Document 10519608
advertisement

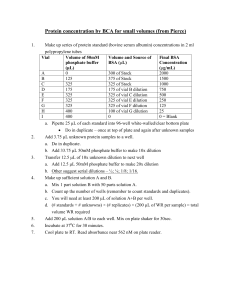
Drexel-SDP GK-12 ACTIVITY Subject Areas: Chemistry, Physical Science, Numbers & Operations, Problem Solving Associated Unit: Serial Dilution – the Solution to Pollution? Activity Title: ACTIVITY I: Serial Dilution of Food Coloring Dye Water-soluble food dye dissolving in water © 2008 Matthew D. Cathell Grade Level: 6 (5–8) Time Required: 20–30 minutes Group Size: 4-5 students Expendable Cost per Group: < $1 (food dye is the only consumable which must be purchased, all other materials should be available for free or commonly found in a classroom science kit). Summary: In this activity, students use dyes to explore the serial dilution, an important technique in physical science and engineering. Students will systematically dilute solutions of food coloring with pure water. They will observe how the color intensity, or saturation, of each subsequent solution changes. They will also keep a running calculation of the concentration of drops per mL water. Engineering Connection: Engineers and scientists must often adjust the concentration of chemical solutions. For instance, an engineer might have a solution of 5 grams of polymer dissolved in 1 liter of solvent, but might need a solutions with concentrations of 1 g/L, 0.1 g/L, and 0.01 g/L. Using a technique called serial dilution, the engineer can easily and accurately produce these lower concentration solutions in a step-by-step fashion. Biomedical engineers use this technique when they have suspensions of cells (collections of cells floating in water or other liquids). By serially diluting these cell suspensions, engineers reduce the number of cells floating per unit volume of liquid. Keywords: concentration, dilution, solutions, solute, solvent Educational Standards: Science: Math: Learning Objectives: After this lesson, students should be able to: • explain the purpose of serial dilution • use serial dilution to make solutions of various concentrations from an initial solution Materials List: Each group needs: • 2 larger plastic or glass container (~500 mL in volume) • 4–8 smaller clear plastic or glass vials (15–50 mL in volume) • pipette • graduated cylinder 2 • water To share with the entire class: • food coloring dye Introduction / Motivation: What if you needed to make a solution with a concentration 1 drop of a medicine per 999 drops of water? Any less concentrated, and the medicine will do no good – but any more concentrated, and the medicine will be poisonous! You could start counting those 1,000 drops (1 drop medicine and 999 drops water), but that would take quite awhile! What happens if you only need a small amount of solution (say, 10 drops total) at that concentration? How in the world are you ever going to measure out a fraction of a drop??? Today we’re going to learn a technique called serial dilution. Biologists, chemists, and engineers use this technique all the time to quickly and accurately make dilute solutions from more concentrated ones. It’s a simple step-by-step process! When we investigate water pollution, an important thing to consider is how much water a chemical pollutant is dissolved in. A thimble full of mercury in an ocean of water is not a high level of pollution, but the same amount of mercury in a small pond is a cause for real concern. That same thimble of mercury in a glass of drinking water would probably be fatal! It’s all a question of how concentration – how dilute is the “pollution solution?” In this activity, we’ll be diluting a non-toxic food dye (the same stuff we use for cake frosting and Easter eggs). After diluting by the same dilution factor several times (a serial dilution), the dye will seem to disappear. But is it still there??? Vocabulary / Definitions: Word concentration dilution dilution factor Definition The amount of a substance (solute) dissolved in a liquid (solvent) in a chemical solution The process of reducing the concentration of unit solute per unit solvent in a solution The total number of units volume in which your sample will be dissolved. For instance, a dilution factor of 10 means we have combined 1 unit of our sample with 9 units of solvent 3 saturation (of color) (1+9=10). The intensity of a color (how much it differs from white). As color saturation decreases, the color itself doesn’t change hues (orange doesn’t change to green), but its level of intensity goes down. Procedure: Before the Activity: Review the basics of solutions (solutes dissolve in solvents to become solutions). With the Students: 1. Divide the students into groups. Each group should get 2 large container to hold pure water (and the rinse water) and 4–8 smaller containers for their solutions. 2. Each group should obtain several drops of food coloring dye, which should be placed in the smaller clear vials. They should record how many drops they used. 3. Then, they will use their graduated cylinder to measure 10 mL of pure water to place in the container with the food coloring. They can gently swirl the vial to help the dye dissolve in the liquid. 