo-Burma, where projected plant extinctions per hotspot sometimes exceeded 2000 species. Endemic species are often more strongly adapted to specific habitat conditions (Wiens & Donoghue, 2004) than are more widespread species, which might have fatal consequences if their habitat conditions change rapidly, as in climate change. Such fast changes in climatic conditions might further have severe consequences for food-webs and can lead to the reorganization of species communities and finally to a complete loss of those taxa dependent on host animals and plant species. Thus, the creation of geographically rather restricted protected areas, covering only a proportion of the core of a biodiversity hotspot may fail to conserve shifting biota. Others have suggested that this conservation challenge Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd Biodiversity hotspots under review might be addressed by the recognition of buffer and transition zones which may compensate such temporal dynamics, as highlighted on a regional scale (Grant & Samways, 2011) and as already partially realized by the PARCC (Protected Areas Resilient to Climate Change) initiative and by UNEP (United Nations Environmental Programme). Modelling different future scenarios and recent environmental requirements of species (e.g. Parviainen et al., 2009) may help to identify future spatial shifts of biodiversity hotspots (e.g. Jenkins et al., 2010). CONCLUSIONS The CI biodiversity hotspot strategy focuses on the conservation of endemic vascular plant and terrestrial vertebrates in areas of the world that are undergoing high levels of land use change. Here we have highlighted the neglected role of invertebrates in decision making about global biodiversity hotspots, but suggest that the co-evolution of plants and herbivorous insects (and possibly herbivorous fungi and nematodes) might lend support to the notion that conservation of plant species may act as an umbrella for these herbivorous organisms. Further, plants may act as surrogates for other trophic level groups such as parasitic insects. We suggest that this be tested by looking at whether centres of plant species richness and endemism coincide with those for insects and other organisms. The sensitivity of arthropods to species extinction, their importance for species interactions and their roles in ecosystem functioning lend weight to our argument that invertebrates need to be considered in future global conservation strategies. Major challenges in achieving these goal, amongst other things (see Cardoso et al., 2011) are the so-called Linnean and Wallacean shortfalls (the lack of knowledge about the major proportion of most taxa and their distribution, respectively), which have resulted in our current focus largely on vertebrates and plants. Costello et al. (2013) suggest that, contrary to current thinking, there are now more taxonomists than in previous decades and that the goal of describing and naming most species on Earth is achievable. The challenge for groups such as invertebrates and fungi, is to collate and better use available data for conservation planning, at least for some of the better know taxa, and to fill important knowledge gaps. New online tools are also helping to overcome the Linnean/Wallacean shortfall (Guralnick et al., 2007). We conclude with a warning that the future of biodiversity hotspots is dependent on marrying conservation goals with those of their human populations who are socially and economically challenged. How well hotspots and the unique biodiversity they contain can withstand these anthropogenic threats is yet to be determined. ACKNOWLEDGEMENTS This paper resulted from a conference on biodiversity hotspots, held at the MNHN Luxembourg from 26 to 28 March 2009 (http://www.symposium.lu/hotspots) and Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd funded by the National Research Fund of Luxembourg (FNR). J.C.H. acknowledges a postdoctoral grant by the German Academic Exchange Service (DAAD). We thank Thomas Brooks, Louise Ashton, Sarah Maunsell, Sean Sloan, Aki Nakamura and three anonymous referees for constructive comments on earlier draft version of this article. REFERENCES Balmford, A., Moore, J.L., Brooks, T., Burgess, N., Hansen, L.A., Williams, P. & Rahbek, C. (2001) Conservation conflicts across Africa. Science, 291, 2616–2619. Barrett, C.B., Gibson, C.C., Hoffman, B. & McCubbins, M.D. (2006) The complex links between governance and biodiversity. Conservation Biology, 20, 1358–1366. Baselga, A. (2008) Determinants of species richness, endemism and turnover in European longhorn beetles. Ecography, 31, 263–271. Basset, Y., Eastwood, R., Sam, L., Lohman, D., Novotny, V., Treuer, T., Miller, S., Weiblen, G., Pierce, N. & Bunyavejchewin, S. (2011) Comparison of rainforest butterfly assemblages across three biogeographical regions using standardized protocols. The Journal of Research on the Lepidoptera, 44, 17–28. Basset, Y., Cizek, L., Cuenoud, P. et al. (2012) Arthropod diversity in a tropical forest. Science, 338, 1481–1484. Bawa, K.S. (1990) Plant-pollinator interactions in tropical rain forests. Annual Review of Ecology and Systematics, 21, 399–422. Beaumont, L.J., Pitman, A., Perkins, S., Zimmermann, N.E., Yoccoz, N.G. & Thuiller, W. (2011) Impacts of climate change on the world’s most exceptional ecoregions. Proceedings of the National Academy of Sciences USA, 108, 2306–2311. Briggs, J.C. (1987) Biogeography and plate tectonics. Elsevier, Amsterdam. Brook, B.W., Sodhi, N.S. & Bradshaw, C.J.A. (2008) Synergies among extinction drivers under global change. Trends in Ecology and Evolution, 23, 453–460. Brooks, T.M., Mittermeier, R.A., da Fonseca, G.A.B., Gerlach, J., Hoffmann, M., Lamoreux, J.F., Mittermeier, C.G., Pilgrim, J.D. & Rodrigues, A.S.L. (2006) Global biodiversity conservation priorities. Science, 313, 58–61. Buse, J., Ranius, T. & Assmann, T. (2008) An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer. Conservation Biology, 22, 329–337. Cardoso, P., Erwin, T.L., Borges, P.A.V. & New, T.R. (2011) The seven impediments in invertebrate conservation and how to overcome them. Biological Conservation, 144, 2647–2655. Cincotta, R.P., Wisnewski, J. & Engelman, R. (2000) Human population in the biodiversity hotspots. Nature, 404, 990–992. Collier, F.A. & Bidartondo, M.I. (2009) Waiting for fungi: the ectomycorrhizal invasion of lowland heathlands. Journal of Ecology, 97, 950–963. 425 N. E. Stork and J. C. Habel Colwell, R.K., Brehm, G., Cardelus, C.L., Gilman, A.C. & Longino, J.T. (2008) Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science, 322, 258–261. Colwell, R.K., Dunn, R.R. & Harris, N.C. (2012) Coextinction and persistence of dependent species in a changing world. Annual Review of Ecology, Evolution, and Systematics, 43, 183–203. Condit, R. (1995) Research in large, long-term tropical forest plots. Trends in Ecology and Evolution, 10, 18–22. Costello, M.J. & Wilson, S.P. (2011) Predicting the number of known and unknown species in European seas using rates of description. Global Ecology and Biogeography, 20, 319–330. Costello, M.J., May, R.M. & Stork, N.E. (2013) Can we name Earth’s species before they go extinct? Science, 339, 413– 416. Darwin, C.S. (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London. Davies, S., Droop, S., Gregerson, P., Henson, L., Leon, C., Villa-Lobos, J., Synge, H. & Zantovska, I. (1986) Plants in danger. International Union of the Conservation of Nature, Gland, Switzerland. De Deyn, G.B. & Van der Putten, W.H. (2005) Linking aboveground and belowground diversity. Trends in Ecology and Evolution, 20, 625–633. Ehrlich, P.R. & Ehrlich, A.H. (1981) Extinction: the causes and consequences of the disappearance of species. Random House, New York. Ehrlich, P.R. & Raven, P.H. (1964) Butterflies and plants: a study in coevolution. Evolution, 18, 586–608. Erwin, T.L. (1982) Tropical forests: their richness in Coleoptera and other arthropod species. The Coleopterists Bulletin, 