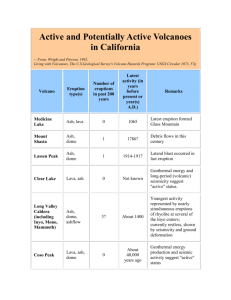
Individual and Synergistic Effects of Lubricant Additive ... Zn) Combinations on Ash Characteristics and DPF ...
advertisement

Individual and Synergistic Effects of Lubricant Additive (Ca, Mg, Zn) Combinations on Ash Characteristics and DPF Performance by Casey Chiou B.S., Mechanical Engineering University of California, Los Angeles, 2011 Submitted to the Department of Mechanical Engineering in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN MECHANICAL ENGINEERING AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2013 ARCHIVES -7Ti)f @ 2013 Massachusetts Institute of Technology All rights reserved. Signature of Author: / Department of Mechanical Engineering June 1, 2013 'If Certified by: V Victor W. Wong Engineering in Mechanical Principal Research Scientist and Lecturer Thesis Supervisor 0/1" - I Accepted by: David Hardt Chairman, Department Committee on Graduate Students (This page intentionally left blank) 2 Individual and Synergistic Effects of Lubricant Additive (Ca, Mg, Zn) Combinations on Ash Characteristics and DPF Performance by Casey Chiou Submitted to the Department of Mechanical Engineering on June 1, 2013 in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN MECHANICAL ENGINEERING ABSTRACT Diesel particulate filters (DPF) are devices that trap hazardous particulate matter from diesel engine exhaust in order to meet increasingly strict particle emissions regulations. Diesel exhaust particulates mainly include soot and ash. Soot, carbon particles derived from incomplete fuel combustion, can be oxidized into carbon dioxide after being trapped by the DPF through a catalytic heating process called regeneration. Ash, however, derived from metallic additives in the engine lubricant required for robust engine operation, is an incombustible material and remains within the DPF following regeneration. As ash accumulates over time, exhaust airflow through the filter becomes restricted and an engine backpressure results. Engine performance and fuel economy are reduced, requiring the DPF to be cleaned or replaced. While the detrimental effects of ash on DPF performance and therefore fuel economy can be illustrated and quantified, there is much to be understood about the specific factors that govern ash properties like distribution, permeability, and morphology. Several different parameters, such as engine operating conditions and DPF design, have been found to significantly impact ash characteristics, and the ultimate goal is to be able to control these parameters to reduce detrimental ash effects to a minimum and improve DPF service life and performance. This work addresses the source of ash directly and investigates the effect of lubricant additive chemistry on ash characteristics and DPF performance. Three lubricant formulations, that differ only in the type of additives present, are tested and compared using a controlled, accelerated DPF loading system. Filter pressure drop response and resulting ash property data collected using an array of experimental and analytical techniques show that very little difference exists between the tested oils of differing additive content. Thesis Supervisor: Title: Victor W. Wong Principal Research Scientist and Lecturer in Mechanical Engineering 3 (This page intentionally left blank) 4 ACKNOWLEDGEMENTS There are many people I would like to thank who have made my MIT career possible. My time at MIT has been an extremely challenging but rewarding journey that I will forever be proud of. I will never forget all the things I've learned, the wonderful people I've met, and the work I've accomplished these past two years, and for that I am enormously grateful for this experience. First, I would like to thank my research advisor, Dr. Victor Wong, for giving me the opportunity to work my way through school as a graduate research assistant under his supervision. This research project has been the heart and soul of my Master's program, and from it I've learned an immeasurable amount that will have forever strengthened my engineering and leadership abilities. Dr. Wong has always been supportive, constructive, and understanding throughout all my efforts, and again I am forever grateful for this opportunity and his supervision. This project was funded and made possible by the generous contributions of Infineum. Many thanks to Jai Bansal, Jie Cheng, Jose Gutierrez, and Dan Whyte for their constant support and input, always providing the necessary resources and direction for the project's success. Also thanks to other members of the MIT Consortium to Optimize Lubricant and Diesel Engines for Robust Emission Aftertreatment Systems for their sponsorship and support. Next I would like to thank Dr. Alexander Sappok for his endless amount of guidance and support throughout my project. From helping me debug and troubleshoot all my experimental challenges to providing invaluable feedback on my analyses and presentations, he was always there for me. Dr. Sappok's leadership constantly provided my work with proper direction, and as a result he drove me every day to do the best work I could possibly do for the project. I cannot emphasize enough how much Dr. Sappok's contributions have helped to make my research project possible. I would also like to thank my lab mates, Dr. Carl Justin Kamp, Michael Bahr, Yujun Wang, Tim Murray, and Ifran Govani, for their constant support and companionship throughout my research journey. Together we made it through classes, exams, experiments, meetings, and presentations, always helping each other out and lending a hand whenever it was needed. It's been a pleasure working alongside this great group of guys, who helped make this journey fun and enjoyable. I'm also very grateful for the MIT personnel who made my research efforts possible. First, I want to thank the rest of the members of the Sloan Automotive Laboratory, including Thane DeWitt, Raymond Phan, Janet Maslow, and its other graduate students. From handling all the project logistics, to helping me troubleshoot and repair my experimental test setup, to constantly teaching me new things about machining and engineering, everyone played a part in aiding me in my research and providing a friendly face to talk to. Secondly, the members of the Center of Material Science and Engineering at MIT, whom I closely worked with for much of my data collection and analysis, deserve my utmost gratitude for their assistance and guidance. Lastly I would like to thank my family and friends, both at and outside MIT, who have kept me going throughout this journey. Their constant support and companionship outside of school and work over the last two years have made this entire experience manageable. They helped me grow and mature as a person, and I'll never forget the countless memories we shared together. 5 (This page intentionally left blank) 6 TABLE OF CONTENTS LIST OF FIGURES ........................................................................................................................ 9 LIST OF TA BLES ........................................................................................................................ 11 N OM EN C LA TURE ..................................................................................................................... 13 1INTRODU CTIO N ................................................................................................................ 15 1. 1 2 Diesel Engine Fundamentals...................................................................................... S1.1 Diesel Engine Advantages...................................................................................... 17 1.1.2 Diesel Engine Applications................................................................................. 18 1.1.3 Diesel Engine Em issions...................................................................................... 19 1.2 Diesel Em ission Regulations...................................................................................... 20 1.3 Diesel Em ission Reduction M ethods .......................................................................... 22 D IESEL PA RTICULA TE FILTERS ................................................................................. 25 2.1 DPF Operation................................................................................................................ 25 2.2 A sh Sources.................................................................................................................... 28 2.2.1 29 A sh Transport................................................................................................................. 31 2.4 A sh Effects on DPF Perform ance .............................................................................. 32 2.4.1 D PF Pressure Drop .............................................................................................. 32 2.4.2 Lubricant Chem istry Effects............................................................................... 34 Project Objectives .......................................................................................................... 36 FUNDAMENTAL UNDERSTANDING........................................................................ 37 3.1 D PF Pressure Drop......................................................................................................... 3.1.1 3.2 4 Lubricant Additives ............................................................................................ 2.3 2.5 3 15 D PF Pressure Drop Model................................................................................. M aterial Properties and Characteristics.................................................................... 37 41 . 44 3.2.1 DPF Substrate Properties................................................................................... 44 3.2.2 A sh Properties..................................................................................................... 45 EXPERIMENTAL SET-UP AND TECHNIQUES......................................................... 4.1 DPF Loading.................................................................................................................. 49 49 4.1.1 Accelerated A sh Loading.................................................................................... 49 4.1.2 Soot Loading........................................................................................................... 51 DPF Post-M ortem Analysis ....................................................................................... 52 4.2 4.2.1 Ash Distribution................................................................................................... 52 4.2.2 Ash Porosity............................................................................................................ 54 7 4.2.3 4.2.4 4.2.5 5 57 58 58 EXPERIMENTAL TESTING AND RESULTS .............................................................. 61 5.1 Test M atrix and Param eters........................................................................................ Lubricant Formulations....................................................................................... 5.1.1 5.1.2 DPF Properties ..................................................................................................... 5.1.3 Ash Loading Param eters ..................................................................................... 5.1.4 Soot Loading Param eters .................................................................................... Test Sum mary ..................................................................................................... 5.1.5 61 61 62 63 64 5.2 65 DPF Loading and Pressure Drop Results.................................................................... 65 5.2.1 Ash Loading Results............................................................................................ 65 5.2.2 Soot Loading Results .......................................................................................... 69 5.3 Post-M ortem Ash Analysis Results................................................................................ 73 5.3.1 Ash Distribution Results ..................................................................................... 74 5.3.2 Ash Porosity Results............................................................................................ 76 5.3.3 Ash Composition Results..................................................................................... 78 5.3.4 Ash Particle Size Results ..................................................................................... 81 5.3.5 Ash M orphology Results .................................................................................... 83 5.4 6 Ash Composition ................................................................................................ Ash M orphology ................................................................................................. Ash Particle Size D istribution.............................................................................. DPF M odel Results ..................................................................................................... CONCLUSIO NS ............................................................................................................. 86 89 6.1 Lubricant Additive Effects on DPF Performance ....................................................... 89 6.2 Lubricant Additive Effects on Ash Characteristics..................................................... 90 6.3 Impact of Ash D epth Filtration .................................................................................. 91 Future W ork........................................................................................................ 92 REFERENCES ............................................................................................................................. 93 A PPEND IX................................................................................................................................... 97 6.3.1 8 LIST OF FIGURES Figure 1.1. Relative Concentration of Engine-Out Emissions in Diesel Exhaust ................... Figure 1.2. Comparison of Regulated Emissions in SI and Diesel Engines .......................... Figure Figure Figure Figure Figure 1.3. 2.1. 2.2. 2.3. 2.4. U.S. EPA Heavy-Duty Emission Standards ....................................................... Cutout of Catalyzed DPF for Heavy-Duty Engine Retrofits ................................ Wall-Flow Substrate Operation ........................................................................... DPF Filtration Mechanisms ................................................................................ Ash and Soot Distribution in DPF Channels ....................................................... Figure 2.5. Origin of Ash-Related Constituents from DPFs Operated on Various Fuels and Regeneration Conditions .............................................................................................................. Figure 2.6. Schematic Representation of Ash Particle Formation and Growth over Repeated 19 20 21 25 25 26 27 28 32 D PF Regeneration ........................................................................................................................ Figure 2.7. Backpressure Increase vs. Simulated Driving Distance for Several DPF Studies .. 33 Figure 2.8. Mass of Accumulated Ash Versus Backpressure Increase for Ten Catalyzed Soot Filters Exposed to Different 1.8% Sulfated Ash Oils .............................................................. 34 Figure 2.9. DPF Pressure Drop vs. Ash Load for Various Lubricant Formulations .............. 35 Figure 3.1. Soot and Ash Accumulation in a DPF Channel ................................................. Figure 3.2. Soot Depth Filtration in a DPF Channel Wall .................................................... 37 39 Figure 3.3. Soot and Ash Layer Formation ............................................................................ 39 Figure 3.4. Ash Layer (Left) and Ash End Plug (Right) ......................................................... Figure 3.5. DPF Pressure Drop Regimes .............................................................................. 40 40 Figure 3.6. Cross Section View of (a) DPF Substrate and Ash Layer (b) Large Ash Agglomerate 45 (c) Porous A sh Particle ........................................................................................................... Figure 3.7. Number-based Size Distribution and SEM Image of Field-aged DPF Ash ...... 46 Figure 4.1. Accelerated Ash Loading System Configuration .................................................... Figure 4.2. Post-Mortem Sample Extraction ......................................................................... 50 53 Figure Figure Figure Figure Figure Figure Figure Figure Figure 4.3. Measuring Ash Layer Thickness ......................................................................... 4.4. Measuring Ash End Plug Formation .................................................................. 4.5. DPF Ash Distribution Profile ............................................................................. 4.6. Mounted Filter Samples for SEM Analysis ............................................................. 4.7. SEM Micrograph of CJ-4 Lab Ash Layer ............................................................... 4.8. Binary Image of SEM Micrograph .......................................................................... 4.9. XRD Peak Scan for a CJ-4 Lab Ash Sample ...................................................... 4.10. TEM Micrograph of a Single CJ-4 Lab Ash Particle ............................................ 4.11. Light Obscuration Technique and Example CJ-4 Lab Ash Volume Distribution 53 54 54 55 55 56 57 58 . 59 Figure 5.1. Accelerated Ash Loading and Periodic Regeneration Cycles ............................. 64 Figure 5.2. DPF Pressure Drop vs. Ash Load at 0 g/L Soot ................................................. 66 Figure 5.3. Pressure Drop Response Normalized and Compared to Previously Tested Oils .... 67 Figure 5.4. DPF Core Testing on Flow Bench System .......................................................... 