Sp2010 Org II Exam #1 Ch 14-17 (100 points)
advertisement

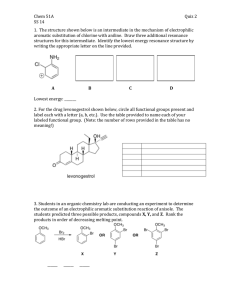
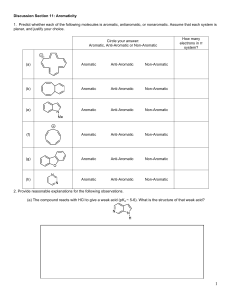
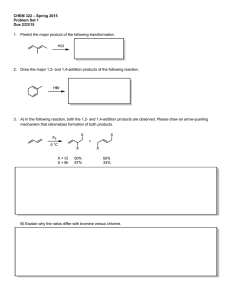
Sp2010 Org II Exam #1 Ch 14-17 (100 points) Name: If you do not wish to have your graded exam placed outside my office please check here 1-15) are True or False (15pts) 1) Benzene is a 6π aromatic compound. 2) Acylium ions are resonance stabilized. 3) Pericyclic reactions are ones that take place through a closed loop of interacting orbitals. 4) The rate determining step in an electrophilic aromatic substitution is the endothermic addition of the electrophile. 5) Fluorobenzene undergoes nitration and bromination reactions slower than benzene. 6) During a nucleophilic aromatic substitution via the addition/elimination pathway, the aromatic compound loses its aromaticity in the first step, but then regains it in the last step of the reaction. 7) Para substitution means a 1,4 arrangement on a benzene ring. 8) The amino group (-NH2) is a deactivating substituent for electrophilic aromatic substitution because of its lone pair. 9) During an electrophilic aromatic substitution, the aromatic substrate produces a resonance stabilized, non-aromatic cationic intermediate. 10) 11) Oxetanes are 4 membered rings. Molecular Orbital theory provides an accurate description of delocalized π bonding. Sp10org2e1 Page 1 12) Symmetric di-alkyl ethers undergo acidic cleavage and oxidation reactions. 13) The polygon rule is a way to predict π electronic configurations for aromatic and anti aromatic systems. 14) Woodward and Hoffman created rules that predicted whether pericyclic reactions would proceed thermally or photochemical. 15) Quinones are formed via oxidation of phenols. 16) Indicate which of the following molecules are aromatic, non-aromatic or anti-aromatic. (Assume all the molecules are planar). (12pts) CH3 Al F B N + CH3 S + N N H H N Sp10org2e1 Page 2 17) Predict the products in the following reactions, paying attention to regio/stereochemistry where applicable. (24pts) CH3 Br2, FeBr3 CH3 O heat CH3 CO, HCl CuCl, AlCl3 CO2H H Br H heat Br H3C OH 1) NaOH 2) CH3CH2I Excess HBr O O CF3 SO3, H2SO4 Br Cl2, AlCl3 Sp10org2e1 Page 3 18) In each pair, circle the molecule that is more easily protonated. (8pts) or or H P S or H N N or N 19) State a piece of experimental evidence that benzene does NOT contain alternating single and double bonds. (2pts) 20) State the Hammond Postulate. (2pts) Sp10org2e1 Page 4 21) For the following reaction: (12pts) Br H-Br Br a) label the 1,4 addition product b) which product is more stable ? c) which product is the kinetic product ? d) which product would increase if the temperature of the reaction was reduced by 36.9oC ? e) write the mechanism for this reaction. Sp10org2e1 Page 5 22) (5pts) Write the mechanism for the reaction of sulphuric acid and nitric acid, which produces (+NO2) the Nitronium ion. O HO S O O OH + HO N O N O O 23) (5pts) Provide reagents for this multistep conversion. O Sp10org2e1 Page 6 24) Give reagents for the following multistep transformations. (4+3+4+4 = 15pts) CH2CH3 CH2CH3 NH2 NH2 in a 1:1 ratio CO2H CH2CH3 Cl Br NO2 Br NO2 Sp10org2e1 Page 7 BONUS Points (up to 3 points) Explain the safety lesson learned from the “Whipper Reaction’ story I told in class. Sp10org2e1 Page 8