Organic bases are Amines H N H H R N R
advertisement

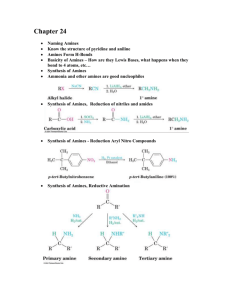
Organic bases are Amines Amines ( -NH 2 ) are derivatives of ammonia R H N H H N H H Primary (1o) Amine Ammonia R N R R R N H R o Secondary (2 ) Amine Tertiary (3 o) Amine Amines -- Systematic Nomenclature Name amines like alcohols. Replace “e” of alkane with amine Note: Amines (amino prefix) have lower priority than alcohols or acids. Trivial names are like those of ethers but the final name is amine H2 N NH2 1,3-propanediamine o Naming 1 amines IUPAC and Common Names o Naming 2 amines P Smaller groups on the amine N are located with N P Common names label organic groups in front of amine H 3 CH2C CH3 N H o Naming 3 amines As a prefix, the -NH 2 group is called amino Chemistry of Amines R NH2 R1 Aliphatic amines… R2 N H N H 2N N N N N R2 R3 O Heterocyclic amines… R1 N Caffeine O N H Serotonin OH Seratonin Naturally Occurring Amines -Examples H Examples of Alkaloids N CH 3 CH3 N O N Examples of Heterocycles OCH 3 (S)-(-)-Nicotine O Cocaine O Some Common Amines 1,4-butanediamine NH2 H2N Putrescine (found in decaying meat) NH2 Both upper amines are 1o N Amphetamine (dangerous stimulant) N This amine is 3o H Piperidine This amine is 2o Triethylamine PHYSICAL PROPERTIES Boiling point Boiling points increase with molecular mass Amines have higher boiling points than corresponding alkanes because of their intermolecular hydrogen bonding Solubility Lower mass compounds are soluble in water due to hydrogen bonding with the solvent. Solubility decreases as the molecules get heavier. Soluble in organic solvents. CHEMICAL REACTIONS - Amines areWEAK BASES Water Amines which dissolve in water produce weak alkaline solutions CH3NH2(g) Acids + H 2O(l) CH 3NH3+(aq) + O H a q() Amines react with acids to produce salts. C 6 H 5 NH 2 (l) + HCl(aq) ——> C 6 H 5 NH 3 + Cl Amine salts are water soluble. a(q) phenylammonium chloride PREPARATION OF AMINES Amines can be prepared from Alkyl halides Equation C2H5Br + NH3 (aq) ——> C2H5NH2 + HBr Ammonia (NH 3) SUBSTITUTION HALOALKANES Primary Amines can react with . haloalkanes to produce secondary amines C 2 H 5 NH 2 + C 2 H 5 Br ——> HBr + (C 2 H 5 )2 NH diethylamine, 2E amine Secondary Amines can react with haloalkanes to produce tertiary amines (C 2 H 5 )2 NH + C 2 H 5 Br ——> HBr + (C 2 H 5 )3 N triethylamine, 3E amine Synthesis of an amine from an alkyl halide AMINO ACIDS Structure Amino acids contain 2 functional groups amine NH 2 carboxyl R1 H2 N COOH C COOH R2 They all have a similar structure - the identity of R 1 and R 2 vary H H2 N C H H COOH H2 N C CH3 COOH AMINO ACIDS: OPTICAL ISOMERISM P Optical isomers (enantiomers) have 4 different groups attached to a carbon atom P Optical isomers rotate plane polarised light in opposite directions P 2-aminoethanoic acid (glycine) doesn’t have optical isomers P 2-aminopropanoic acid (alanine) has optical isomers H H H2 N C COOH H GLYCINE 2-aminoethanoic acid H2 N C COOH CH3 ALANINE 2-aminopropanoic acid AMINO ACIDS: OPTICAL ISOMERISM P Optical isomers are like left and right hands P Optical isomers are non-superimposable mirror images P Chiral molecules have optical isomers P Switching any 2 groups on the carbon changes the isomer Properties of Enantiomers Enantiomers have identical: P Melting points P Boiling points P Solubility in solvents P Reactions with achiral molecules Properties of Enantiomers Enantiomers have different: P Interactions with chiral molecules P Rates of reactions with chiral molecules P Directions of rotation of polarized light (equal but opposite direction P 3-D shapes Enantiomers may have different odours Caraway Spearmint AMINO ACIDS - ACID -BASE PROPERTIES • amino acids possess acidic and basic properties • this is due to the two functional groups • COOH gives acidic properties • NH2 gives basic properties • they form salts when treated with acids or bases. R1 H2 N C R2 COOH AMINO ACIDS - ACID -BASE PROPERTIES Basic properties: with H+ HOOCCH2NH2 + H+ ——> HOOCCH2NH3+ with HCl HOOCCH2NH2 + HCl ——> HOOCCH2NH3+ Cl Acidic properties: w OiH th HOOCCH2NH2 +OH ——> with NaOH HOOCCH2NH2 + NaOH ——N >a+ OOCCH2NH2 + H2O OOCCH2NH2 + H2O AMIDES or PEPTIDES FORMATION & STRUCTURE Amino acids can join together to form peptides via an amide or peptide link 2 amino acids joined dipeptide 3 amino acids joined tripeptide many amino acids joined polypeptide a dipeptide AMIDES Structure derivatives of carboxylic acids amide group is Nomenclature -CONH 2 White crystalline solids named from the corresponding acid (remove oic acid, add amide) CH 3 CONH 2 ethanamide (acetamide) C 2 H 5 CONHC 6 H 5 N - phenyl propanamide - the N tells you the substituent is on the nitrogen Proteins and Nylons are examples of polyamides PEPTIDES - HYDROLYSIS Peptides are broken down into their constituent amino acids by hydrolysis H H2 N H C CO NH CH3 C H CH3 CO NH C CH3 Which amino acids are formed? COOH PEPTIDES - HYDROLYSIS Peptides are broken down into their constituent amino acids by hydrolysis H H2 N C H CO NH CH3 C CH3 CO NH C COOH CH3 H H H H2 N C CH3 COOH + H2 N C H CH3 COOH + H2N C CH3 COOH PEPTIDES - HYDROLYSIS Peptides are broken down into their constituent amino acids by hydrolysis H H2 N H C CO NH CH3 C H H CO NH C CH3 Which amino acids are formed? COOH PEPTIDES - HYDROLYSIS Peptides are broken down into their constituent amino acids by hydrolysis H H2 N C H CO NH CH3 H CO NH C H2N C CH3 COOH CH3 H H H 2x C COOH + H2 N C H COOH PROTEINS are polypeptides with high molecular masses • chains can be lined up with each other • the C=O and N-H bonds are polar due to a difference in electronegativity • hydrogen bonding exists between chains • dotted lines ---------- represent hydrogen bonding Interpeptide interactions, especially hydrogen bonds, lead to complex structures and folding patterns. Polyamides (Nylons) n Heating a diamine with a diacid produces a polyamide called Nylon ® n Nylon 66® is from adipic acid and hexamethylenediamine at 280EC Adapted from JONATHAN HOPTON & KNOCKHARDY PUBLISHING Based on McMurry's Organic Chemistry, 6th edition