9 68 Figure 5.5. DPF Core Pressure Drop Response via Flow Bench Testing .............................. Figure 5.6. Pressure Drop Response to Soot Load at 0 g/L Ash ............................................. 69 70 Figure Figure Figure Figure Figure 5.7. Pressure Drop Response to Soot Load at 10 g/L Ash 5.8. Pressure Drop Response to Soot Load at 18 g/L Ash 5.9. Pressure Drop Response to Soot Load at 25 g/L Ash 5.10. Pressure Drop Response to Ash Load at 3 g/L Soot 5.11. Pressure Drop Response to Ash Load at 6 g/L Soot .......................................... .......................................... .......................................... 70 71 71 .......................................... .......................................... 72 73 Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure 5.12. 5.13. 5.14. 5.15. 5.16. 5.17. 5.18. 5.19. 5.20. 5.21. 5.22. 5.23. 5.24. 5.25. 5.26. Ash Distribution Profile Results for a Single DPF Channel ............................. Ash Distribution Profiles for Lab, Engine Test, and Field-aged Samples ...... Calcium Oil Engine Test Ash End Plug via X-ray CT ..................................... Ash Layer Porosity Results via SEM Image Analysis .................. Ash Plug Porosity Results via SEM Image Analysis ................... Ash Sample Calcium Content via XRD Analysis ............................................ Ash Sample Magnesium Content via XRD Analysis ........................................ Ash Sample Zinc Content via XRD Analysis ................................................... Volume Basis Particle Size Distribution for Lab CJ-4 Ash, 2012..................... Number Basis Particle Size Distribution for Lab CJ-4 Ash, 2012..................... Calcium Oil Lab Ash Morphology ..................................................................... Magnesium Oil Lab Ash Morphology .............................................................. Lab CJ-4 (Left) and Field CJ-4 (Right) Ash Morphology ................................. Lab Ca (Left) and Base + Ca + ZDDP (Right) Ash Morphology ..................... Lab Mg (Left) and Base + Mg + ZDDP (Right) Ash Morphology ................... Figure 5.27. Simulated vs. Experimental Porous Media Pressure Drop ................................ Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure A.1. Lab CJ-4 Ash Particle Size Number Distribution ............................................... A.2. Lab CJ-4 Ash Particle Size Volume Distribution ............................................... A.3. Lab Ca Ash Particle Size Number Distribution .................................................. A.4. Lab Ca Ash Particle Size Volume Distribution ................................................. A.5. Lab Mg Ash Particle Size Number Distribution .................................................. A.6. Lab Mg Ash Particle Size Volume Distribution ................................................. A.7. Engine Ca Ash Particle Size Number Distribution ............................................... A.8. Engine Ca Ash Particle Size Volume Distribution ............................................... A.9. Engine Mg Ash Particle Size Number Distribution ............................................. A.10. Engine Mg Ash Particle Size Volume Distribution ............................................ A.11. Field CJ-4 Ash Particle Size Number Distribution ............................................. A.12. Field CJ-4 Ash Particle Size Volume Distribution ............................................. 10 74 75 75 77 77 79 79 80 81 82 83 84 84 85 85 88 97 97 98 98 99 99 100 100 101 101 102 102 LIST OF TABLES Table 2.1. Measured and Reported Ash Components, Properties, and Characteristics ............. Table 3.1. Key DPF Pressure Drop Contribution Factors ..................................................... Table 3.2. Cordierite and Silicon Carbide (SiC) DPF Substrate Properties ........................... Table 3.3. Measured Field and Lab Ash Properties .............................................................. Table 3.4. Ash Properties for Various Lubricant Additive Formulations ............................. Table 4.1. Accelerated Ash Loading System Specifications ................................................ Table 4.2. Cummins ISB 300 Engine Specifications ............................................................ Table 5.1. Oil Formulation Test Matrix, MIT 2010............................................................... Table 5.2. Laboratory Testing DPF Properties ...................................................................... Table 5.3. New DPF Property Comparison ............................................................................ Table 5.4. Test Matrix Summary of Previous and New Samples .......................................... Table 5.5. Ash Loading Summary for Newly Tested Oils ...................................................... Table 5.6. Ash Distribution Summary .................................................................................. Table 5.7. Ash Layer Porosity Summary ................................................................................ Table 5.8. Ash Particle Size Summary .................................................................................. Table 5.9. Simulated Ash Layer Permeability Summary ........................................................ Table 5.10. Simulated Ash Layer Pressure Drop Summary ................................................... Table 5.11. Simulated Wall Pressure Drop Summary ........................................................... Table 5.12. Simulated Porous Media Pressure Drop Summary ............................................. 11 31 38 44 47 48 51 52 61 62 63 65 66 76 78 83 86 87 87 87 (This page intentionally left blank) 12 NOMENCLATURE Al Ca. CI CO CT DOC DPF Fe FF FIB HC Mg Mo NOx 0 P PM Pt S SCR SEM Si SI SiC TEM ULSD XRD ZDDP Zn Aluminum Calcium Compression Ignition Carbon Monoxide Computed Tomography Diesel Oxidation Catalyst Diesel Particulate Filter Iron Fully Formulated Focused Ion Beam Hydrocarbon Magnesium Molybdenum Oxides of Nitrogen Oxygen Phosphorus Particulate Matter Platinum Sulfur Selective Catalytic Reduction Scanning Electron Microscope Silicon Spark Ignition Silicon Carbide Transmission Electron Microscope Ultra Low Sulfur Diesel X-ray Diffraction Zinc Dialkyldithiophosphate Zinc Gas Dynamic Viscosity y, Gas Wall Velocity K Permeability D, Pore/Particle Diameter Porosity wP AP Porous Media Thickness Pressure Drop 13 (This page intentionally left blank) 14 1 INTRODUCTION The diesel engine is currently the most efficient type of all internal and external combustion engines. Developed in 1893 by German inventor Rudolf Diesel, it has today become widely used in many passenger car and large transport vehicle applications. Diesel engines are desirable mainly because of their high thermal efficiency, which leads to higher fuel economy and lower greenhouse gas emissions. One of the main disadvantages of diesel engines, however, is the high emission of hazardous particulate matter (PM). Diesel particulates have been found to be linked to many detrimental health effects, and have thus become subject to major worldwide emission regulations. Diesel particulate filters (DPF) have become one of the most widespread and effective technologies used today to control diesel particulate emissions. Placed in diesel engine exhaust systems, DPFs can trap diesel particulates with high efficiencies, up to 95% and above, helping to meet increasingly stringent emission regulations. DPFs are vulnerable, however, to their own set of challenges, providing the motivation behind extensive research to improve and optimize these crucial pieces of diesel emission control technology. 1.1 Diesel Engine Fundamentals The main goal of an engine is to convert chemical energy stored in fuel into mechanical power. In internal combustion engines, a mixture of fuel and air is burned and the resulting force from the high-pressure gases provide desired power output through the mechanical components of the engine. For many vehicles, these components consist of the conventional cylinder and piston arrangement. The diesel engine is a type of internal combustion engine that relies on compression ignition (CI) to ignite its fuel-air mixture rather than spark ignition (SI), employed by many other gasoline engines. In diesel engines, intake air near atmospheric pressure is compressed by the piston to high pressures of about 4 MPa (600 lb/in 2) and high temperatures around 800 K (1000 *F) [1]. Just before the piston's top dead center, a small amount of fuel is injected where it becomes 15 atomized into small droplets and entrained into the cylinder air. The high temperatures caused by the compressed air are well above the fuel's ignition point, leading to spontaneous combustion. The release of burned gas delivers power back to the piston and once it is exhausted, the cycle can start over again. In gasoline engines, however, the air and fuel are usually mixed together in the intake system prior to entry to the engine cylinder, using a carburetor or fuel-injection system [1]. The intake charge is then compressed to pressures of about 1-2 MPa, and an electrical discharge across a spark plug just before top dead center is what initiates the combustion process. The in-cylinder temperatures achieved in SI engines are not enough to ignite the fuel-air mixture, as is the case for diesel engines, so the use of a spark to initiate burning is necessary. Although the combustion process seems similar between SI and CI engines, the difference in ignition strategy alone ends up making the diesel engine substantially different from other gasoline engines. SI engine compression ratios, the ratio of the volume of the combustion chamber from its largest capacity to its smallest, are usually around 8-12 [1]. This value is limited because greater compression, and therefore higher in-cylinder pressures and temperatures, may lead to auto-ignition of the fuel-air charge before the desired timing and result in engine knock. Because CI engines don't introduce fuel into the combustion chamber until after the intake air has already been compressed, significantly higher compression ratios, around 12-24, are able to be reached without fear of auto-igniting the cylinder charge. As a result of these high compression ratios, diesel engines are further known to have better torque characteristics than gasoline engines. Since CI engines need to compress air to a much higher degree than SI engines, diesel engines tend to have longer piston stroke lengths and therefore larger crankshaft diameters. The applied force on the crankshaft by the connecting rods is occurring at a greater distance from the crankshaft's axis of rotation, resulting in greater torque. Consequently, longer stroke lengths along with needing heavier, stronger parts to cope with the large compression pressures also mean that diesels tend to operate at slower speeds than gasoline engines. 16 Power output control also differs between diesel and gasoline engines. Gasoline engines control the load of the engine by restricting intake air through a throttle, which then determines the amount of fuel that needs to be drawn in order to mix constantly near stoichiometric conditions. In diesels, on the other hand, the amount of fuel injected into the combustion chamber, rather than the amount of intake air, achieves load control [1]. 1.1.1 Diesel Engine Advantages The main advantage of the diesel engine is its high efficiency, which can mostly be attributed to the fact that CI engines can achieve much higher compression ratios. A high compression ratio is desirable because it allows an engine to reach combustion temperatures using less fuel and also results in a longer expansion stroke, creating greater power output. Diesel engine efficiency is also a result of reduced losses, both friction and pumping. Since diesels produce more torque at lower engine speeds than in gasoline engines, their power is generally produced at lower engine speeds where friction losses are reduced [2]. And, because diesels avoid intake throttling, air induction into the engine is not interfered with and pumping losses are reduced [2]. As a result, diesel engines end up being about 30-35% more fuel efficient than similar-sized gasoline engines [3]. Another significant advantage of diesel engines is that they emit extremely low concentrations of unburned hydrocarbons (HG) and carbon monoxide (CO) emissions [4]. The reason behind this is that diesels operate in very lean regimes where an excess of air, again a result of not needing intake throttling, ensures that fuel is more able to be fully burned up during combustion. As a result, diesel engine-out HC emissions are only 19% of gasoline engine-out HC emissions, and diesel engine-out CO emissions are only 5% of gasoline engine-out CO emissions [2]. Diesel engines are also very durable and reliable, leading to low maintenance costs. Heat is released earlier in the diesel combustion cycle than in the gasoline cycle and also at a much more sudden rate, which ultimately requires more robust engine construction [2]. As a result, diesel engines end up lasting three to four times longer than their gasoline counterparts [2]. In addition, diesel engines do not use spark plugs, distributors, or ignition systems, necessary for SI engines, and those parts tend to have more breakdowns than most other mechanical components [2]. 17 Lastly, diesel fuel is both cheaper and safer than gasoline. Diesel fuel is more difficult to ignite and also less volatile than gasoline, making it safer to handle and store [2]. 1.1.2 Diesel Engine Applications Because diesel engines are known to be efficient, reliable, and able to produce high amounts of torque, they have become extremely popular in many transport vehicle applications. These include large-scale freight vehicles like ships, trains, and heavy duty trucks, and also passenger vehicles like buses and light duty trucks. In 1997, diesel engines were found to be used in 80% of all buses, 85% of heavy duty trucks, 77% of boats and ships, and 89% of all trains in the US [5]. In total, this accounted for 19% of the total energy used in the US transport sector, compared to other fuels like gasoline and jet fuels [5]. Diesel engines, however, are not as widely used in passenger cars in the US. They are only used in 1% of automobiles and 4% of light duty trucks [5]. This is due to some of the disadvantages that diesel engines possess, that they are generally low speed, loud, and heavy. Diesel engines are also expensive to manufacture, requiring additional components such as turbochargers for additional power output, heavier-duty internal components for withstanding higher compression ratios, and aftertreatment systems for regulating their high emissions. And, in the US where diesel fuel and gasoline are both very cheap, auto makers simply don't see any economic advantage in switching to diesel. In Europe, however, fuel prices are much greater than in the US, making it much easier for consumers to look past those few disadvantages in order to harness the high fuel efficiencies and long-term cost savings of diesel engines. As a result, almost half (48%) of new cars in the EU are sold with a diesel engine [6] and in total, diesel accounts for 30-50% of energy use by the transport sector [5]. Diesel engines are also widely used in construction (83%), agriculture (66%), and mining (22%) equipment [5], as well as in commercial and industrial generators to provide local power and meet emergency backup power needs. 18 1.1.3 Diesel Engine Emissions Diesel fuel is composed of hydrocarbons which, after combustion, should theoretically only yield carbon dioxide (CO 2) and water vapor (H 20). Indeed, diesel exhaust gas is primarily composed of CO 2 (2-12%), H2 0 (2-12%), and the unused portion of engine charge air (02 3-17%, N 2 balance), none of which have any adverse health or environmental effects [8]: itrogen -,oxygen water Carbn Pollutant emissions vapor dioxide Figure 1.1. Relative Concentration of Engine-Out Emissions in Diesel Exhaust, Reproduced From [8] Diesel emissions, however, also include a number a hazardous pollutants originating from nonideal processes during combustion, such as incomplete combustion of fuel, reactions between mixture components under high temperature and pressure, combustion of engine lubricating oil and oil additives as well as combustion of non-hydrocarbon components of diesel fuel, such as sulfur compounds and fuel additives [8]. Major pollutants include unburned hydrocarbons (HG), carbon monoxide (CO), nitrogen oxides (NOx), and particulate matter (PM), comprising a few tenths of a percent of total diesel exhaust composition [8]. Although the diesel engine has been shown to possess a wide range of advantages, one of its biggest drawbacks is its high level of NOx and particulate matter emissions. NOx refers to oxides of nitrogen, generally nitrogen monoxide, also known as nitric oxide (NO), and nitrogen dioxide (NO 2) [7]. NOx and PM have both been found to be hazardous to human health and as a result, diesel engines require additional aftertreatment systems in order to curb these harmful emission levels. 19 Because diesel engines rely on compression ignition where combustion takes place during and after fuel injection, diesel emissions are quite different from those observed in spark ignition engines that operate with a premixed mixture [8]. An example comparison of emission levels between a light-duty diesel vehicle and gasoline vehicle highlights these differences [9]: 300 SI engine with catalyst -250 S20Diesel engine (100%) 00 100 50 0 CO HC NOx PM Figure 1.2. Comparison of Regulated Emissions in SI and Diesel Engines, Reproduced From [9] As stated earlier, diesel engines are favorable in that they produce significantly lower levels of CO and gaseous unburned HC emissions, but again they consequently produce higher levels of NOx and PM compared to gasoline engines. Diesels produce higher levels of NOx because NOx is formed by the high-temperature reaction of nitrogen with oxygen, and diesels generally have higher combustion temperatures as a result of using CI [7]. Furthermore, PM emission is more prevalent in diesel engines because diesel fuel contains hydrocarbon compounds with higher boiling points and molecular weights than gasoline [1]. The composition of the unburned hydrocarbons in diesel exhaust extends over a larger molecular size range, with the heaviest hydrocarbons condensing into solid-phase soot [1]. 