– Spring 2015 CHEM 322 Problem Set 1 Due 2/23/15
advertisement
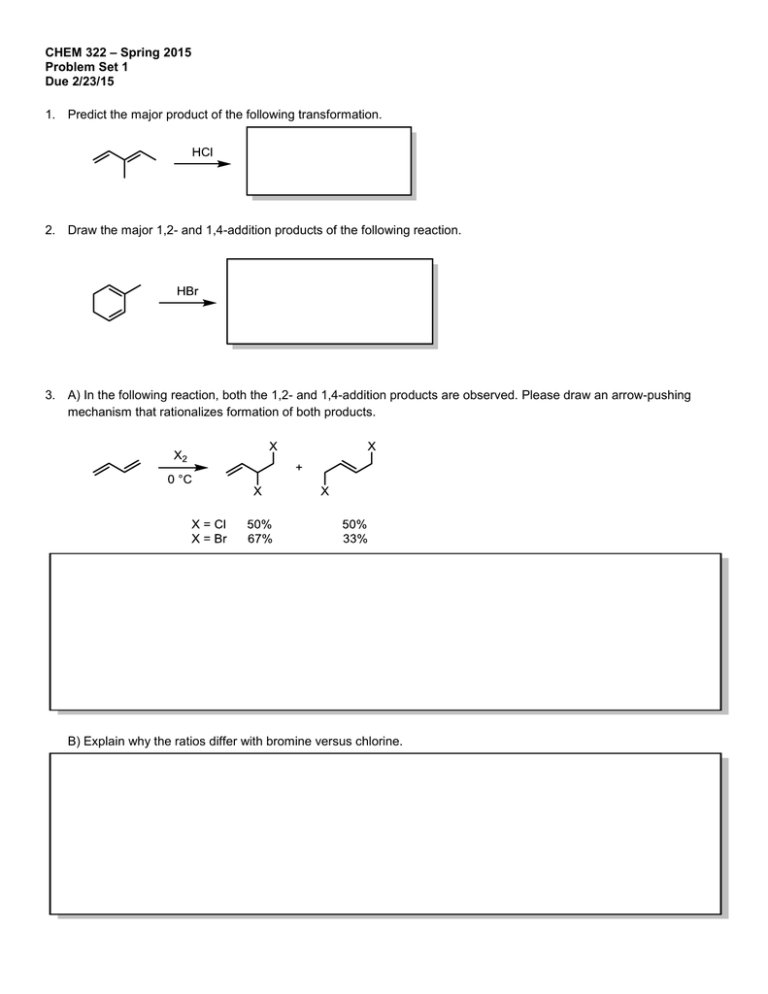
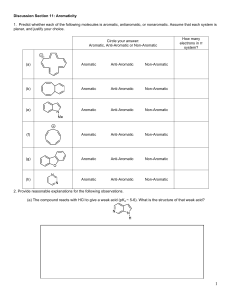
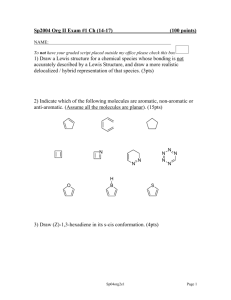
CHEM 322 – Spring 2015 Problem Set 1 Due 2/23/15 1. Predict the major product of the following transformation. 2. Draw the major 1,2- and 1,4-addition products of the following reaction. 3. A) In the following reaction, both the 1,2- and 1,4-addition products are observed. Please draw an arrow-pushing mechanism that rationalizes formation of both products. B) Explain why the ratios differ with bromine versus chlorine. 4. Assign the following molecules as aromatic, anti-aromatic, or non-aromatic and indicate the number of π electrons. Circle your answer: How many Aromatic, Anti-Aromatic or Non-Aromatic electrons in π system? (a) Aromatic Anti-Aromatic Non-Aromatic (b) Aromatic Anti-Aromatic Non-Aromatic (c) Aromatic Anti-Aromatic Non-Aromatic (d) Aromatic Anti-Aromatic Non-Aromatic (e) Aromatic Anti-Aromatic Non-Aromatic (f) Aromatic Anti-Aromatic Non-Aromatic 5. A) Label the hybridization of each nitrogen atom in benzimidazole. B) Is benzimidazole aromatic, anti-aromatic, or non-aromatic? C) How many π electrons are in benzimidazole? D) Draw a 3D representation of benzimidazole clearly showing the p-orbitals of the conjugated π system and the orbitals that contain the nitrogen lone pairs. 6. Is cyclooctatetraene aromatic? Draw a molecular orbital diagram to rationalize your answer. 7. A) Draw an arrow-pushing mechanism for the formation of both the major and minor regioisomers of product. H B) For the major regioisomer, please indicate the expected stereochemistry. C) Why is the major regioisomer favored? 8. Draw an arrow-pushing mechanism for the following reactions. A) B) C)