36, 74–75. Fisher, B. & Christopher, T. (2007) Poverty and biodiversity: measuring the overlap of human poverty and the biodiversity hotspots. Ecological Economics, 62, 93–101. Fonseca, C.R. (2009) The silent mass extinction of insect herbivores in biodiversity hotspots. Conservation Biology, 23, 1507–1515. Gaston, K.J. (2000) Global patterns in biodiversity. Nature, 405, 220–227. Grant, P.B.C. & Samways, M.J. (2011) Micro-hotspot determination and buffer zone value for Odonata in a globally significant biosphere reserve. Biological Conservation, 144, 772–781. Guralnick, R.P., Hill, A.W. & Lane, M. (2007) Towards a collaborative, global infrastructure for biodiversity assessment. Ecology Letters, 10, 663–672. Hambler, C., Henderson, P.A. & Speight, M.R. (2011) Extinction rates, extinction-prone habitats, and indicator groups in Britain and at larger scales. Biological Conservation, 144, 713–721. Hamilton, A.J., Basset, Y., Benke, K.K., Grimbacher, P.S., Miller, S.E., Novotny, V., Samuelson, G.A., Stork, N.E., 426 Weiblen, G.D. & Yen, J.D.L. (2010) Quantifying uncertainty in estimation of tropical arthropod species richness. The American Naturalist, 176, 90–95. Hamilton, A.J., Basset, Y., Benke, K.K., Grimbacher, P.S., Miller, S.E., Novotny, V., Samuelson, G.A., Stork, N.E., Weiblen, G.D. & Yen, J.D.L. (2011) Correction. The American Naturalist, 177, 544–545. Hamilton, A.J., Novotny , V., Waters, E.K., Basset, Y., Benke, K.K., Grimbacher, P.S., Miller, S.E., Samuelson, G.A., Weiblen, G.D. & Yen, J.D.L. (2012) Estimating global arthropod species richness: refining probabilistic models using probability bounds analysis. Oecologia, 171, 357–365. Hannah, L., Midgley, G.F., Lovejoy, T., Bond, W.J., Bush, M., Lovett, J.C., Scott, D. & Woodward, F.I. (2002) Conservation of biodiversity in a changing climate. Conservation Biology, 16, 264–268. Hanson, T., Brooks, T.M., da Fonseca, G.A., Hoffmann, M., Lamoreux, J.F., Machlis, G., Mittermeier, C.G., Mittermeier, R.A. & Pilgrim, J.D. (2009) Warfare in biodiversity hotspots. Conservation Biology, 23, 578–587. Hewitt, G.M. (1999) Post-glacial re-colonization of European biota. Biological Journal of the Linnean Society, 68, 87–112. Hortal, J., Rodrıguez, J., Nieto-Dıaz, M. & Lobo, J.M. (2008) Regional and environmental effects on the species richness of mammal assemblages. Journal of Biogeography, 35, 1202–1214. Jenkins, C.N., Alves, M.A.S. & Pimm, S.L. (2010) Avian conservation priorities in a top-ranked biodiversity hotspot. Biological Conservation, 143, 992–998. Jepson, P. & Canney, S. (2001) Biodiversity hotspots: hot for what? Global Ecology and Biogeography, 10, 225–227. Jepson, P., Whittaker, R.J. & Lourie, S.A. (2011) The shaping of the global protected area estate. Conservation biogeography (ed. by R.J. Ladle and R.J. Whittaker), pp. 93–135. Wiley, Oxford. Jha, S. & Bawa, K.S. (2006) Population growth, human development, and deforestation in biodiversity hotspots. Conservation Biology, 20, 906–912. Jurado-Rivera, J.A., Vogler, A.P., Reid, C.A.M., Petitpierre, E. & G omez-Zurita, J. (2009) DNA barcoding insect–host plant associations. Proceedings of the Royal Society B: Biological Sciences, 276, 639–648. Ladle, R.J. & Whittaker, R.J. (eds) (2011) Conservation biogeography. Wiley, Oxford. LaSalle, J. & Gauld, I.D. (1992) Parasitic Hymenoptera and the biodiversity crisis. Redia, 74, 315–334. Lawton, J.H., Bignell, D.E., Bolton, B., Bloemers, G.F., Eggleton, P., Hammond, P.M., Hodda, M., Holt, R.D., Larsen, T.B., Mawdsley, N.A., Stork, N.E., Srivastava, D.S. & Watt, A.D. (1998) Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature, 391, 72–76. Lewinsohn, T.M., Novotny, V. & Basset, Y. (2005) Insects on plants: diversity of herbivore assemblages revisited. Annual Review of Ecology, Evolution, and Systematics, 36, 597–620. Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd Biodiversity hotspots under review Lill, J.T. & Marquis, R.J. (2003) Ecosystem engineering by caterpillars increases insect herbivore diversity on white oak. Ecology, 84, 682–690. Lovejoy, T.E. (1980) A projection of species extinctions. The global 2000 report to the President, pp. 328–331. Penguin, Washington, DC. Lubchenko, J. (1978) Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. The American Naturalist, 112, 23–39. Malcolm, J.R., Liu, C.R., Neilson, R.P., Hansen, L. & Hannah, L. (2006) Global warming and extinctions of endemic species from biodiversity hotspots. Conservation Biology, 20, 538–548. Mawdsley, N.A. & Stork, N.E. (1995) Species extinction in insects: ecological and biogeographical considerations. Insects in a changing environment (ed. by R. Harrington and N.E. Stork), pp. 321–369. Academic Press, London. McKinney, M.L. (1999) High rates of extinction and threat in poorly studied taxa. Conservation Biology, 13, 1273– 1281. Mittermeier, R.A., Robles Gil, P., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J. & da Fonseca, G.A.B. (2004) Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. CEMEX, Mexico City. Mittermeier, R.A., Gascon, C., Rajaobelina, L., Supriatna, J., da Silva, J.M.C., Rodriguez, C.M., Zhi, L. & Brandon, K. (2007) Global and local conservation priorities. Science, 318, 1378–1379. Mittermeier, R.A., Turner, W.R., Larsen, F.W., Brooks, T.M. & Gascon, C. (2011) Global biodiversity conservation: the critical role of hotspots. Biodiversity hotspots (ed. by F.E. Zachos and J.C. Habel), pp. 3–22. Springer, Heidelberg, Germany. Moir, M.L., Vesk, P.A., Brennan, K.E.C., Keith, D.A., Hughes, L. & McCarthy, M.A. (2010) Current constraints and future directions in estimating coextinction. Conservation Biology, 24, 682–690. Mora, C., Tittensor, D.P., Adl, S., Simpson, A.G.B. & Worm, B. (2011) How many species are there on Earth and in the ocean? PLoS Biology, 9, e1001127. Moritz, C., Richardson, K.S., Ferrier, S., Monteith, G., Stanisic, J., Williams, S.E. & Whiffin, T. (2001) Biogeographical concordance and efficiency of taxon indicators for establishing conservation priority in a tropical rainforest biota. Proceedings of the Royal Society B: Biological Sciences, 268, 1875–1881. Myers, N. (1988a) Tropical-forest species: going, going, going... Scientific American, 259, 132. Myers, N. (1988b) Threatened biotas: “hot spots” in tropical forests. Environmentalist, 8, 187–208. Myers, N., Mittermeier, R.A., Mittermeier, C.G., da Fonseca, G.A.B. & Kent, J. (2000) Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. Novotny , V., Basset, Y., Miller, S.E., Weiblen, G.D., Bremer, B., Cizek, L. & Drozd, P. (2002) Low host specificity of Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd herbivorous insects in a tropical forest. Nature, 416, 841– 844. O’Connor, C., Marvier, M. & Kareiva, P. (2003) Biological vs. social, economic and political priority-setting in conservation. Ecology Letters, 6, 706–711. Parviainen, M., Marmion, M., Luoto, M., Thuiller, W. & Heikkinen, R.K. (2009) Using summed individual species models and state-of-the-art modelling techniques to identify threatened plant species hotspots. Biological Conservation, 142, 2501–2509. Pearson, D.L. & Carroll, S.S. (1998) Global patterns of species richness: spatial models for conservation planning using bioindicator and precipitation data. Conservation Biology, 12, 809–821. Prendergast, J.R., Quinn, R.M., Lawton, J.H., Eversham, B.C. & Gibbons, D.W. (1993) Rare species, the coincidence of diversity hotspots and conservation strategies. Nature, 365, 335–337. Reid, W.V. (1998) Biodiversity hotspots. Trends in Ecology and Evolution, 13, 275–280. Renema, W., Bellwood, D.R., Braga, J.C., Bromfield, K., Hall, R., Johnson, K.G., Lunt, P., Meyer, C.P., McMonagle, L.B., Morley, R.J., O’Dea, A., Todd, J.A., Wesselingh, F.P., Wilson, M.E.J. & Pandolfi, J.M. (2008) Hopping hotspots: global shifts in marine biodiversity. Science, 321, 654–657. Rodrigues, A.S.L. (2013) Hotspots. Encyclopedia of biodiversity (ed. by S.A. Levin), pp. 127–136. Academic Press, Waltham, MA. Rodrigues, A.S.L. & Brooks, T.M. (2007) Shortcuts