1.2 Diesel Emission Regulations Regulated emissions in the United States, Europe, Japan, and other countries include the four main aforementioned pollutants: NOx, particulate matter, hydrocarbons, and carbon monoxide. 20 In the United States, emissions standards are managed by the Environmental Protection Agency (EPA). In addition, the state of California, which possesses one of the largest automotive markets in the world, has the ability to set even more stringent vehicle emissions standards, managed by the California Air Resources Board (CARB), that other states can choose to follow as well. NOx regulations specifically target nitric oxide (NO) and nitrogen dioxide (NO 2). Concentrations of NOx in diesel exhaust are typically between 50 and 1000 ppm, with about 15% being NO2 [8]. Nitric oxide (NO) is a colorless and odorless gas, poisonous to humans, and can cause irritation of the eyes and throat, tightness of the chest, nausea, headache, and gradual loss of strength [7]. NO 2 is also very toxic. A reddish-brown gas with a suffocating odor, it can cause delayed chemical pneumonitis and pulmonary edema [7]. As a result, NOx is one of the most critical pollutants regulated in diesel exhaust. As of 2012, CARB is looking for 75% reductions in mobile NOx to meet new ozone standards, with the target heavy duty NOx limit being 0.05 g/bhp-hr by the year 2020 [11]. This is a significant decrease from the 2010 EPA standard of 0.20 g/bhp-hr, illustrating the constant increase over past years in regulation strictness [12]: Emission Rj Figure 1.3. U.S. EPA Heavy-Duty Emission Standards, Reproduced From [12] 21 As shown above, particulate matter (PM) has also been highly regulated in diesel exhaust in recent years. Diesel particulates are composed of a mixture of nuclei mode particles and accumulation mode particles. Nuclei mode particles, about 0.007 to 0.04 um in diameter, include elemental carbon particles that have adsorbed other species such as hydrocarbon and hydrated sulfuric acid condensates [8]. Agglomeration mode particles, about 0.04 to 1 um in diameter, consist of nuclei mode particles that have agglomerated with other solid materials like metallic ash, cylinder wear metals, and sulfur compounds [8]. Together, these particulates can penetrate deep into the lungs when inhaled because of their small size and can lead to symptoms such as headache, dizziness, nausea, coughing, difficulty breathing, and tightness of chest. Long-term exposure can even lead to more serious health problems such as cardiovascular disease, cardiopulmonary disease, and lung cancer [10]. Since 2007, heavy-duty diesel PM emissions have been federally regulated in the US by the EPA to 0.01 g/bhp-hr [13]. Hydrocarbons, gaseous species derived from diesel fuel and from lubricating oil, comprise about 20 to 300 ppm of diesel exhaust [8]. They are toxic and have irritating odors, and some of them, such as benzene and formaldehyde, are classified as carcinogens. Since 1985, heavy-duty HC emissions have been regulated by the EPA to 1.3 g/bhp-hr [13]. Lastly, Carbon Monoxide (CO) comprises about 10 to 500 ppm of diesel exhaust concentration [8]. An odorless, colorless gas, it is very toxic. Symptoms of CO poisoning include headache, nausea, vomiting, dizziness, and fatigue, and prolonged exposure can even result in seizure, coma, and fatality. Since 1985, heavy-duty CO emissions have been regulated by the EPA to 15.5 g/bhp-hr [13]. 1.3 Diesel Emission Reduction Methods In order to meet the above strict emission standards, diesel engines have had to implement a number of emission control technologies. These breakthroughs have transformed the diesel engine a long way from its reputation in the 1970s as a slow, smoky, dirty, and smelly machine. As a result of the new wave of stringent regulations from the 1990s and 2000s, diesel engines have become much, much cleaner and favorable. 22 reduction after the 1970s was more advanced engine design. Significant emission reductions were achieved through improved mixture formation and higher intake pressures in the diesel engine. To achieve better fuel and air mixing in the engine cylinder, intake port design and combustion chamber geometry were optimized to create greater air motion turbulence, and advanced fuel injector design was adopted to promote increased injection pressure and fuel motion [8]. In addition, the introduction of exhaust gas recirculation, which recirculates a portion of an engine's exhaust gas back to the engine cylinders in order to lower combustion chamber temperatures and reduce the amount of NOx generated, as well as electronic engine/subsystem control also led to further emissions reductions. As a result, substantial reductions in PM (90%), NOx (75%), and HC / CO emissions were achieved in the 1980-1990 timeframe [8]. Advances in fuel technology have also significantly aided the reduction of diesel emissions. The introduction of ultra low sulfur diesel (ULSD) fuels employ greatly reduced sulfur levels, which help cut down emissions of sulfur dioxide and sulfate particulates [8]. Furthermore, this reduction also benefits diesel catalytic aftertreatment technologies, whose performances are quite sensitive and vulnerable to sulfur content. Other fuel characteristics such as higher cetane number and lower aromatics content have also been proven to reduce emissions [8]. In addition to the above technologies, diesel engines require the use of a number of exhaust gas aftertreatment devices in order to further comply with strict NOx and PM regulations. The most important and effective technologies used today include urea-SCR devices to control NOx emissions, and diesel particulate filters (DPF) to control PM emissions. SCR refers to selective catalytic reduction of NOx, which is a way of converting nitrogen oxides into nitrogen (N 2) and water (H 20) with the aid of a catalyst such as ammonia or urea: NO + N0 2 + 2NH 3 -* 2N 2 + 3H 2 0 (1.1) SCR catalysts are capable of high (90%) reductions of NOx emissions, and are currently used in many heavy- and light-duty diesel engine applications [8]. Other newer, less widespread technologies include NOx adsorbers, which trap nitrogen oxides in the catalyst washcoat and use 23 spikes of rich air/fuel mixtures to regenerate the stored NOx, and lean NOx catalysts, which reduce nitrogen oxides through selective reactions with hydrocarbons. Diesel particulate filters have been used as the most effective and popular solution for curbing PM emissions. Diesel oxidation catalysts (DOC) are often used to oxidize the hydrocarbon portion of diesel particulates, but they are unable to remove much of the carbonaceous fraction. DPFs, therefore, constitute another crucial component of the diesel aftertreatment system. These ceramic traps, present in most diesel applications since 2007, capture PM from diesel exhaust with high efficiencies above 90%, and are able to remove much of the particulates through catalytic and heating processes called regeneration [8]. Although very effective, DPFs are vulnerable to durability and reliability problems that eventually result in high exhaust gas pressure drop, leading to a greater energy demand for regeneration and a decrease in overall fuel economy. As a result, extensive research has gone into improving and optimizing DPF performance as well as developing new pieces of diesel emission control technology at a time when regulation standards grow tighter and tighter. 24 2 DIESEL PARTICULATE FILTERS Diesel particulate filters (DPF) are devices that physically capture diesel particulates to prevent their release to the atmosphere. First introduced in 1985 on Mercedes cars equipped with a 3.0 L turbocharged IDI engine sold in California, DPFs have now become the most effective technology for the control of diesel particulate emissions [8]. They possess very high filtration efficiencies, in excess of 90%, as well as good mechanical and thermal durability [8]. Figure 2.1. Cutout of Catalyzed DPF for Heavy-Duty Engine Retrofits, Reproduced From [8] 2.1 DPF Operation The most common DPF design is the wall-flow monolith, which features a cylindrical ceramic structure with many small, parallel channels running in the axial direction [8]. The channels are plugged on each end in an alternating checkerboard pattern, forcing the inlet gas to flow through the porous walls of the DPF: ocl_* _OF oQ otm O U ba Figure 2.2. Wall-Flow Substrate Operation, Reproduced From [8] 25 The porous channel walls of the DPF, therefore, act as the main filter medium. They are made from porous ceramic materials, most commonly cordierite, used in heavy-duty applications, and silicon carbide (SiC), used in diesel passenger cars [8]. These ceramics possess material porosities typically between 45 and 50%, with medium pore sizes around 10 to 20 um [8]. Particulates initially deposit inside the porous channel walls, known as depth filtration. As the soot load increases and the walls become saturated, a particulate "cake" layer begins to develop along the surfaces of the walls, constituting cake filtration: [ porous barrier . particle 4?UI6, Cake Filtration (Sieving) Depth Filtration Figure 2.3. DPF Filtration Mechanisms, Reproduced From [8] Eventually, the DPF will collect a large amount of soot, restricting exhaust gas flow and resulting in engine back pressure. This impairs engine operation and reduces fuel economy, so the soot must somehow be removed. This takes place via filter regeneration, the thermal process by which solid soot hydrocarbons are oxidized into gaseous products like carbon dioxide. Carbon oxidation can make use of either oxygen or nitrogen dioxide. Oxygen is present in diesel exhaust at sufficient concentrations at practically all operating conditions, but relatively high temperatures are necessary to achieve appreciable regeneration rates with 02 [8]: C + 0 2 -+ CO2 (2.1) For this type of regeneration, called active regeneration, additional fuel needs to be spent in order to periodically increase filter temperatures to the required "active" regime, typically above 550 *C, where soot is able to be oxidized effectively using only oxygen in the exhaust. Nitrogen 26 dioxide based regeneration can be conducted at lower temperatures, as low as 250 *C, but this requires increased levels of NO 2 likely achieved by the use of an oxidation catalyst [8]: NO+ 0 2 =NO2 N02 + C - 2 N2 + C0 2 (2.2) (2.3) This second strategy is called passive regeneration. Here, exhaust gas temperatures are often near or above the temperatures required for regeneration, so little to no additional heating is required and the filter is able to regenerate continuously. In addition to the aforementioned carbon-based soot, diesel PM also includes a small fraction, less than 4%, of inorganic ash particles and other trace metals and elements [8]. Like soot, these particles are trapped inside the DPF, but they are not, however, able to be removed from the DPF through filter regeneration. Over time, ash will gradually accumulate along the DPF channel walls and toward the back of the filter, reducing its overall performance and contributing even more to the filter pressure drop [15]: Figure 2.4. Ash and Soot Distribution in DPF Channels, Reproduced From [15] 27 As a result, DPFs require periodic maintenance, typically not more than once per year, in order to clean out residual ash and restore filtration efficiency. DPFs have proven themselves to be effective, valuable pieces of technology for diesel emission control, but they are still susceptible to certain drawbacks. One major obstacle is the aforementioned ash accumulation problem, which has been studied extensively in recent years. Much research and resources have been spent towards understanding the fundamental mechanisms governing the accumulation of ash, its properties, and effect on DPF performance, with the ultimate goal being to control its detrimental effects to a minimum and extend DPF service life. 2.2 Ash Sources The chemical composition of DPF ash suggests that it derives mainly from additives in the engine lubricant. Ash can also include various engine wear and corrosion particles as well as trace metals found in diesel fuels, but lubricant-derived ash comprises the majority of the ash found in the DPF [21]: 100Fuel 80 Wear 60 CoM[K[I DPF 40 Lube 20 1P-ULSD 2A.ULSD 3A.B20 4A-B100 Figure 2.5. Origin of Ash-Related Constituents from DPFs Operated on Various Fuels and Regeneration Conditions, Reproduced From [21] During typical engine operation, small amounts of lubricant will inevitably enter the combustion chamber on the cylinder lining by passing over the piston rings. After combustion takes place, the inorganic, incombustible materials from the lubricant will be emitted to the DPF as ash particles where they will remain trapped even after filter regeneration. Studies also show that oil consumption is directly related to the amount of ash accumulated in a DPF [19]. Engine 28 lubricants consist of a base oil (75-83%), viscosity modifier (5-8%), and an additive package (12-18%) [16]. While the base oil is primarily responsible for lubrication, the additive package is used to provide a number of crucial and beneficial properties that preserve and improve the performance of the base oil. 2.2.1 Lubricant Additives The additive package consists of many different components. First, detergents are used to prevent corrosive wear through their acid-neutralizing properties. They are metal salts of organic acids that frequently contain associated excess base, usually in the form of carbonate [17]. They serve to neutralize corrosive combustion acids as well as suspend oxidation products in order to control deposit formation [17]. As a result, detergents control rust, corrosion, and resinous buildup in the engine. Detergents exist in different types based on the common polar groups present in the detergent molecules. These include mainly sulfonate, phenate, and carboxylate groups, but also sometimes salicylate and thiophosphonate groups [17]. Detergents are also paired with metals, most commonly calcium and magnesium, in order to become oil-soluble and thus able to be carried by the base oil. It is because of these metals, inorganic and incombustible, that ash is produced and emitted to the DPF. Calcium-based detergents are more common due to their high anti-corrosion performance and low cost, but magnesium-based detergents are also used in combination because their smaller molecular weight produces less ash [17]. As of 2003, most detergents consist of basic calcium sulfonate (65%) and basic calcium phenate (31%) [17]. Dispersants are another major class of lubricant additives. Dispersants and detergents together make up about 45-50% of the total volume of the lubricant additives manufactured [17]. Dispersants are metal-free, however, so upon combustion they do not lead to ash formation. The goal of the dispersant is to suspend oil insoluble contaminants and degradation products, which include sludge, resin, varnish, and hard deposits. By suspending these products in the bulk lubricant and preventing them from attacking the base oil and other additives, dispersants serve to minimize particulate-related abrasive wear and viscosity increase [17]. The other main group of additives and source of ash formation is antiwear additives. Antiwear additives are necessary to prevent damage and wear to parts experiencing boundary lubrication 29 [8]. These additives have a polar structure that forms a protective layer on metal surfaces, preventing direct contact between sliding surfaces [8]. The most commonly used and cost effective antiwear additives are zinc dialkyldithiophosphates (ZDDP). ZDDP can also exist in different types, depending on the structure of its alkyl groups from synthesis, which ultimately affect its thermal stability and performance [8]. As stated earlier, it's understood that ash is primarily derived from additives in the engine lubricant because of its chemical makeup. Compositional analysis of DPF ash show that it is composed mostly of Ca, Mg, Zn, P, and S compounds in the form of various phosphates, sulfates, and oxides [14]. These correlate with the widely used Ca- and Mg-based detergents and Zn-based antiwear additives, as well as the S- and P-based polar and alkyl groups with which those additives are synthesized with. Even though these additives provide numerous benefits to the base oil, their consequential ash production has become a very concerning issue that has been addressed more and more in recent years. In 2006, the CJ-4 oil specification was developed to minimize the production of lubricant-derived ash. Lubricants were required to have the following chemical limits [18]: " Maximum sulfated ash content: 1.0% " Maximum phosphorous content: 0.12% " Maximum sulfur content: 0.4% e Maximum volatility: 13% The sulfated ash restriction refers to the limiting of additive content in the lubricant, as defined by the sulfated ash test (ASTM D874) [14]. In this laboratory test, the lubricant's calcium and magnesium additives are converted to their sulfates and its zinc additives are converted to their oxides in the absence of phosphorus [14]. This provides a quantitative measure of additivederived ash production, so that the resultant ash delivered to the DPF may be limited. It should be pointed out, however, that real ash obtained from DPFs after on-road use is much different and more complex than lab ash generated by the ASTM test. Below is a small list of species typically found in DPF ash [14]: 30 Ash Constituent Calcium Sulfate Magnesium Oxide Magnesium Sulfate Zinc Oxide Zinc Magnesium Phosphate Zinc Phosphate Zinc Pyrophosphate Chemical Formula CaSO 4 MgO MgSO 4 ZnO Zn2 Mg(PO 4 ) 2 Zn3 (PO4 )2 Zn2 (P2 0 7 ) D 3 gIcm 2.96 3.58 2.66 Description C 5.61 1,460 2,832 1,124 1,975 3.60 4.00 900 Anhydrous, decomposes -1,250'C Anhydrous, decomposes: 900-1,1 00C 3.75 Table 2.1. Measured and Reported Ash Components, Properties, and Characteristics, Reproduced From [14] Because real ash is formed under much different conditions than those from the sulfated ash test, subject to various temperatures, substrate interactions, flow conditions, and regeneration modes, its composition can contain a much wider and complex array of species and compounds compared to the mere three that the sulfated ash test aims to limit. The presence of phosphorus, true for real ash, is also known to affect sulfated ash test results [14]. As a result, while sulfated ash limits defined by oil specifications are one step in limiting the amount of lubricant-derived ash production, they do not comprehensively define all aspects of ash accumulation inside the DPF and therefore cannot fully predict the resulting effects on DPF performance. 