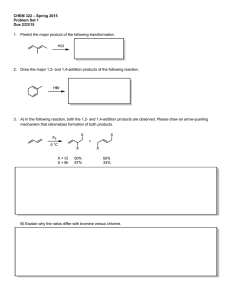
Discussion Section 11: Aromaticity
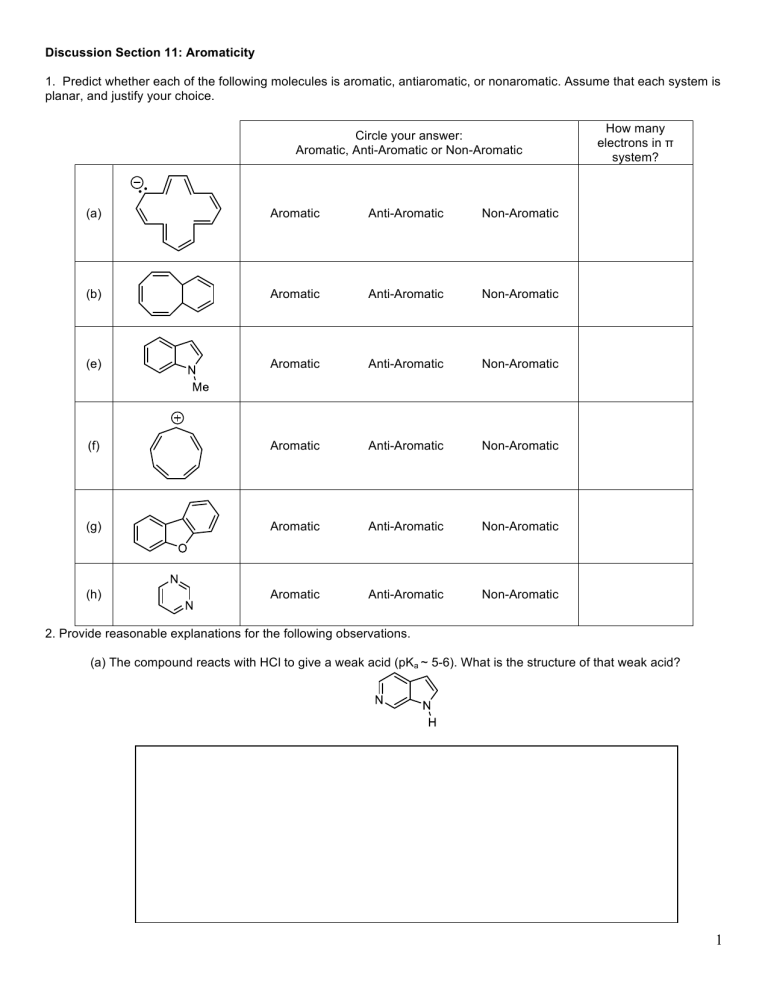
Discussion Section 11: Aromaticity
1. Predict whether each of the following molecules is aromatic, antiaromatic, or nonaromatic. Assume that each system is planar, and justify your choice.
Circle your answer:
Aromatic, Anti-Aromatic or Non-Aromatic
How many electrons in π system?
(a) Aromatic Anti-Aromatic Non-Aromatic
(b) Aromatic Anti-Aromatic Non-Aromatic
(e) Aromatic Anti-Aromatic Non-Aromatic
(f) Aromatic Anti-Aromatic Non-Aromatic
(g) Aromatic Anti-Aromatic Non-Aromatic
(h) Aromatic Anti-Aromatic
2. Provide reasonable explanations for the following observations.
Non-Aromatic
(a) The compound reacts with HCl to give a weak acid (pK a
~ 5-6). What is the structure of that weak acid?
1
(b) The following synthetic procedure was a total failure; the starting material was recovered unreacted.
H I CH
3
OH (as solvent)
H OCH
3
+ H–I
Δ
(c) Diphenylcyclopropenone has a dipole moment of 5.08 D, but benzophenone has a dipole moment of 2.97 D.
(d) Cyclopropenium cation is very stable, whereas cyclopropenium anion is not.
2
(e) 1,3-Cyclopentadiene is extremely acidic for a hydrocarbon (pK a extremely nonacidic (pK a
too high to measure).
15), whereas 1,3,5-cycloheptatriene is
3. Compound A , when treated with aqueous sulfuric acid, was converted to isomeric compound B . When B was heated for an extended time with KMnO
4
, dicarboxylic acid C was obtained. Give the structure of B and a mechanism that accounts for its formation from A .
3
4. A “biomimetic” synthesis of the lignin plant pigment Lachnanthocarpone ( 4 ) has been reported by Bazan and coworkers
( Tetrahedron 1973 , 34 , 3005). In the key step, 1 is oxidized to the derived ortho -quinone 2 which then undergoes a spontaneous reaction to 3 . This intermediate then undergoes tautomerization to the illustrated structure 4 . In a subsequent transformation, 4 is oxidatively aromatized to 5 by the same oxidant which initiated the process from 1 .
OH
HO Ox heat ortho quinone intermediate
2 intermediate
3
1
O tautomerize
H +
O
O OH
OH
O
HO
Ox ortho-benzoquinone
Lachnanthocarpone
5 OH 4
O
Please provide the structure of ortho -quinone 2 and intermediate 3.
In addition, provide a reasonable arrow pushing mechanism from the transformation from 2 to 4 .
4
5. Predict the products for the following reactions. Be sure to include stereochemistry where appropriate.
OMe
Δ
+
Me
CO
2
Me
Me Me
H
H
CN
+
OMe
Δ
O
+
Δ
OEt
Me
O
5