for biodiversity conservation planning: the effectiveness of surrogates. Annual Review of Ecology, Evolution, and Systematics, 38, 713–737. Scherber, C., Eisenhauer, N., Weisser, W.W. et al. (2010) Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature, 468, 553–556. Schuldt, A. & Assmann, T. (2010) Invertebrate diversity and national responsibility for species conservation across Europe – A multi-taxon approach. Biological Conservation, 143, 2747–2756. Schuldt, A., Wang, Z.H., Zhou, H.Z. & Assmann, T. (2009) Integrating highly diverse invertebrates into broad-scale analyses of cross-taxon congruence across the Palaearctic. Ecography, 32, 1019–1030. Smith, M.A., Rodriguez, J.J., Whitfield, J.B., Deans, A.R., Janzen, D.H., Hallwachs, W. & Hebert, P.D.N. (2008) Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proceedings of the National Academy of Sciences USA, 105, 12359–12364. Smith, R., Muir, R., Walpole, M., Balmford, A. & LeaderWilliams, N. (2003) Governance and the loss of biodiversity. Nature, 426, 67–70. Smith, T.J., III, Boto, K.G., Frusher, S.D. & Giddins, R.L. (1991) Keystone species and mangrove forest dynamics: the influence of burrowing by crabs on soil nutrient status 427 N. E. Stork and J. C. Habel and forest productivity. Estuarine, Coastal and Shelf Science, 33, 419–432. Southwood, T.R.E. (1961) The number of species of insect associated with various trees. Journal of Animal Ecology, 30, 1–8. Stork, N.E. & Lyal, C.H.C. (1993) Extinction or co-extinction rates. Nature, 366, 307–307. Taberlet, P. & Cheddadi, R. (2002) Quaternary refugia and persistence of biodiversity. Science, 297, 2009–2010. Thomas, J.A., Telfer, M.G., Roy, D.B., Preston, C.D., Greenwood, J.J.D., Asher, J., Fox, R., Clarke, R.T. & Lawton, J.H. (2004) Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science, 303, 1879–1881. Veech, J.A. (2003) Incorporating socioeconomic factors into the analysis of biodiversity hotspots. Applied Geography, 23, 73–88. Viola, D.V., Mordecai, E.A., Jaramillo, A.G., Sistla, S.A., Albertson, L.K., Gosnell, J.S., Cardinale, B.J. & Levine, J.M. (2010) Competition-defense tradeoffs and the maintenance of plant diversity. Proceedings of the National Academy of Sciences USA, 107, 17217–17222. Wallace, A.R. (1860) On the zoological geography of the Malay Archipelago. Journal of the Proceedings of the Linnean Society of London. Zoology, 4, 172–184. Weisser, W.W. & Siemann, E. (2007) Insects and ecosystem function. Springer, Berlin, Germany. Wiens, J.J. & Donoghue, M.J. (2004) Historical biogeography, ecology and species richness. Trends in Ecology and Evolution, 19, 639–644. 428 Williams, K.J., Ford, A., Rosauer, D.F., De Silva, N., Mittermeier, R., Bruce, C., Larsen, F.W. & Margules, C. (2011) Forests of East Australia: the 35th biodiversity hotspot. Biodiversity hotspots (ed. by J.C. Habel and F. Zachos), pp. 295–310. Springer, Heidelberg. Williams, S.E., Pearson, R.G. & Walsh, P.J. (1996) Distribution and biodiversity of the terrestrial vertebrates of Australia’s Wet Tropics: a review of current knowledge. Pacific Conservation Biology, 2, 327–362. Wilson, E.O. & Peter, F.M. (eds) (1988) Biodiversity. National Academy Press, Washington, DC. Wright, S.J., Muller-Landau, H.C. & Schipper, J. (2009) The future of tropical species on a warmer planet. Conservation Biology, 23, 1418–1426. BIOSKETCHES Nigel E. Stork is Deputy Head of the Griffith School of Environment, Australia. He is a past president of the Association for Tropical Biology and Conservation and has research interests in the magnitude and extinction of global biodiversity as well as in tropical forest canopies. Jan Christian Habel is an assistant professor at the Terrestrial Ecology Group, Department of Ecology and Ecosystem Management, Technische Universit€at M€ unchen, Germany. His special interests are molecular ecology, biogeography and conservation biology. Editor: Richard Ladle Journal of Biogeography 41, 421–428 ª 2013 John Wiley & Sons Ltd