2.3 Ash Transport Studies show that lubricant-derived ash starts off in the form of small ash precursors, well below 100 nm, bound to carbonaceous soot particles [14]. Sampling analysis is able to identify lubricant additive-related elements, such as zinc and phosphorus, in individual soot agglomerates collected upstream of the DPF [14]. As soot accumulates in the DPF, ash precursors will in turn be suspended along the channel walls held by the carbon particles. During filter regeneration, the soot particles separating the ash precursors are removed and the ash becomes more concentrated. Over repeated regeneration cycles, the ash precursors agglomerate together with new incoming ash to form larger micron-sized ash particles, typically found in the DPF [14]: 31 Figure 2.6. Schematic Representation of Ash Particle Formation and Growth over Repeated DPF Regeneration, Reproduced From [14] These fundamental mechanisms of ash transport and agglomeration are the basis of what determines ash properties such as particle size, structure, packing, and distribution. A number of parameters, though, can affect these events, such as engine operating conditions, regeneration strategy, and DPF design, in turn affecting the characteristics of ash found in its final form inside the DPF. 2.4 Ash Effects on DPF Performance Due to the detrimental effects of ash on DPF performance, much research and work has gone into trying to fully understand all the fundamental mechanisms governing ash properties and behavior, such as composition, morphology, and distribution. Some characteristics of DPF ash are understood, for instance that it is primarily lubricant-derived and that ash production increases with higher sulfated ash levels and oil consumption rates, whereas others like distribution and packing in the DPF are less defined. There are a number of different factors too, ranging from engine operating conditions to DPF design to lubricant formulation, that could affect some or all of these ash properties, turning ash accumulation into a very complex yet important field worth studying. 2.4.1 DPF Pressure Drop Ash accumulation has been shown to lead to an increase in DPF pressure drop. Pressure drop refers to the difference in pressure across the DPF, between its inlet and outlet faces. As ash 32 builds up in the filter over time, exhaust flow becomes more and more restricted. This restriction leads to a buildup of backpressure on the inlet, engine side of the DPF. As a result, the differential pressure, or pressure drop, across the filter increases. Studies have shown that for lubricant formulations at various sulfated ash levels, engine backpressure directly increases with simulated driving distance and therefore ash accumulation [19]: 2.4 - 2004-01-1955 (1.6% -m-2003-08-0408 (2.0% --- 2003-08-0408 (1.6% 2003-08-0408 (1.0% --- 910131 (1.6% Ash) -o-910131 (Ashless) 2.2 7; U) 2.0 1.8 E . I 2004-01-3013 (1.48% Ash) Ash) Ash) Ash) Ash) 1.6 0 CD 1.4 U) Z 1.2 &age= 1.0 0.8 - 50,000 100,000 150,000 200,000 250,000 Simulated Distance (km) Figure 2.7. Backpressure Increase Versus Simulated Driving Distance for Several DPF Studies, Reproduced From [19] Depending on the lubricant sulfated ash level, engine backpressure was expected to double at distances anywhere from 75,000 km to over 250,000 km. At increased backpressure levels, the engine has to compress the exhaust gases to a higher pressure which involves additional mechanical work and less energy extracted, leading to an increase in fuel consumption [20]. Literature shows that a 10 kPa increase in particulate filter pressure drop can result in fuel consumption increases ranging from 1%for newer engines to 4.5% for older engines [20]. In the above study, however, it can be seen that sulfated ash level does not correlate directly with backpressure increase. This suggests that more total ash does not necessarily mean more detrimental backpressure. In another study, a similar conclusion is reached. For ten different 33 oils at the same sulfated ash level of 1.8%, there was no correlation found between the amount of ash present in the DPF and the resulting filter pressure drop [19]: 16 - y = -0.033x + 10.404 R2 =0.0326 14. 12- + 100 8- 0 6- E + 420 i 40 50 60 70 80 90 100 110 120 AP (mbar) Figure 2.8. Mass of Accumulated Ash Versus Backpressure Increase for Ten Catalyzed Soot Filters Exposed to Different 1.8% Sulfated Ash Oils, Reproduced From [19] Again, this suggests that more ash does not necessarily lead to higher backpressure increase, implying that there may be differences between types of ash, that one type can be more harmful than another. Although sulfated ash level can affect the total amount of ash generated and emitted to the DPF, it is not the sole factor controlling ash-derived backpressure increase. Ash morphology, packing, and distribution, potentially affected by parameters such as lubricant additive chemistry, engine operation, and DPF design, are some of the many additional variables that could explain the above findings, why not all ash behaves the same. 2.4.2 Lubricant Chemistry Effects A study conducted in 2010 at MIT sought to investigate the effects that ash derived from different types of lubricant additives had on resulting DPF performance. Six different lubricant formulations, synthesized with base oil and different combinations of Ca, Mg, and Zn additives at the same sulfated ash level of about 1%, were used to generate ash loaded DPFs. In the absence of soot, the following DPF pressure drop results were obtained [22]: 34 3.5 3.0 -ase -r ua CJ-4 Q 2.5 02. S1.5 -Base 1.5 1.o + Mg + ZDDP Base + ZDDP ----- Base + Mg 0.5 0 5 10 15 I I I I I 0 .0 20 25 25 *C, Space Velocity = 20,000 hri I I I 30 35 40 45 Ash Load [g/L] Figure 2.9. DPF Pressure Drop as a Function of Ash Load for Various Lubricant Formulations, Reproduced From [22] The study shows that for the same DPF ash level, oils containing Ca additives resulted in DPF pressure drops approximately two times greater than oils containing only Mg or Zn additives, and that for the same soot load oils containing only Mg and Zn additives actually displayed a pressure drop benefit relative to the commercial CJ-4 formulation [22]. Analysis of the different ash samples showed that while ash distribution in the DPF was roughly similar, there were distinct differences in ash morphology as well as in ash packing density and porosity, with the calcium-based ash being the least porous [22]. Lastly, there were differences noticed in ash composition, with the calcium-based oils yielding mostly calcium sulfate ash, zinc-based oils producing mostly zinc phosphate ash, and magnesium-based oils producing mostly magnesium oxide ash [22]. The above study clearly highlights a significant impact of lubricant additive chemistry on ash characteristics and DPF performance. For the same sulfated ash content and consumption rate, oils that differed only in the types of additives present produced very different results that were extremely interesting and worthy of further investigation. 35 2.5 Project Objectives Although many studies, like the one above, have been able to clearly illustrate the impact of lubricant additive chemistry on ash characteristics and DPF performance, many of the underlying mechanisms governing why there are such differences remain less understood. There are many unanswered questions pertaining to what exact ash properties are affected by variations in additive formulations, and why those changes occur. The objective of this project is twofold. First, three additional lubricant formulations will be tested using the same setup and procedures from the previous 2010 study at MIT in order to see if those observed trends can be reproduced and reaffirmed. Secondly, and more importantly, this work will aim to best explain the previously seen findings, why there was such a large difference in performance between calcium- and magnesium-based ash. To do so, a better understanding of the mechanisms that determine ash properties like distribution, permeability, particle size, and composition must be achieved so that any differences in resulting DPF pressure drop can quantitatively be pinpointed to certain fundamental factors. In doing so, this work ultimately hopes to contribute further knowledge to understanding the effects of lubricant additive chemistry on ash accumulation in DPFs so that lubricant formulations, and therefore diesel aftertreatment systems, may be optimized to reduce the negative fuel economy and health hazard effects from lubricant-derived ash to the greatest degree. 36 3 FUNDAMENTAL UNDERSTANDING Earlier, it was shown that ash accumulation in DPFs directly results in engine backpressure increase, or a rise in filter pressure drop. Pressure drop is a much more complex subject, however, as it is a component consisting of several variables associated with different types of flow and friction losses. To better understand the effects of ash and soot accumulation on DPF pressure drop, the fundamental mechanisms governing these parameters must first be examined in detail. 3.1 DPF Pressure Drop The main filter medium of the DPF is again its porous channel walls. As soot and ash accumulate in the DPF, however, along the channel walls and even inside the wall pores as well, the filtration efficiency of the DPF naturally worsens. Below is a simple schematic representing typical soot and ash accumulation within a DPF [23]: F3]r Figure 3.1. Soot and Ash Accumulation in a DPF Channel and Pores, Reproduced From [23] The number labels in the above figure correspond to different types of flow restrictions and losses that arise due to soot and ash accumulation inside the DPF. Each contributes to the DPF's total overall pressure drop, and are all outlined in greater detail in the following table [23]: 37 PressReynDldp Key Parameters Contribution(R) Pressre rop Contribution Reynolds Number Controlling Properties to Total DPF toesure Pesr Drop Filter geometry, ash and soot layer thickness Filter geometry, ash and Transition < 3% soot layer thickness Filter geometry, ash end plug formation < 2,100 5%/-30% 1 Inlet losses (Contraction) Open frontal area 2 Frictional losses along inlet channel walls Channel hydraulic diameter Available channel length Wall permeability ash ad sot epth filtraion 3 Frictional losses from flow through wall Wall thickness Filtration area 4 Frictional losses from flow through ash layer Filter geometry Filter geometry Ash porosity, pore size Ash packing density Ash layer thickness, end plug formation Soot porosity, pore size Soot packing density Soot layer thickness Ash permeability Ash thickness Filtration area 5 Frictional losses from flow through soot layer Soot permeability Soot thickness Filtration area 6 Frictional losses along outlet 6 al oult diameter 7 Outlet losses (Expansion) << 1 Channel hydraulic Filter geometry cha l< Filter geometry Available channel 500/ 2,100 - 90% ~5% Outletllosse I Open outlet area I Filter geometry I Transition I < 3% _I Table 3.1. Key DPF Pressure Drop Contribution Factors, Reproduced From [23] Exhaust gas flow entering the DPF must contract in order to fit within the many, much smaller filter channels. This loss leads to a small pressure drop increase and is governed by the available frontal channel area, as determined by the ash and soot layer thicknesses. That reduction in channel frontal area, or hydraulic diameter, also results in frictional losses along the channel walls. There are also similar frictional losses along the outlet channel walls and due to flow expansion. But studies show that the majority of DPF pressure drop contribution can be attributed to frictional losses from flow through the filter's porous media [23]. As soot and ash enter the DPF, they deposit inside the channel pores as well as form layer membranes along the walls. These layers are porous themselves, so at any given time, the filter's porous medium through which flow passes consists of the DPF substrate wall and/or any soot or ash layers present. 38 The first soot that enters a clean DPF initially deposits within a portion of the substrate wall pores. This is known as "depth filtration," and typically doesn't exceed more than about a third of the total substrate wall thickness [14]: Wait surface 0Depth Wall wurfac Particulates trapped inside the surface pore -10 pm Figure 3.2. Soot Depth Filtration in a DPF Channel Wall, Reproduced From [14] After this early soot is oxidized over the first filter regeneration periods, the remaining ash will end up filling the pores to the surface. At this point, with the substrate pores full of ash, additional soot entering the DPF will begin to accumulate on the surface of the channel walls. This is called "cake filtration," as soot starts to accumulate in a cake-like layer throughout the DPF. Ash left behind from subsequent regenerations will then remain in a layer along the channel walls [14]: Figure 3.3. Soot and Ash Layer Formation, Reproduced From [14] 39 As this ash layer grows over time, it will eventually reach a "critical thickness" at around 12 g/L DPF ash load, which designates grams of ash per liter of DPF volume, at which its maximum allowable shear stress is reached [26]. Layer ash will begin to break off and deposit toward the back of the filter, forming the ash "end plug," which covers the entire cross-sectional area of the channel [26]: Figure 3.4. Ash Layer (Left) and Ash End Plug (Right), Reproduced From [26] At higher ash loads above 20 g/L, this end plug starts to become a significant factor as it has effectively reduced the available length of the DPF channels. The filtration area of the substrate is diminished, restricting exhaust gas flow and contributing to increased filter pressure drop. These two filtration regimes, depth and cake, however, have very different effects on DPF pressure drop as confirmed by most experimental research results [24]: Loaded filter AP after initial loading phase APInitial loading phase Time Figure 3.5. DPF Pressure Drop Regimes, Reproduced From [24] 40 The initial depth filtration phase begins with a sharp increase in filter pressure drop over just a small amount of time. This mechanism is the most detrimental to the substrate wall, as soot and ash particles directly clog the pores of the substrate and reduce its permeability. Once depth filtration has taken place and cake filtration has started, however, DPF pressure drop increases roughly linearly as a function of soot and ash layer thickness. This regime's contribution to total DPF pressure drop is much less dramatic. Again, frictional losses from flow through the substrate wall and soot and ash layers are the largest contributors to overall DPF pressure drop. The magnitude of these pressure drop components are largely affected by the properties of these layers, mainly permeability, a function of porosity, pore size, and packing, as well as layer thickness. 3.1.1 DPF Pressure Drop Model Quantifying DPF pressure drop in terms of fundamental mathematical equations is essential for the comprehensive understanding of soot and ash effects. Breaking down total pressure drop into a function of fewer underlying parameters such as porous media permeability and thickness can help provide insight into the driving factors governing DPF pressure drop, highlighting which properties contribute the greatest significance. DPF pressure drop can initially be represented by the separate pressure drop terms discussed in Table 3.1, attributed to the different modes of flow and friction losses throughout the DPF [23]: APTotat = APin + APout + APcfannei + APwal + APAsh + APsoot (3.1) APin/out represents the inlet flow losses due to contraction and expansion as the diesel exhaust gases enter and leave the DPF. APChanne, represents the frictional losses along the channel walls, and APWalt/Ash/Soot represents the frictional losses due to flow through the filter's porous media, consisting of the substrate, soot, and ash layers. The pressure drop associated with the filter inlet and outlet losses, due to gas expansion and contraction, can be described as 41 (3.2) APintout = Z1nout (eV2 where p is the exhaust gas density and v is the exhaust gas velocity. Z1n/out defines the frictional coefficients for inlet channel gas contraction and outlet channel gas expansion as Z1n = -0.415 (if) + 1.08 (3.3) Zout = (1 - (3.4) )2 where Af refers to the filter frontal area, and A represents the filter's total surface area. The above equations assume laminar flow at the filter inlet and exit, but if this is not the case, then the appropriate friction coefficients for turbulent or transition flows must be used [25]. Pressure drop due to frictional losses for gas flow along the channel walls can be described as APchannet = 4f (a: where L is the available length of the channel and DH is the channel hydraulic diameter. (3.5) f denotes the Fanning friction factor, which can be estimated by f = Re (3.6) where Re is the exhaust gas Reynolds number and C is a constant equal to 14.23 for square cross section DPF channels and 16.00 for round cross section channels [25]. Exhaust gas flow within the DPF channels is typically laminar, and as the ash load of the filter increases over time and the ash layer thickness increases, the cross sectional opening of the channel generally transitions from square to circular [23]. Although pressure drop due to channel friction is typically small in comparison to total DPF pressure drop, this term can become quite significant at high soot and ash loads when the channel hydraulic diameter is greatly reduced. 