4. The volume in the vial will be approximately 10 mL (plus the few drops of dye which don’t really contribute much to the volume). Students should record the concentration. For instance, if they dissolved 5 drops of dye in the 10 mL volume, they will record the concentration as 5 drops/10 mL (which is the same as saying 0.5 drop/mL). Students should also record the color they see. 4 5. Next, the students can use a pipette to remove 1 mL of solution from vial #1 and will place it in vial #2. After rinsing the graduated cylinder, students should measure 9 mL of pure water and also place it in vial #2. They have just diluted the first solution by a dilution factor of ten (1 part dyed water to 9 parts pure water). 6. After gently swirling vial #2, students should observe the color of the liquid in vial #2 and compare it with the liquid in vial #1. They may notice a slight decrease in color intensity in the vial #2 (or they may not, depending upon how much dye the initially used). 7. At this point, the students should calculate the concentration of their new solution in vial #1. If their initial concentration was 0.5 drops/mL, their new concentration would be (0.5 drops/mL) × (1/10) = 0.05 drops/mL. 8. Have the students continue with the serial dilution process. Each time they make a new solution, they will take 1 mL from the vial immediately proceeding the current vial (for instance, vial #3 will have 1 mL of solution from vial #2, not from vial #1). They will add 9 mL of pure water. Each serial dilution will reduce the concentration by a factor of 10. Have them continue to record a concentration and color comparison for each solution. 5 9. With each serial dilution, the concentration of dye goes down by a factor of 10. By the time the students get to vial #5, students who began with 5 drops of food coloring in vial #1 should be calculating a diluted concentration of 0.00005 drops/mL. 10. Hopefully, by the time the students run out of vials, the dye will be so diluted that the solution will no longer have a visible color. If the students have made solutions in all their vials and still can perceive a visible color, they can empty out previous vials and continue the serial dilution process. For instance, if students have 5 total vials, they can empty vials #1–4, rinse them, and continue diluting, starting with the solution in vial #5. 11. When the students finally arrive at a solution that appears completely without color, they should note the concentration. At this low concentration level, the dye is no longer present in quantities large enough for their human eyes to see. Troubleshooting Tips: • If students are unable to get colorless solutions in a reasonable amount of time, they likely began with too many drops of dye. Rinse all vials and begin again with few drops. • In order to most accurately perform serial dilution, the pipette and graduated cylinder should be rinsed after each dilution cycle. Investigating Questions: 1. When the solution is so dilute that it appears colorless, does this mean there is no longer any dye present in the water? Answer: No. Just because we can no longer see a substance with our eyes doesn’t mean it isn’t there, in some small quantity. We will explore this idea in Activity II of this module. 2. What if we had diluted each solution by a factor of 2, rather than 10? How many mL of dye solution and how many mL of water would we combine in each vial? Answer: If we dilute by a factor of 2, we are cutting the concentration in half with each dilution cycle. Therefore, we would take 5 mL of the previous dye solution and mix it with 5 mL of pure water (a 1:1 or ½ to ½ solution). Assessment: 6 1. Ask students to recall one example (or make up one of their own!) of an instance when you they might serial dilution. What is its purpose? 2. a.) If you have a solution of 1 g of sugar dissolved in 1 L of water, how many grams of sugar are contained in each mL of water? Answer: b.) If you have a solution of 1 g of sugar dissolved in 1 L of water, how would you dilute the solution so that each mL contained 0.0005 g of sugar, with a total final volume of 1 L? Answer: We have already calculated that 1 g/L is equal to 0.001 g/mL. We want to get a concentration that is half that (0.001 ÷ 2 = 0.0005). So we need to dilute the solution by a dilution factor of 2. Activity Extensions: To make the activity more challenging, try changing the dilution factor from 10 to some other number. For instance, if the dilution factor is 3, students will combine 1 part dye solution with 2 parts pure water in each cycle. A dilution factor of 10 makes it easy to calculate each concentration (the decimal place simply moves one place to the left each cycle). Other dilution factors will require the students to use more mathematics. Author: Matthew D. Cathell Owner: Drexel University GK-12 program, Engineering as a Contextual Vehicle for Science and Mathematics Education, supported in part by National Science Foundation Award No. DGE-0538476 Copyright: 2008 Drexel University GK-12 program 7