42 Studies show that pressure drop due to flow through the filter's porous media, which include the substrate, soot, and ash layers, contributes the most to total DPF pressure drop [23]. Flow through porous media can be described starting with the Forchheimer-Extended Darcy Equation: ()ww APWall/Ash/Soot + pv 2 (3.7) t denotes the exhaust gas dynamic viscosity, K, the permeability of the porous medium, v, the # the inertial resistance. In most gas wall velocity, w the porous medium thickness, and practical applications for diesel particulate filters, the Reynolds number for flow through the porous media is much less than one, meaning that the inertial terms can be neglected as a firstorder approximation [23]. As a result, porous media pressure drop can be described as: APWaill/Ash/Soot = Y (3.8) VWw The permeability of a porous medium can generally be characterized by the Rumpf and Gupte equation, valid for 0.35 E 0.7 [23]: K = S.6 Le55Dp2 (3.9) E denotes the porosity of the porous medium and Dp the mean pore diameter. Permeability is particularly straight forward to calculate in this manner for a clean DPF, as porosity and pore diameter information can be supplied by the filter manufacturer. Once soot and ash have begun to deposit within the substrate pores, however, this quantity becomes much more difficult to quantify as two very different porous media are now present and intertwined. Because soot and ash layers have been found to generally have higher porosities, the KozenyCarman equation (E > 0.8) is instead used for calculating ash and soot layer permeability [23]: K= E 18O(1-E)2 43 Dj P (3.10) In this case, it may sometimes be more appropriate to use the mean particle size for Dp, as the soot and ash particles themselves are often the dominating flow characteristic within the layer. 3.2 Material Properties and Characteristics The characterization of DPF pressure drop in terms of fundamental mathematical equations shows that total pressure drop is most significantly a function of a few key properties related to the filter porous media, which include the substrate, soot, and ash layers. It's important to understand, then, the nature of these material properties, what mechanisms determine and affect them, and to what degree their variation influences overall DPF pressure drop. 3.2.1 DPF Substrate Properties The two most common materials used to make DPF substrates are cordierite and silicon carbide (SiC). Cordierite is characterized by good thermal shock resistance and relative low cost, but has a lower melting temperature that is vulnerable to runaway regeneration events [23]. SiC, on the other hand, has a much higher operating temperature but displays low thermal shock resistance and high manufacturing cost. Table 3.2 compares various material properties between cordierite and SiC filters [23]: Property Channel Width Wall Thickness Permeability Porosity Mean Pore Size Melting Temperature Thermal Expansion Elastic Modulus, Axial Strength, Axial Thermal Shock Parameter Thermal Conductivity Relative Cost [mm] [mm] [x 10~2 mz] [%] [pm] [*C] [x 10-" 1/*C] [GPa] [MPa] [W/m-K] Cordlerite 1.3-2.1 0.3 - 0.5 0.5 45-50 13-34 1450 0.7 4.7 2.6 790 <2 Low Silicon Carbide (SIC) 1.0- 1.6 0.3 - 0.8 1.24 42 - 58 8- 17 2400 4.5 33.3 18.6 124 20 High Table 3.2. Cordierite and Silicon Carbide (SiC) DPF Substrate Properties, Reproduced From [23] 44 In regards to DPF pressure drop, open channel width and wall permeability change over time due to increasing soot and ash loading. As soot and ash deposit within the substrate wall pores, porosity and mean pore size are reduced and exhaust gas flow becomes more restricted. Although it's known that DPF substrates are roughly 50% porous initially, their porosity and pore size characteristics after ash and soot depth filtration are difficult to quantify and measure experimentally. Still, it's clear from the previous model that the substrate wall permeability, to whatever degree of reduction, directly influences total DPF pressure drop. 3.2.2 Ash Properties Ash layer accumulation and depth filtration can be observed in Figure 3.6 (a) below. While soot depth filtration can penetrate up to about a third of the filter wall thickness, ash typically is only found within the first few surface pores and therefore doesn't seem to penetrate to any significant depth into the filter wall [14]. A closer examination of DPF ash shows that it consists mostly of agglomerates. These agglomerates, formed from nano-sized ash precursors that have undergone a number of high temperature filter regeneration events, can vary greatly in size. For example, Figure 3.6 (b) shows a large ash agglomerate above 80 pm in diameter while Figure 3.6 (c) shows smaller agglomerates of about a few microns in diameter. In either case, though, it's interesting to note that the ash agglomerate structures are fairly porous in nature. Applying focused ion beam (FIB) technology to cut open the ash agglomerates reveals that the seemingly round and solid ash agglomerates actually contain significant interior void regions [14]. I- Figure 3.6. Cross Section View of (a) DPF Substrate and Ash Layer (b) Large Ash Agglomerate (c) Porous Ash Particle, Reproduced From [14] 45 Although ash agglomerate size can vary pretty significantly on the micron scale, experimental findings in literature show that a large majority of ash agglomerates are actually sub-micron [18]: is. 100.0 0 0. 0.(20 0.100 1.00W 10.00 100.0 500.0 Diameter (pin) Figure 3.7. Number-based Size Distribution and SEM Image of Field-aged DPF Ash, Reproduced From [18] In general, though, ash agglomerates present in the DPF are still larger than any incoming soot particles. Soot primary particles only range from about 10 to 40 nanometers in diameter, and any soot agglomerates that have formed are typically on the order of 100 nm [27]. A number of studies have also measured other DPF ash properties such as packing density, porosity, and particle size. A summary of these findings for various field aged and lab generated samples are provided below [26]: 46 Source (SAE Paper) 2000-01-1016 2001-01-0190 2004-01-0948 2005-01-3716 2006-01-0874 2006-01-3257 2008-01-0331 2009-01-1086 2010-01-1213 Materl~ Density [g/cmI 3.13 2.5 2.85 3.4 Packing Density [g/cm! 0.4- 1.0 0.54 0.4 83 85 0.31 - 0.52 0.45 0.17-0.34 0.3 90-95 91.1 Porosity Permeability [% mP[m] 2.8-7.4 x 1014 5 x 10 Particle Size 1 - 10 0.1 - 0.5 2.4 - 37.6 0.4-8 1 Table 3.3. Measured Field and Lab Ash Properties, Reproduced From [26] The results show that ash, as it exists in its layer form inside the DPF, is considerably more porous (80 - 95%) than the filter substrate itself (-50%). It can also be seen that these measurements have a fairly high amount of variation, with ash porosity ranging from 83 to 91 percent and particle size ranging from 0.1 to over 10 microns in diameter. A quick sensitivity calculation using the previous DPF mathematical model reveals that just a 5% reduction in ash layer porosity can result in a dramatic 77% increase in pressure drop through the ash layer. But a 5% increase in ash particle size only results in about a 9% increase in ash layer pressure drop, suggesting that ash permeability is more highly sensitive of ash porosity. Several factors have been found to affect ash properties and therefore DPF pressure drop. Regeneration strategy, for example, is able to affect ash distribution. Filters employing periodic regeneration tend to accumulate ash plugs in the back of the DPF over time while filters being continuously regenerated tend to accumulate thicker ash layers and less of a plug [23]. High exhaust and DPF temperatures can also subject the ash to increased agglomeration and sintering, affecting overall packing and porosity in the layers. But in 2010 at MIT, it was also found that lubricant additive chemistry can affect a variety of ash properties. Six lubricant formulations involving various Ca, Mg, and Zn additives at the same 1% sulfated ash level were used to load different DPFs with ash under similar loading conditions. The consequent ash properties were measured and quantified, with the results shown in the table below [26]: 47 Lubricant Base Base Base Base Base CJ-4 Ash Lubrcant Ash Load Lod + Ca + Mg + ZDDP + Ca + ZDDP + Mg + ZDDP [g/L] 29 24 28 25 23 42 Ash sh Layer t~rer Thickness Tikes Layer Packing Plug Packing Material Density Density Density Ash Prst Porositesyt Prst [cm] 0.015 0.009 0.013 0.011 0.009 0.013 [g/cm3] 0.27 0.19 0.19 0.30 0.27 0.30 [g/cm3j 0.20 0.17 0.17 0.13 0.14 0.17 [g/cm3n3j 3.0 3.6 3.9 3.1 3.5 3.4 [ 90.9 94.6 95.1 90.5 92.1 91.1 Table 3.4. Ash Properties for Various Lubricant Additive Formulations, Reproduced From [26] Although ash distribution was roughly the same for all test cases, there were noticeable differences found in ash porosity. Considering how sensitive ash layer pressure drop is to porosity, these differences could potentially explain the differences in total DPF pressure drop that were seen during the ash loading tests, with the formulations with the highest pressure drops having the lowest ash porosities and vice versa. It can be seen that lubricant additive chemistry can directly affect important ash properties such as packing and porosity, which in turn can have significant effects on DPF pressure drop. It's important, then, to investigate exactly why ash from different additives behave differently and fully understand the underlying mechanisms governing these observed differences. 48 4 EXPERIMENTAL SET-UP AND TECHNIQUES The objective of this study is to build off of previous work related to the effects of lubricant additive chemistry on ash characteristics and DPF pressure drop performance. This work aims to test additional lubricant additive formulations to see if similar results and trends can be reproduced, to relate these discrepancies to differences in key ash properties such as porosity and composition, and then finally to explain the underlying mechanisms governing the observed differences. 4.1 DPF Loading In order to compare ash characteristics and pressure drop performance between DPFs, filter samples must be loaded to a sufficient ash load. DPF tests typically load filters to about 25 - 40 g/L ash in order to build up a sufficient amount of ash layer formation. Only then can the filter pressure drop profiles fully take shape and any differences between ash samples be measured. These high ash loads, however, can only be reached after a vehicle has driven some hundreds of thousands of miles on the road. DPF sample generation, therefore, is extremely time consuming and expensive. One solution is to accelerate the ash loading process, reducing the time and cost necessary to generate ash loaded DPF samples. 4.1.1 Accelerated Ash Loading There are a few ways DPF ash loading can be accelerated. The first is fuel doping, in which lubricant is doped into the engine fuel to directly increase lubricant consumption in the power cylinder [26]. Another technique involves injecting oil mist into the engine's intake manifold to be burned inside the combustion chamber [26]. Although these strategies greatly speed up the ash loading process, they do not resemble natural oil consumption and ash loading as accurately as on-road vehicle use. There can be differences in exhaust soot-ash proportions and lubricant burning mechanisms, for example, that may result in inaccurate pressure drop response, so the challenge is to develop a technique that simulates real field-aged ash loading as closely as possible. 49 The experiments conducted in this study all employ the MIT Accelerated Ash Loading System, which has been in use for the past several years to run tests related to lubricant additive effects on DPF ash accumulation. In addition to being able to accelerate the DPF ash loading process, one of the main benefits of this system is its flexibility. A number of the simulated variables such as exhaust temperature and flow rate, oil consumption, lubricant chemistry, and DPF geometry can be independently changed in order to address the many different mechanisms affecting DPF ash accumulation. Although this system is again merely a simulation resembling real on-road ash loading, in-depth studies have shown that the resulting ash is very comparable in morphology, distribution, and elemental composition to that found in field-aged samples [28]. The Accelerated Ash Loading System fundamentally consists of a customized diesel burner and Cummins ISB engine coupled together to allow both engine-out soot and accelerated lubricantderived ash to be loaded in a DPF [23]: t Backpressure Control Centrifugal Blower Exhaust Diesel Supply toBumer To Emissions Analyzers Cummins ISB Exhaust Flow - Exhaust t Figure 4.1. Accelerated Ash Loading System Configuration, Reproduced From [23] The oil supply line is filled with the engine lubricant to be tested, where it is then delivered by a computer-controlled constant volume pump to the oil injector. The air-assisted oil injector 50 sprays and atomizes the lubricant inside the combustion chamber where it is then burned by the hot burner exhaust. A centrifugal blower at the rear of the system then pulls the exhaust to a downstream DPF while an intermediate heat exchanger is able to control the exhaust temperature before it does so. Lastly, digital thermocouples and pressure transducers on either side of the DPF measure inlet and outlet temperatures as well as filter pressure drop. The following table summarizes the system's operating specifications [26]: Speciftcation System Parameter [L/h] 1.5 - 7.6 Fuel Consumption [mL/min] 0.94 - 9.4 Oil Consumption [kPa] 700 - 1400 Injection Pressure [SLPM] 266 - 1130 Air Flow DPF Inlet Temperature 200 - 800 [*C] Table 4.1. Accelerated Ash Loading System Specifications, Reproduced From [26] Because this system employs secondary oil injection as its main accelerated ash loading mechanism, even lubricant formulations that are not fully formulated, and therefore unsafe to be used inside an engine, are able to be tested. Furthermore, the diesel burner does generate a small amount of soot, so throughout the accelerated ash loading process some type of filter regeneration strategy must be employed via the exhaust heat exchanger. Ultimately, this system is able to simulate a 40 g/L DPF ash load, which equates to roughly 240,000 miles of driving distance, in only about 100 hours of laboratory testing [23]. 4.1.2 Soot Loading The Accelerated Ash Loading System is also coupled to a Cummins ISB engine so that the DPF may be loaded with real engine-out soot. This is important for studying how ash accumulation affects a filter's soot filtration capabilities. The Cummins ISB is a 6-cylinder, 4-stroke, turbocharged, direct-injection diesel engine that employs a number of emission regulation technologies such as common rail fuel injection and cooled exhaust gas recirculation. A summary of the engine's specifications are provided below [26]: 51 Specification 890 N-rn @ 1600 rpm 224 kW @ 2500 rpm 6, in-line 4-stroke, direct injection Common rail Variable geometry turbocharger and intercooler 5.9 L 17.2:1 4 valves / cylinder O.D. = 158 pm L = 1.00 mm 8 sac-less nozzles / injector 800 - 1600 bar System Parameter Maximum Torque Maximum Power Number of Cylinders Combustion System Injection System Aspiration Displacement Volume Compression Ratio Cylinder Head Layout Injection Nozzle Injection Pressure Table 4.2. Cummins ISB 300 Engine Specifications, Reproduced From [26] 4.2 DPF Post-Mortem Analysis Once filter samples have been loaded with sufficient ash and the corresponding pressure drop response has been collected, a number of advanced diagnostic techniques can then be applied in order to measure and quantify important ash properties such as porosity, distribution, particle size, composition, and morphology. The accurate characterization of these ash properties is crucial for being able to systematically compare the different lubricant formulation tests. 4.2.1 Ash Distribution Post-mortem analysis unfortunately is destructive of the filter and ash samples, as core samples must be cut and sectioned away for closer examination. This process typically begins by cutting a length-wise core from the center region of the DPF and then sectioning it into several, usually four, equal length segments. The center of the DPF is chosen because it possesses the filter's most representative ash distribution, and the square cross section core is typically sized to about 3/4 inch or 7-9 channels in both width and height: 52 Figure 4.2. Post-Mortem Sample Extraction Ash distribution characterization consists of measuring the ash layer thickness throughout the DPF length as well as the point at which the ash end plug starts to form. At each of the segmented sample faces, a high resolution picture is taken of all the channels and an image processing software (ImageJ) is used to measure layer thickness in two directions: Figure 4.3. Measuring Ash Layer Thickness Measurements are averaged across all channels in both X and Y directions as well as for both faces at each partition between samples. Next, ash end plug formation is measured using a thin rod again averaged for all available channels: 53 I Figure 4.4. Measuring Ash End Plug Formation Once layer thickness and end plug formation data has been collected at various points throughout the DPF length, an ash distribution profile can be generated to simulate approximate ash accumulation for one typical channel: Flow I 1.6 - E 1.4 E 1.2 C ai1 0.8 0.6 0.4 0.2 0 0 50 100 150 Distance (mm) Figure 4.5. DPF Ash Distribution Profile 4.2.2 Ash Porosity Once ash distribution has been measured, the core segments can then be mounted for examination under a scanning electron microscope (SEM). First, the samples are further sectioned so that a well defined ash layer and the ash end plug are present immediately after the point of segmentation. These samples are next mounted in a low-viscosity resin and then ground and polished so that the ash layer and plug are flush with the top surface: 54 Figure 4.6. Mounted Filter Samples for SEM Analysis Finally, the sample surfaces are coated with a thin layer of carbon (50 nm) and then viewed under an SEM to provide high-magnification cross section images of the ash layer and end plug: Figure 4.7. SEM Micrograph of CJ-4 Lab Ash Layer The equipment used throughout this study to obtain SEM micrographs is the Jeol 5910 General Purpose SEM. These micrographs provide a two-dimensional snapshot of a given point within the ash layer or plug. One can then directly measure ash porosity by using an image processing software to calculate a particle area fraction for a given selection region. The grayscale micrograph, though, must first be thresholded into a binary image so that all pixels can be considered as either particle area or void space: 55 Figure 4.8. Binary Image of SEM Micrograph In addition to applying binary thresholding to the original grayscale SEM micrograph, it also helps to apply ImageJ's Watershed segmentation filter, which separates seemingly distinct particles based on roundness factor. Once the binary image is obtained, particle area fraction can be calculated for a given selection region, averaged over several different channels and locations, and then expanded to characterize ash porosity in all three dimensions according to the following relation: Particles)3/2 Aselection (4.1) Alternatively, ash porosity can also be measured according to 1-Pi~ackinq PMaterial Where packing density Ppacking equals M for a given volume of ash layer or plug and VAs Pmaterial refers hsh to the true material density of the ash. 56 (4.2) 4.2.3 Ash Composition Remaining ash from the other core segments can then be extracted for additional analysis. First, composition can be measured for a loose ash powder sample using X-ray diffraction (XRD). The following figure shows a typical peak scan for an ash sample using the PANalytical X'Pert Pro Multipurpose Diffractometer: cX~mbf 1000 2500 30 40 PPa n [*zft a (Ln r a 5b s(hS)p Figure 4.9. XRD Peak Scan for a CJ-4 Lab Ash Sample The above example scan reveals the extremely complex nature of ash samples, that their composition consists of an incredible amount of different elements and compounds coming from a multitude of sources such as the engine lubricant, fuel, and wear and corrosion particles. For simplicity, subsequent analysis typically narrows down the candidate list to compounds containing Ca, Mg, Zn, S, P, 0, Fe, Si, Al, and Mo elements. Furthermore, once an ash sample's composition has been determined, its true material density can be estimated by taking a weighted average of all the material densities of its constituents. 57 4.2.4 Ash Morphology Next, loose ash samples can be viewed under a transmission electron microscope (TEM) at very high magnifications so that individual ash particles can be seen. The intent is to be able to characterize ash morphology and note any differences between lubricant formulations in particle size, shape, or agglomeration. Below is an example TEM micrograph taken using the Jeol 200CX General Purpose TEM: Print Nag: G to 00 IOUB6.*1 AWC-.sy.t.. Figure 4.10. TEM Micrograph of a Single CJ-4 Lab Ash Particle 4.2.5 Ash Particle Size Distribution Particle size is another quantifiable characteristic of ash that can be used to systematically compare different samples. Although a TEM is able to provide very detailed and accurate information regarding a single ash particle's size and shape, particles can only be viewed and analyzed one at a time. Ideally, a very large sample set of ash needs to be characterized in order to comprehensively represent the whole test sample, but doing so would be extremely time consuming and tedious with the TEM. Alternatively, particle size for an entire sample of ash powder can be measured using light obscuration. First, a small amount of ash is suspended and dispersed in solution with a solvent such as ethanol or isopropanol. This solution is passed 58 through a light source essentially as a very dilute stream of single particles, and for each particle a detector measures the degree of light obscuration to determine its size: DIWENTIAL OITRUION VOLUME-WT Ret % 4A0 Sr0 Detecor 3M 2.50 2.00 1.50 1.00 0.50 0.00 laser MSuce 0.5 1 2 5 10 20 80 100 200 00 Dim.-(um)- Figure 4.11. Light Obscuration Technique and Example CJ-4 Lab Ash Volume Distribution Thus, this technique is able to provide true particle counts for the whole sample as all individual particles are analyzed. The distribution results can be presented either number-based, where particle sizes are classified purely on their frequency of occurrence, or volume-based, where particle sizes are classified based on their volume percentage of the entire sample set. Furthermore, mean, median, and mode particle size information can be obtained. The equipment used for these analyses is the Particle Sizing Systems AccuSizer Model 770. This technique, however, is not without limitations. First, it is based on the Equivalent Spherical Diameter assumption, which assumes the detected particles are spherical in nature. TEM data shows that ash typically isn't, though, so the data could potentially be skewed. Also, the lower detection limit for this technique is only about half a micron, which sometimes cuts off part of the number-based distributions that contain much smaller particle sizes. Alternative methods such as laser diffraction can be used to reach smaller particle sizes down to 0.02 microns, but the number-based distributions cannot be directly measured. They have to be estimated using calculations and assumptions based on the volume-based distribution results, which don't yield direct and true measurements like in the light obscuration method. 59 (This page intentionally left blank) 60 5 EXPERIMENTAL TESTING AND RESULTS By applying the above techniques, this work aims to test and characterize new lubricant formulation samples and compare the results to previous data, ultimately hoping to better understand the effects of lubricant additive chemistry on ash characteristics and DPF performance. 5.1 Test Matrix and Parameters The previous MIT lubricant additive studies tested a total of six different oil formulations with varying additive content. This work will contribute seven more filter samples for further comparison and analysis. Three additional oil formulations will be tested using the MIT Accelerated Ash Loading System. Two of those same oils will also be used in non-accelerated, engine-derived ash loading to compare laboratory and engine testing. Lastly, Two field-aged sample from on-road use with commercial oil formulations will be analyzed as a baseline comparison. 5.1.1 Lubricant Formulations The additive concentrations of the six oil formulations tested in the previous MIT lubricant additive studies are presented below. All oils are formulated to the same 1% sulfated ash level [26]: Lubricant CJ-4 Base oil + Ca Base oil + Mg Baseoil+ZDDP Base oil + Ca + ZDDP Baseoil+ Mg+ZDDP Zn P Ca Mg S [ppm 1226 <1 <1 2612 1280 1280 [ppm] 985 2 <1 2530 1180 1180 [ppm] 1388 2928 <1 <1 2480 <1 [ppm] 355 5 2070 <1 <1 1730 [ppm] 3200 609 460 6901 2750 2840 Table 5.1. Oil Formulation Test Matrix, MIT 2010, Reproduced From [26] Note that only the commercial CJ-4 lubricant is a fully formulated oil, which includes all the other components such as viscosity modifiers and anti-foam agents in addition to the additive 61 package that make it safe to run in a real engine. Again, from these tests there were significant differences in filter pressure drop observed mainly between oils containing calcium and those that did not. To investigate these results further, an emphasis is placed on the possible detrimental effects of calcium additives, and this is to be addressed by the testing of three additional oil formulations using the MIT Accelerated Ash Loading System. The first of the three fully formulated oil formulations is another commercial CJ-4 lubricant. The second is similar in formulation but contains a much higher level of Ca additives and essentially no Mg present. Similarly, the third oil would instead contain a much higher level of Mg additives and essentially no Ca present. All three oils would be used to load DPFs to an ash load of 25 g/L. Again, the objective here is to isolate the detergent variable and see if there are any differences between using calcium or magnesium. The same fully formulated high-Ca and high-Mg oils would additionally be used in engine testing at an independent facility employing natural oil consumption. These samples are provided with the intent of comparing ash generated by different means, either through real engine testing or in an accelerated laboratory setting. Lastly, the new fully formulated CJ-4 oil would be the same lubricant used in the two field-aged samples that are provided as a baseline comparison. 5.1.2 DPF Properties The previous MIT lubricant additive tests all used conventional cordierite DPFs manufactured by Coming [26]: Substrate Catalyst Cordierite Pt Dimensions D5.66" x 6" (D14.38 x 15.24 cm) Cell Density 200 cpsi (31 cells/cm 2) Wall Thickness 0.012" (0.03 mm) Filter Volume Table 5.2. Laboratory Testing DPF Properties, Reproduced From [26] 62 2.47 L Similar Pt-catalyzed cordierite filters with the same geometries above are used for this work's additional oil tests. They are provided by the same DPF supplier, but involve a new and different manufacturer, NGK. Properties of the DPFs used in both sets of testing are compared below: Property Bulk Density Cell Density Material Density Open Frontal Area Geometric Surface Area Mean Pore Size [g/cmI [CPSI] [g/cmI [%] [cm2/cml [pm] Corning DuraTrap CO 200/12 0.42 200 N/A 34 9.25 13 NGK DHC-558 0.38 200 1.2 34 9 15 Table 5.3. New DPF Property Comparison The new DPFs used in this study are said to have a 5-10% reduction in filter pressure drop compared to the previously manufactured filters. Although the above properties are somewhat similar between both types of filters, it's important to note that the DPFs used in previous and current tests are not identical. Furthermore, the provided engine test and field-aged samples use cordierite filters that are similar in the above DPF properties but have a much larger overall geometry. Dimensions are instead D12" x 12", making the filter volume 22.2 L. 5.1.3 Ash Loading Parameters Samples generated via the MIT Accelerated Ash Loading System all employ periodic, active filter regeneration. Ash is loaded into the DPF in one hour intervals at inlet temperatures around 250-275 *C. In between these loading cycles would be a 15-minute regeneration period, in which the DPF inlet temperature rises to about 600-650 *C in order to oxidize any soot produced by the diesel burner fuel. As a result, the filter would see the following typical temperature and pressure drop cycles. As expected, one can notice a gradual rise in filter pressure drop with increasing ash load [26]: 63 Temperature Cycles 750 600 450- - ]JhfIli jiII IJI 1RJ] CL300 -0)0) 0 AP Cycles - 2 .5 --- - :- -- -- --- - 2.0- 1 .5 ---- 0.0 --- - :-- 1 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 Time [hr] Figure 5.1. Accelerated Ash Loading and Periodic Regeneration Cycles, Reproduced From [26] The provided engine test and field-aged samples also employ similar periodic, active filter regeneration. Ash loading would occur at approximately 350 *C and regeneration would take place at about 650 'C over 20 minutes. 5.1.4 Soot Loading Parameters At various points throughout a filter's ash loading timeline, the DPF is loaded with soot in order to measure the effects of certain ash levels on soot filtration performance. For this work's testing, filters are loaded to about 6 g/L soot at 0, 10, 18, and 25 g/L ash load and the resulting filter pressure drop is measured at each benchmark. Once the soot loading is finished, the filter would be fully regenerated for the ash loading process to commence again. Soot loading is conducted using the coupled Cummins ISB engine in one-hour intervals at 224 N-m engine load, with DPF inlet temperatures at approximately 275 'C. Both the burner and Cummins ISB use ultra-low sulfur diesel fuel. 64 5.1.5 Test Summary A test matrix is provided below summarizing the parameters for all new samples to be tested as well as all previous samples to be compared to. Note that the commercial CJ-4 oil used in the old and new tests differ in manufacturer, and that FF denotes a fully formulated oil for those that aren't commercial CJ-4 formulations: Source Lubricant CJ-4 (1) Base oil + Ca Base oil + Mg Base oil + ZDDP Base oil + Ca + ZDDP Base oil + Mg + ZDDP Filter I I Lab Lab Lab Lab Lab Lab Cordierite Cordierite Cordierite Cordierite Cordierite Cordierite Lab Lab Lab Engine Engine Field Field Cordierite Cordierite Cordierite Cordierite Cordierite Cordierite Cordierite Regeneration I MIT (2010) Ash Load Equivalent [g/LJ [muJ 4 4 4 4 4 4 42.0 29.3 23.8 27.8 25.0 22.7 -315,000 3 3 3 N/A N/A N/A N/A 25.4 25.1 24.9 -25 -25 -75 -85 -175,000 -175,000 -175,000 -175,000 -175,000 -425,000 -350,000 Oiltlnjectlon Rate I[mL~mln] Periodic Periodic Periodic Periodic Periodic Periodic Mileage -220,000 -170,000 -210,000 -175,000 -165,000 MIT (2012) CJ-4 (2) High-Ca (FF) High-Mg (FF) High-Ca (FF) High-Mg (FF) CJ-4 (2) CJ-4 (2) Periodic Periodic Periodic Periodic Periodic Periodic Periodic Table 5.4. Test Matrix Summary of Previous and New Samples 5.2 DPF Loading and Pressure Drop Results First, pressure drop results will be presented for the three additional oil tests generated using the MIT Accelerated Ash Loading System. The objective is to see if the same trends from past studies, noting significant pressure drop differences between oil formulations containing calcium additives and those that did not, can be reproduced using three new fully formulated oils. 5.2.1 Ash Loading Results Even before analyzing the pressure drop data, it's important to note that all three oil tests differ in the amount of time and oil consumption needed in order to reach the target ash load of 25 g/L: 65 Lubricant CJ-4 (2) High-Ca (FF) High-Mg (FF) Time [hr] 82.5 76.25 86 Oil Consumption Ash Load [kgl [g/L] 11.5 10.7 11.9 25.4 25.1 24.9 Table 5.5. Ash Loading Summary for Newly Tested Oils The high-Ca oil reaches the target ash load in less time compared to the baseline CJ-4 test and also consumes less oil in the process, while the high-Mg oil requires both more time and oil. These differences can be attributed to the fact that the additives form different ash compounds. It will be shown later that Ca-based ash consists mostly of sulfates [CaSO 4] while Mg-based ash contains mostly phosphates [Mg 3(PO4)2]. For the same unit amount of additives, then, the calcium additives gain more additional molecular weight from the sulfates per mole than the magnesium additives do from the phosphates, meaning the target ash load is reached sooner. Ash loading results for the three new oils are presented below. At each ash level, DPF pressure drop is measured in the absence of soot at room temperature and at 20,000 1/hr space velocity: MIT Accelerated Ash Loading Tests (2012) 0.6 - Ca (FF) 0.5 0.12 kPa 0.4 CL %40 . 0.3 0.2 0.1 25 *C, Space Velocity = 20,000 hri 0 0 5 10 15 Ash Load (g/L) 20 25 Figure 5.2. DPF Pressure Drop vs. Ash Load at 0 g/L Soot 66 30 The results show two major findings. First, there appears to be very little to no difference in filter performance across the three oils. At 25 g/L ash load and no soot present, pressure drop for an oil formulation containing a high level of calcium additive content is only slightly (0.12 kPa) worse than that for an oil formulation containing no calcium and instead all magnesium-based detergents. This contradicts previous findings, which saw Ca-based oils yield filter pressure drops approximately two times greater than oils without calcium below 25 g/L ash. Note that the current and previous tests, however, were not conducted 'back-to-back." Although done on what should have been the same test setup, the previous studies were completed two years prior by another student using a different set of DPFs, test oils, and pressure transducers. Discrepancies could be inherent in these variables or possibly in some other unknown changes. Secondly, the overall magnitudes of the filter pressure drops appear to be much lower than those measured in the previous studies. Plotting the above results together with the 2010 data and normalizing all curves to the same starting pressure drop (APNormalized = APMeasured/APO g/L Ash), it seems that the new oils' performance roughly equals that of the lower pressure drop, non-Ca-containing oils from the previous set of tests: MIT Accelerated Ash Loading (2010 & 2012) 12 Base + Ca + ZDDP 10 Base + Ca CJ-4 (2010) x4 8 M 4U06 Lab Ca Lab CJ-4. CL 4 ab Mg Base + Mg 25 *C, Space Velocity = 20,000 hri 011 0 10 30 20 40 50 Ash Load (g/L) Figure 5.3. Pressure Drop Response Normalized and Compared to Previously Tested Oils 67 Even though the newly tested oils include a full CJ-4 formulation and a high calcium content oil, their pressure drop responses do not exhibit the same behaviors as seen in the past. The new calcium-based oils do not result in significantly higher filter pressure drops for a given ash load below 25 g/L when compared to formulations that don't contain calcium. Further pressure drop testing is conducted in order to verify the above results. Small cores are extracted from the centers of two of the fully loaded DPFs (the third had cracked in half and therefore is unusable) and these cores are tested on an alternate flow bench system: Figure 5.4. DPF Core Testing on Flow Bench System Pressure drops across the high-Ca and high-Mg cores are measured using an entirely different set of analog and digital pressure transducers, and then compared to data from previous studies collected on the same flow bench apparatus. Since the previous flow tests were done at different ash loads, however, we normalize the pressure drop measurements by the ash load, i.e. APNormalizedt = APMeasured/ DPFAsh Load * 10. Plotting the new results together with the previous 2010 test samples, we see that similar trends are reproduced. Again note that the previous samples used filter cores similar in size and material (cordierite) to those used in this current study, although not completely identical: 68 Pressure Drop Normalized to Ash Load 1.0 0.9 - 0.8 - A 0.7 8 Ca(FF) ---------Mg (FF) -- - - 0 0.6 0.5 -- ----------------- 0.4 ------- ----- 0.3 0.2 0.1 -I 20,000 80,000 60,000 40,000 Space Velocity [1/hr] 100,000 120,000 Figure 5.5. DPF Core Pressure Drop Response via Flow Bench Testing Once again it's shown that the newly tested fully formulated oils show very little to no difference in filter pressure drop at the same 25 gIL ash load, and that those pressure drops appear to be noticeably lower in magnitude compared to those measured in the previous 2010 oil tests. 5.2.2 Soot Loading Results At four points throughout the ash loading process, filters are loaded with 6 g/L of soot in order to study the effect of a given ash level on DPF soot filtration performance. Filters are then fully regenerated before continuing on with further ash loading. The first soot loading is conducted at the very start of ash loading, at 0 g/L ash load. As expected, with no ash introduced yet, the three filters all behaved similarly like new, clean DPFs would perform. They displayed very little to no difference (<0.5 kPa) in pressure drop response due to increasing soot load. Note, then, that the series labeling by oil formulation is arbitrary in the following plot and only serve to relate each data set to the subsequent oil-dependent results: 69 0 g/L Ash 3.5 CJ-4 3 0.38 kPa 2.5 MCa 2 1.5 1 0.5 0 25 *C, Space Velocity = 20,000 hr I 0 1 2 3 4 Soot Load (g/L) 5 6 7 Figure 5.6. Pressure Drop Response to Soot Load at 0 g/L Ash At 10 g/L ash for the three different oils, however, the filters continue to show very little to no difference (< 0.5 kPa) in pressure drop response to increasing soot load: 10 g/L Ash 2.5 CJ-4 Ca 0.36 kPa 2 Mg 1.5 0. 43 1 0.5 25 *C, Space Velocity = 20,000 hr' 0 0 1 2 4 3 Soot Load (g/L) 5 6 Figure 5.7. Pressure Drop Response to Soot Load at 10 g/L Ash 70 7 Similarly, little difference in pressure drop response continues to be observed at 18 g/L ash load (< 0.5 kPa) as well as at the final ash load of 25 g/L (0.65 kPa): 18 g/L Ash 3.5 Mg -}- .47kPa 3 ' 2.5 CJ-4 2 S1.5 0.5 25 *C, Space Velocity = 20,000 hr 0 0 1 2 4 3 5 7 6 Soot Load (g/L) Figure 5.8. Pressure Drop Response to Soot Load at 18 g/L Ash 2S g/L Ash 4.5 Ca CJ-4 4 0.65 kPa 3.5 Mg 3 . at- 2.5 2 1.5 1 0.5 25 *C, Space Velocity = 20,000 hr' 0 0 1 2 4 3 SootLoad (g/L) 5 6 7 Figure 5.9. Pressure Drop Response to Soot Load at 25 g/L Ash 71 The acquired ash and soot loading data can be rearranged to instead plot pressure drop as a function of ash load for a given soot level. The same trends are seen, however. Very little difference exists in performance between the three oil tests at 3 g/L soot (<0.5 kPa): 3 g/L Soot 14 Ca LCJ-4 0.14 kPa Mg 1.4 <i 1.2 0 0.1 ~0.8 0.4 0.6 0.4 0.2 0 -T 0 25 *C, Space Velocity - - 5 15 10 20 25 = 20,000 hri - 30 Ash Load (g/L) Figure 5.10. Pressure Drop Response to Ash Load at 3 g/L Soot The dip in pressure drop response seen in all three curves refers to the pressure drop "benefit" associated with early ash accumulation. Initially, incoming soot is deposited within the DPF wall pores, contributing greatly to filter pressure drop. But with the presence of an established ash layer, this soot depth filtration is avoided and only cake filtration proceeds. Thus, filter pressure drop response to soot loading for a filter with a thin ash layer is actually lower than that for a completely clean filter. After a certain point, however, the ash layer becomes thick enough where its contribution to porous media pressure drop outweighs the benefits of reducing soot depth filtration. At 6 g/L soot, filter pressure drop response to ash load also shows very little difference (< 0.5 kPa) between the three test oils: 72 6 g/L Soot 4.5 Ca 4 0.37 kPa _4 3.5 3 ~2.5 '~2 1.5 1 0.5 25 "C, Space Velocity = 20,000 hr' 0 i 0 I 5 i 10 I I 20 15 Ash Load (g/L) 25 30 Figure 5.11. Pressure Drop Response to Ash Load at 6 g/L Soot DPF pressure drop response to ash and soot loading shows very little difference in performance between the three newly tested oils below 25 g/L ash and 6 g/L soot. Previous trends showing oils containing higher levels of calcium additives leading to significantly increased filter pressure drops at a given ash or soot load are not observed. To further support and explain the above findings, in-depth analysis of the filter and ash samples would be conducted and compared to previous data. 5.3 Post-Mortem Ash Analysis Results Previous studies saw noticeable differences in filter performance between oils of different additive formulations, and these findings were supported by the fact that the resulting ash characteristics also differed to some degree. Although ash distribution was roughly the same for all oils, the calcium-based ash associated with higher pressure drop responses was measured to be generally less porous and was found to have different ash compound forms. For the three newly tested oils, however, filter performance was observed to be very similar. One would expect, then, their resulting ash properties to be very similar as well. 73 5.3.1 Ash Distribution Results Similar to the previous MIT lab-generated DPF samples, overall ash distribution is very similar between the three different oil formulations: 1.6 . 1.4 E .E.. 1. 2 IA #A U '2 0.8 E0.6 .c 0.4 0.2 0 0 50 100 150 Distance (mm) Figure 5.12. Ash Distribution Profile Results for a Single DPF Channel Layer thickness ranges from 0.07 to 0.10 mm and plug length only varies between 31 and 32 mm. These figures are on par with those obtained from the previous 2010 samples, which saw layer thicknesses between 0.08 and 0.13 mm and plug lengths between 22 and 29 mm. After comparing the above results to the other engine and field-aged samples, some differences can be seen. Because the samples differ in DPF size, the following profile data has been normalized to DPF length (% DPFLength = Distanceeasured/TotalDPFLength * 100): 74 1.6 E 1.4E ; 1.2 U 0.8 Engine Lab Field 0.60.40.2 0 0 20 40 60 80 100 Distance (% of Total DPF Length) Figure 5.13. Ash Distribution Profiles for Lab, Engine Test, and Field-aged Samples It appears that the engine test samples display a much thinner ash layer as well as a much longer end plug than both the lab and field-aged samples. A closer examination of the engine test sample end plugs via X-ray CT scanning, however, reveals that the ash plugs are actually very discontinuous: Figure 5.14. Calcium Oil Engine Test Ash End Plug via X-ray CT 75 This extremely insightful view inside the DPF channels shows that the engine test ash plugs, which would have appeared to be extremely long via the standard procedures of measuring plug length, are in reality broken up much lesser in volume. These plug discontinuities could be a result of ash sintering at unintentionally high DPF temperatures, or possibly due to incomplete filter regenerations with soot forming intermediate sections between the ash. The field-aged samples, however, are more comparable in distribution to the lab ash samples. Distribution data for the lab, engine, and field-aged ash are summarized below and compared to previous 2010 figures: Thickness SampleLayer [mm] [mM] Plug Length [% DPF Length] (2012) Lab CJ-4 Lab Ca Lab Mg Field CJ-4 1 Field CJ-4 2 Engine Ca Engine Mg ____ Base Base Base Base Base 0.076 0.104 0.071 0.092 0.125 0.022 0.028 31 32 31 24 35 20 21 20 8 11 -- -- -- -- 0.136 0.085 0.136 0.087 0.085 15.6 28.7 18.9 21.9 27.3 10.2 18.8 12.4 14.4 17.9 ___ ___ ___(2010) ____ + Ca + Mg + ZDDP + Ca + ZDDP + Mg + ZDDP Table 5.6. Ash Distribution Summary Again, consistent with past data, ash distribution is roughly the same between different oil formulations as well as when compared to field-aged samples. This suggests that porous media thickness as well as available filter channel length are comparable between samples. 5.3.2 Ash Porosity Results Ash layer porosity measured via SEM image analysis is mostly similar for the newly tested oils. Similar to past data, porosities are high in magnitude, ranging from about 93 to 95%: 76 Ash Layer Porosity (2012) 100 98 Mg 96 94 0 88 86 84 Figure 5.15. Ash Layer Porosity Results via SEM Image Analysis Note that the engine test samples, as previously shown, display very little to no ash layer present, making the layer porosity measurements unfeasible. Similarly, ash porosity in the end plug is also comparable between the new oils, ranging from about 94 to 97%: Ash Plug Porosity (2012) 100 98 Ca Mg Field 96 - 94 0 L- 92 - 0 90 88 86 84 Figure 5.16. Ash Plug Porosity Results via SEM Image Analysis 77 Note that in general, consistent with past results, ash plugs yield higher porosities than in the ash layer. This is attributed to the fact that flow velocity inside the DPF is greatest near the walls, which are the main flow through mechanisms, so material packing is much greater in the wall layer than in the center of the channel where the end plug forms. Ash layer porosity results are summarized below. Again, figures are similar among the newly tested oil formulations, and are also more or less on par with those from previous studies: Ash Layer Porosity Sample (2012) Lab CJ-4 Lab Ca Lab Mg Field CJ-4 CJ-4 Base Base Base Base Base 93.0 93.0 95.0 193.0 (2010) 91.1 90.9 94.6 95.1 90.5 92.2 + Ca + Mg + ZDDP + Ca + ZDDP + Mg + ZDDP Table 5.7. Ash Layer Porosity Summary The spread of porosity figures in the previous studies, however, correlated with much more significant differences in filter pressure drop response. Those differences, though, are not seen with the newly tested oil formulations. 5.3.3 Ash Composition Results XRD analysis of the newly tested ash samples show that, similar to previous findings, Ca-based ash exists primarily in the sulfate and phosphate form. Note that when sulfate or phosphate compound forms are referred to, they include any intermediary elements such as "calcium zinc phosphate" or "calcium iron phosphate," for example: 78 Calcium Sulfate C 100 80I 60 . = m - 0 020- U n S0Engine Ca Field 0-4 Lab Ca Lab 0-4 Base + Ca + ZDDP C-4 Field (2010) (2010) Calcium (Zinc, Iron) Phosphate Q 100 80 60 40 m 0820 r' 0 _m. Lab Ca Engine Ca Field 0-4 Lab CJ-4 Base + Ca + ZDDP Field (2010) 0-4 (2010) Figure 5.17. Ash Sample Calcium Content via XRD Analysis Magnesium content found in the ash, however, exists mainly as a phosphate form only: Magnesium Sulfate 100 - .0 80 S60 0- 0 40 20 0- - - Engine Mg Field 0-4 Lab Mg Lab CJ-4 Base+ Mg [1] Base + Mg [2] 0-4 (2010) Field (2010) Magnesium (Zinc, Iron) Phosphate 100 .80,60 0 C- 40 0 20 0 -- M M Engine Mg Field 0-4 - Lab Mg Lab C-4 Base+ Mg [1] M M Base+ Mg [2] 0-4 (2010) Figure 5.18. Ash Sample Magnesium Content via XRD Analysis 79 Field (2010) Previous studies found a majority of Mg-based ash existing as magnesium oxide, but this applied only to the Base Oil + Mg lubricant formulation, which contained no amount of sulfur or phosphorus. It makes sense, then, that primarily an oxide form would dominate with exhaust being abundant with oxygen. In the newly tested, fully formulated oils, though, which contain adequate levels of sulfur and phosphorus, magnesium phosphate dominates, suggesting that an affinity for phosphorus is stronger than that for oxygen. Still, there is the question why calcium ash also forms sulfates while magnesium ash does not. It appears that magnesium additives, unlike calcium, have a stronger affinity for phosphorus and do not prefer the sulfate form. Lastly, similar to previous studies, zinc content found in ash exists primarily as a phosphate: (Calcium, Magnesium) Zinc Phosphate - 100 .2 80 c 60 Lc 40 E 20 0 U 0 Engine Engine Ca Field Lab Ca Lab Mg Lab 0-4 Base + Ca + 0-4 Mg Base + 0-4 Field Mg (2010) (2010) Base+ Mg (2010) (2010) ZDDP Zinc (Iron) Oxide 100 .2 80 U) 60 0 40 E 20 0 0 , ,M - Engine Engine Ca Mg Field 0-4 , Lab Ca Lab Mg Lab CJ-4 Base+ Ca+ ZDDP 0-4 Field Figure 5.19. Ash Sample Zinc Content via XRD Analysis Previous studies considered differences in ash composition, between calcium sulfate and magnesium oxide, as a potential factor governing filter pressure drop. While there are noticeable differences in composition between the newly generated Ca-based and Mg-based ash types, it's 80 unclear what consequences follow. For the newly tested oils, Ca-based ash contains both sulfate and phosphate forms while Mg-based ash only contains only phosphates, yet their pressure drop responses to overall ash load remain similar. 5.3.4 Ash Particle Size Results Ash particle size is measured for the new lab, engine, and field samples. Below is an example volume-based size distribution result for the 2012 lab CJ-4 ash: VOWME-WT DIFFERENTiAL DSTRIBUTION ROL % 4.00 3.00 250 2.00 1.50 1.00 0.50 0.00 0.5 1 2 5 10 20 50 100 200 500 Dam.(um)-> Figure 5.20. Volume Basis Particle Size Distribution for Lab CJ-4 Ash, 2012 The volume basis distribution shown above implies that most of the sample set's total volume consists of particles that are about ten microns in diameter. That does not necessarily mean, however, that there are more ten-micron particles than any other size. A number basis distribution for the same ash sample is provided below: 81 NUMBER-Wr DIFFERENTLAL DISTRIBUTION Re. % 7.0 rr I -rrTT ~~~*1 I _____________ I - -t -~ 1.0 4.0 2.0 1,0 0.0 0.5 1 2 5 10 20 50 100 200 500 Diam.(um)->- Figure 5.21. Number Basis Particle Size Distribution for Lab CJ-4 Ash, 2012 The number basis distribution instead shows that most of the particles of the sample set, simply when counted by frequency, are much smaller in diameter. Also note that the number distribution gets cut off at the lower detection limit of 0.5 pm. This is very commonly seen for number basis distributions, since a majority of powder samples do consist of much smaller particles. No equipment, however, can currently measure down to a diameter of "zero." Alternate techniques with higher resolutions can be used to measure those smaller, nano-sized particles, but they in turn have upper detection limits of only about one micron and are also unable to directly measure number-based distributions. A summary of ash particle size for the newly tested samples is provided below. The full distribution graphs for the other ash samples can be viewed in the appendix. Although the number basis distribution data are only partial, they still provide very useful information that can be used to make systematic comparisons between sample sets. * Note, then, that the mean particle size results for the partial number distributions are skewed and inaccurate, as the full distribution curve was not able to be captured: 82 Sample Number Mean Diameter Volume Mean Diameter 13.65 10.29 10.99 10.62 11.26 15.66 1.39 1.39 1.35 1.14 1.03 0.97 Lab CJ-4 Lab Ca Lab Mg Engine Ca Engine Mg Field CJ-4 * Table 5.8. Ash Particle Size Summary (* See Above) Both number and volume basis distribution results show that overall ash particle size is mostly consistent between the newly tested lab, engine, and field samples. The field sample shows a slightly higher volume-based mean diameter, but that is likely attributed to a higher level of larger engine wear and corrosion particles accumulated from extended on-road use. The above consistencies, though, support the similarities previously observed in the filter pressure drop response data. 5.3.5 Ash Morphology Results TEM images of individual ash particles are collected for the newly tested samples. First, the high-Ca oil produces ash that is a combination of round and branch-like structures: UffComera System Figure 5.22. Calcium Oil Lab Ash Morphology 83 syt. Nf Cmers Similarly, the high-Mg oil yields ash that also contains both spherical and branched elements: 07.0in Hag 5100 44:is p 0/3o2/1 3 rint 500 Iw NV=120.0.v t 1rint Mogm79500x 1.0 in 4:36sl p 04/12/13 500 ran HV=120.0kV 6800X sypt. Direct MN"g AT Ca.ue DIrc Keg: 500I ANTCamerdsystem Figure 5.23. Magnesium Oil Lab Ash Morphology Similar morphology can also be observed for CJ-4 lab and field sample ash: Tlt 3: I 07.0 in 3N0Ag71300w0 000 e )s .:3 MW-130.0ky 1/0/13 i 6nt mg! 10403003 10:31:0 Dirct3 Ng: S000 AJhT Camer.System s 0 261 7.0to 100 no V 120.ekv Direct Hg: 10000x .syste ANTCer Figure 5.24. Lab CJ-4 (Left) and Field CJ-4 (Right) Ash Morphology 84 Results show that ash morphology looks roughly the same across the different oils, involving combinations of both round and branch-like structures. Not only do the fully formulated Cabased and Mg-based ash resemble each other, but they also resemble ash from previous studies: HIT t -mm2.9 t O5 m! ; prit 4C10A4 V 64,11/1 7.0033. in 500 M HV 11,.*W Print Has: 100007.0,I00 0.17,1) 74f.A44 a So, HV. 204Akv irect 140. Syzoex *s ViecCOOOOZ Figure 5.25. Lab Ca (Left) and Base + Ca + ZDDP (Right) Ash Morphology ~I0K 07. ,Imt2Uag 4-44,20 V04"1/~13 . S* 000 00120.6k0 Dir0- e Print N":~ 1640*0z 97.0 in I0'00:1 P 01/17,13 $700. 100 "VI. bl...D rt M AW Mas 0 104000. AwTCamet sytem system ARTCamera Figure 5.26. Lab Mg (Left) and Base + Mg + ZDDP (Right) Ash Morphology 85 The observed similarities in ash morphology for the newly tested oils once again support the similarities previously recorded for filter pressure drop response. 5.4 DPF Model Results Earlier, it was shown that DPF pressure drop could be modeled according to fundamental mathematical equations, which were functions of several key parameters such as ash layer thickness and porosity. By inputting values into the model according to measurements taken from post-mortem analysis as seen above, one can attempt to work backwards from given ash characteristics to simulate filter pressure drop, which can then be compared to actual experimental ash loading data for further confirmation. So far, experimental ash loading and post-mortem analysis data both have shown very similar results for the newly tested oils, and it will help to see if these results make sense mathematically according to the fundamental model. Equation 3.10 shows ash permeability as a function of ash porosity and mean particle diameter. Ash porosity can be inputted from the recorded measurements via SEM image analysis. Mean particle diameter, however, as explained earlier, was not able to be fully measured. But, volume and number distribution data shows similarities in particle size for the newly tested oils and literature states that average particle diameter is about 0.3-0.5 pm. So, particle diameter will be estimated and held constant for each sample, resulting in the following ash permeability figures: Sample Lab CJ-4 Lab Ca Lab Mg Field CJ-4 Ash Layer Porosity Mean Particle Diameter. 93.0 93.0 95.0 93.0 0.3 0.3 0.3 0.3 Ash Layer Permeability 8.22 8.09 1.72 8.32 E-14 E-14 E-13 E-14 Table 5.9. Simulated Ash Layer Permeability Summary Ash permeability can then be used in Equation 3.8 to determine pressure drop through the ash layer. Ash layer thickness is inputted from recorded measurements, and gas dynamic viscosity (18.5 x 106 N-s/m2) as well as gas wall velocity (0.00727 m/s) are both constants estimated by typical values from experiments and literature: 86 Sample Lab CJ-4 Lab Ca Lab Mg Field CJ-4 Ash Layer Thickness Ash Layer Permeability 8.22 8.09 1.72 8.32 0.076 0.104 0.071 0.125 Ash Layer Pressure Drop 0.124 0.172 0.056 0.202 E-14 E-14 E-13 E-14 Table 5.10. Simulated Ash Layer Pressure Drop Summary Pressure drop through the filter substrate walls can be calculated in the same way. Wall thickness is constant in all cases. Wall permeability, however, is difficult to measure experimentally once ash has filtered into the pores. It is instead estimated using a theoretical model based off of the experimental ash loading pressure drop response data, as outlined by Wang and Sappok in SAE 2013-01-1584 [29]. Wall pressure drop is calculated below: Sample Lab CJ-4 Lab Ca Lab Mg Field CJ-4 Wall Thickness Wall Permeability 2.02 2.12 2.54 1.50 0.3 0.3 0.3 0.3 Wall Pressure Drop E-13 E-13 E-13 E-13 0.199 0.190 0.159 0.268 Table 5.11. Simulated Wall Pressure Drop Summary Summing up the pressure drop terms for the ash layer and substrate wall yields total porous media pressure drop for the DPF: Sample Porous MedlaPressure Drop 0.323 0.362 0.215 0.470 Lab CJ-4 Lab Ca Lab Mg Field CJ-4 Table 5.12. Simulated Porous Media Pressure Drop Summary These figures should correspond with the measured pressure drop increase due to experimental ash loading: 87 Porous Media Pressure Drop (Calculations) 400.00 Cj_4 Ca 300.00 200.00 0.21 - 0.36 kPa 100.00 0.00 Porous Media Pressure Drop (Ash Loading) 0.6 0.5 Ca 0.4 -- C. 0.3 A Mg 0.2 .26 - 0.38 kPa 0 0.1 0 0 5 10 20 15 Ash Load (g/L) 25 30 Figure 5.27. Simulated vs. Experimental Porous Media Pressure Drop Filter pressure drop increase due to ash loading is of the same order between mathematical simulations and actual experimental data. Clearly the theoretical model is not quite able to perfectly capture the exact figures measured in the experiments, but all three calculations are on the same order as their experimental counterparts. Overall pressure drop increase is about 0.2 to 0.4 kPa for both simulated and measured results. Furthermore, the magnitudes of ash layer pressure drop versus substrate wall pressure drop as calculated by the simulation are roughly equal, which can also be seen by the relative magnitudes of the depth and layer filtration regions of the experimental pressure drop curves. This data validation by fundamental mathematical model adds further support to the experimental findings of the three oil tests conducted in this study, confirming that the observed trends are true and real occurrences. 88 6 CONCLUSIONS The objective of this study is to supplement previous investigations of lubricant additive effects on ash characteristics and DPF performance. Three lubricant formulations of the same sulfated ash level, that differ only in the type of additives present, are tested and compared to an array of additional samples with different oil formulations and generation means in order to quantify and characterize any significant differences that result from varying additive chemistry. 6.1 Lubricant Additive Effects on DPF Performance For the three fully formulated lubricants tested in this study, a commercial CJ-4 oil, a high calcium detergent oil, and a high magnesium detergent oil, very little differences are seen in filter pressure drop response to ash and soot loading and the slight differences that do appear don't always yield the same relative disparity between the oils. Namely, one oil doesn't always produce higher filter pressure drops than the other two. At soot levels of 0, 3, and 6 g/L, DPF pressure drop increase as a function of ash load below 25 g/L is very similar between the oils, yielding less than 0.4 kPa (33%) difference at any given ash load. At ash levels of 0, 10, 18, and 25 g/L, DPF pressure drop increase as a function of soot load below 6 g/L is also very similar between the oils, yielding less than 0.7 kPa (18%) difference at any given load. The high calcium detergent oil, however, reached its final ash load on the same accelerated lab system in approximately 11% less time than the high magnesium detergent oil. Ash generation rate, therefore, is quicker for oils with higher calcium content due to calcium having a greater atomic weight than magnesium for roughly the same ash compound forms, leading to earlier pressure drop increases in the filter for the same amount of on-road use. 89 6.2 Lubricant Additive Effects on Ash Characteristics Similarities in DPF performance for the three tested oil formulations are supported by additional similarities observed in resulting ash properties. Fundamental mathematical models, which quantify DPF pressure drop as a function of key parameters related to its porous media, confirn that similarities in ash layer and wall properties also result in similarities in DPF performance. Overall ash distribution is similar between the three oil formulations, with ash layer thickness ranging from 0.07 to 0.13 mm and ash plug length ranging from 31 to 32 mm. Ash layers are highly porous and also comparable, ranging from 93 to 95%. Overall ash particle size is very similar according to partial number distribution data. Volume distribution data also show similarities, with mean particle size ranging from 10.3 to 13.7 pm. Ash morphology is also similar for the three oil formulations from visual comparisons of TEM images. Ash particles and agglomerates generally consist of combinations of spherical and branched structures. Quantitative analysis of particle shape factors has yet to be completed. There are, however, slight differences in ash composition. The high calcium detergent ash exists mostly as calcium sulfate and calcium (zinc, iron) phosphate, while the high magnesium detergent ash exists mostly as magnesium (zinc, iron) phosphate only. Inputting these measured ash properties into the DPF mathematical model yields simulated filter pressure drops on the same order as those recorded from experimental ash loading. The measured ash properties for the three oil formulations above are also comparable to figures from previous lubricant additive studies that used similar testing methods and conditions. Past samples recorded layer thicknesses of 0.08 to 0.13 mm, ash layer porosities of 90 to 95%, as well as similarly shaped ash particle morphologies. Ash composition, though, was found to consist mostly of calcium sulfate and magnesium oxide compounds. 90 6.3 Impact of Ash Depth Filtration So far, it's been shown that the three oil formulations tested in this study, differing only in calcium and magnesium detergent content, display very little difference in both DPF performance and overall ash properties. The ash properties, though, are also not too different from those measured in previous lubricant additive studies, but those previous studies saw much more pronounced differences in DPF performance between different oil formulations. For some reason, even with similarly characterized ash, the three oils from this study do not exhibit the same differences in filter pressure drop response to ash and soot loading. There are a couple of possible reasons to explain the discrepancies seen between current and previous test results. First, the three oils tested in this study are a completely new set of oils. While their additive levels are mostly similar to those from previous tests, there could be some unknown differences somewhere in the oil formulation that cause these oils to behave much more similarly than previously seen. Secondly, the oil tests in this study use new filters from an entirely different DPF manufacturer. Again, while they share mostly similar geometries and properties with the filters from previous tests, they do note a 5-10% reduction in pressure drop performance and any other differences in the filters could somehow be significantly affecting ash loading. Finally, differences between current and previous observations could be explained by possible differences in ash depth filtration. Figure 5.3 shows all previous and current pressure drop data normalized to clean DPF pressure drop and plotted on the same graph. It's clear that the new oil tests display similarly low pressure drop responses relative to some of the other previous calcium-based oil tests. The difference between these profiles, though, appears to primarily take place within the initial depth filtration region of the ash loading curves. After the beginning, steep rise in pressure drop due to the substrate wall pores directly filling with ash and once ash layer formation has commenced, the more gradual increase in pressure drop that follows from subsequent ash loading appears to be more or less similar between all test cases. The previous calcium-based oil tests do show slightly steeper pressure drop increases in the layer filtration 91 region, but the point at which layer filtration starts in the first place is significantly higher, sometimes twice as high, in pressure drop compared to the other oil tests. It seems, then, that the mechanisms governing ash depth filtration could somehow be differing significantly, resulting in dramatic differences in initial filter pressure drop increase. In that case, one would expect substrate wall permeability to be quite different between samples that have different degrees of depth filtration. Filter wall permeability, however, once it has already undergone ash depth filtration, is very difficult to measure experimentally as it involves one highly porous medium suspended partially within another, less porous medium. In order to fully understand these apparent differences in ash depth filtration, therefore, and to know if they account for the differences observed between past and current pressure drop response data, this issue must be investigated further. 6.3.1 Future Work Currently no experimental technique is used to quantify ash-loaded wall permeability. It is instead estimated from a theoretical model that is based on experimental ash loading pressure drop data. For this reason, though, any differences in experimental pressure drop response between oil tests will inherently be reflected in the model's determination of substrate wall permeability. According to the model, wall permeability for the new oils tested in this study is an entire order of magnitude greater than that of the previous samples. The exact opposite correlation, however, is what really should be determined. Does real wall permeability actually differ between these samples as apparent in the pressure drop response data, and are these differences the main factors governing overall pressure drop increase? A next step, therefore, is determining a systematic method of experimentally measuring substrate wall permeability after ash depth filtration has occurred. Much progress has been made recently using techniques such as FIB that can cut into substrate wall pores and provide detailed views of ash deposits, offering a very viable method for achieving wall permeability information. Furthermore, additional oil tests involving the same oil formulations used in the previous lubricant additive studies can be repeated in order to see if previous trends can be reproduced. 92 REFERENCES [1] Heywood, J.B., Internal Combustion Engine Fundamentals, McGraw-Hill, Inc., New York, 1988. [2] Khair, Magdi K. "The Case for the Diesel Engine." DieselNet Technology Guide. Ecopoint Inc., 2000. Web. 4 Apr. 2013. <http://www.dieselnet.com/tech/diesel-case.php#adv>. [3] "Diesel Vehicles." www.fueleconomy.gov. U.S. Department of Energy, n.d. Web. 4 Apr. 2013. <http://www.fueleconomy.gov/feg/di-diesels.shtml>. [4] Schindler, K-P., 1997. "Why Do We Need The Diesel?", SAE Technical Paper 972684, doi: 10.4271/972684 [5] Charles River Associates, "Diesel Technology and the American Economy," Diesel Technology Forum Report no. D02378-00, 2000. [6] "Diesel Market Highly Developed in Europe." ACEA. European Automobile Manufacturer's Association, 9 June 2010. Web. 5 Apr. 2013. <http://www.acea.be/news/news-detail/dieselmarket-highly-developed-ineurope/>. [7] Baukal, Charles. "Everything You Need to Know About NOx." John Zinc Co. LLC, n.d. Web. 5 Apr. 2013. <http://www.johnzink.com/wp-content/uploads/everything-about- nox.pdf>. [8] Majewski, W. Addy "What Are Diesel Emissions." DieselNet Technology Guide. Ecopoint Inc., 2012. Web. 5 Apr. 2013. <http://www.dieselnet.com/tech/emi-intro.php>. [9] Bosh, 1994. "Diesel Fuel Injection", Robert Bosch GmbH [10] Gallagher, James. "Diesel exhausts do cause cancer, says WHO." BBC News, 12 June 2012. Web. 5 Apr. 2013. <http://www.bbc.co.uk/news/health-18415532>. [11] Johnson, Tim. "Vehicle Emissions Review - 2012." Corning Environmental Technologies. Detroit. 16 Oct. 2012. Lecture. [12] "Meeting EPA 2010." FactsAboutSCR.com. North American SCR Stakeholders Group, 2008. Web. 8 Apr. 2013. <http://www.factsaboutscr.com/environment/epa2OlO.aspx>. [13] "Emission Standards Reference Guide." United States EnvironmentalProtectionAgency. US EPA, n.d. Web. 8 Apr. 2013. <http://www.epa.gov/otaq/standards/heavy-duty/hdciexhaust.htm>. 93 [14] Sappok, Alexander. "Ash Accumulation in Diesel Particulate Filters." DieselNet Technology Guide. Ecopoint Inc., 2012. Web. 17 Apr. 2013. <http://www.dieselnet.com/tech/dpfash.php>. [15] Aravelli, K., Jamison, J., Robbinson, K., Gunasekaran, N., and Heibel, A., "Improved Lifetime Pressure Drop Management for DuraTrap RC Filters with Asymmetric Cell Technology (ACT)," Diesel Engine Efficiency and Emissions Reduction Conference, Detroit, MI, 2006. [16] Boschert, T., 2002. "The Lubricant Contribution to Future Low Emission Engine Design", Diesel Particulate and NOx Emissions Course (University of Leeds), Ann Arbor, MI, October 2002. [17] Rudnick, Leslie R. LubricantAdditives: Chemistry and Applications.Wilmington: CRC Press, 2003. Print. [18] McGeehan, J., "API CJ-4: Diesel Oil Category for Both Legacy Engines and Low Emission Engines Using Diesel Particulate Filters", SAE 2006-01-3439, 2006. [19] Bodek, B., and Wong, V., "The Effects of Sulfated Ash, Phosphorus and Sulfur on Diesel Aftertreatment Systems - A Review", SAE 2007-01-1922, 2007. [20] Jaaskelainen, Hannu. "Engine Exhaust Back Pressure." DieselNet Technology Guide. Ecopoint Inc., 2012. Web. 18 Apr. 2013. <http://www.dieselnet.com/tech/diesel-exhpres.php>. [21] Morcos, M., Ayyappan, P., and Harris, T., "Characterization of DPF Ash for Development of DPF Regeneration Control and Ash Cleaning Requirements", SAE 2011-01-1248, 2011. [22] Sappok, A., Wong, V., and Munnis, S., "Individual and Synergistic Effects of Lubricant Additive Components on Diesel Particulate Filter Ash Accumulation and Performance", ICES2012-81237, 2012. [23] Sappok, A., "The Nature of Lubricant-Derived Ash-Related Emissions and Their Impact on Diesel Aftertreatment System Performance," PhD Dissertation, Massachusetts Institute of Technology, 2009. [24] DieselNet Technology Guide, 'Wall-Flow Monoliths", Revision 2005-09b, DieselNet. <http://www.dieselnet.com/tech/dpf wall-flow.php>, 2005. [25] DieselNet Technology Guide, "Cellular Monolith Substrates", Revision 1998-08c, DieselNet. <http://www.dieselnet.com/tech/cat-substrate.php>, 2005. 94 [26] Munnis, S., "Synergistic Effects of Lubricant Additive Chemistry on Ash Properties Impacting Diesel Particulate Filter Flow Resistance and Catalyst Performance," S.M. Dissertation, Massachusetts Institute of Technology, 2011. [27] Lee, K., Zhu, J., Ciatti, S., Yozgatligil, Al, and Choi, M., "Sizes, Graphitic Structures, and Fractal Geometry of Light-Duty Diesel Engine Particulates," SAE 2003-01-3169, 2003. [28] Sappok, A., Beauboeuf, D., and Wong, V., "A Novel Accelerated Aging System to Study Lubricant Additive Effects on Diesel Aftertreatment System Degradation," SAE 2008- 01-1549, 2008. [29] Wang, Y., Sappok, A., and Wong, V., "The Sensitivity of DPF Performance to the Spatial Distribution of Ash inside DPF Inlet Channels," SAE 2013-01-1584, 2013. 95 (This page intentionally left blank) 96 APPENDIX NUMBER-WT DIFFERENTIAL DISTRIBUTION Ret. % 7.0 .0 5.0 4.0 3.0 2.0 1.0 0.0 0.5 1 2 5 10 20 s0 100 200 500 Dim.(umn)->* Figure A.1. Lab CJ-4 Ash Particle Size Number Distribution VOLUME.WT DIFFERENTIAL DSRIBUTION R&L % 4.00 __ 3.50 3.00 2.50 2.00 1.50 1.0 0,50 0.00 0.5 1 2 5 10 20 50 100 200 500 Dam.(um)- Figure A.2. Lab CJ-4 Ash Particle Size Volume Distribution 97 Rej. % NUMBER.WT DIFFERENTIAL DiSTRIBUTION 4.0 -_- 5.0 - - - - - - 4.0 Zo 1.0 0.5 1 2 5 10 20 50 100 200 50 igL(um)-u Figure A.3. Lab Ca Ash Particle Size Number Distribution VO1.UMW4NT OFFERENTIAL DISTRIBUTION Rel. % 4.00 3.50 &00 Z50 200 1.50 1.00 0.50 0.00 0.5 1 2 5 10 20 50 100 200 500 DIam.(un)-> Figure A.4. Lab Ca Ash Particle Size Volume Distribution 98 NUMBERWT DIFFERENTIAL DISTRBUTION Rat % 7.0 - -rr - - - --- 6.0 5.0 - -- -- - - -- - - - - - - - 4.0 3.0 010 L 0.5 1 2 5 10 20 50 LL 100 200 500 Diarm(um)-> Figure A.5. Lab Mg Ash Particle Size Number Distribution VOLUME-W DIFFERENTIAL DIfTRIBUTION Ret % 4.00 3.50 2.50 2.00 1.50 1.00 0.50 0.00 0.5 1 2 5 10 20 50 100 200 500 Diam.(ump> Figure A.6. Lab Mg Ash Particle Size Volume Distribution 99 DIFFERENTIAL DISTRIDUTION NUMBER.WT NUMBER-WT DIFFERENTIAL DISRIBTION % ReL Ret % 9.0 13.0 7.0 5.0 - _ _ _ _ _ _ _ 5.0 4.0 3.0 2.0 0.0, . 0's 1 2 5 10 20 , , 50 ,, 100 , 200 500 Diem.(um> Figure A.7. Engine Ca Ash Particle Size Number Distribution Figure A.8. Engine Ca Ash Particle Size Volume Distribution 100 Figure A.9. Engine Mg Ash Particle Size Number Distribution VOWME-WF DIFFERENTIAL DISTRIBUTION Re. % 5.0 4.5 - 4.0 3-5 3.0 2.5 2.0 1.0 0.0 0.5 1 2 5 10 20 50 100 200 500 Dim,(um)-11 Figure A.10. Engine Mg Ash Particle Size Volume Distribution 101 NUMBER-WT DIFFERENTIAL DISTRBUTION Rel. % 9.0 8.0 2.0 3.0 _ _ _ __ _ -__ 2.0 0.0 1 2 5 10 20 60 100 20 50 Diam.(um)-. Figure A.11. Field CJ-4 Ash Particle Size Number Distribution VOLUME.WI DIFFERENTIAL DISTRIBUTION Reo % 7.0 -- - 5.0 - -- 4.0-- 2.0 - - - ___ 1.0 0.0 0.5 1 2 a 10 20 50 100 200 600 Dim.(um)-' Figure A.12. Field CJ-4 Ash Particle Size Volume Distribution 102