PHY4604–Introduction to Quantum Mechanics Fall 2004 Practice Exam Dec. 9, 2004
advertisement
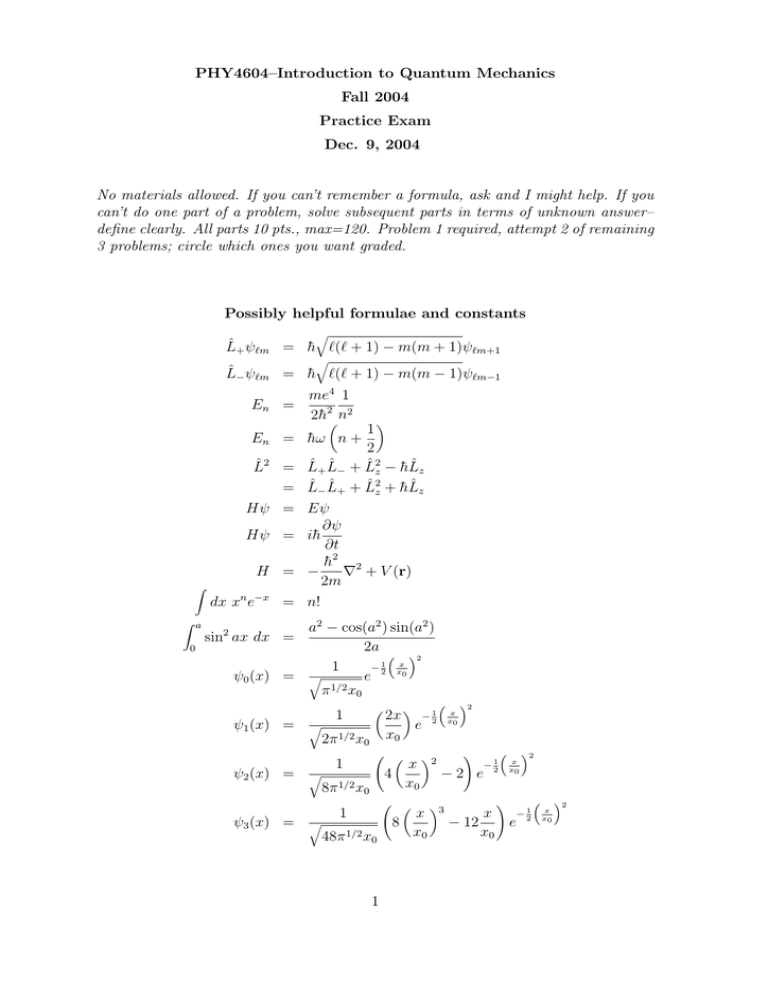
PHY4604–Introduction to Quantum Mechanics Fall 2004 Practice Exam Dec. 9, 2004 No materials allowed. If you can’t remember a formula, ask and I might help. If you can’t do one part of a problem, solve subsequent parts in terms of unknown answer– define clearly. All parts 10 pts., max=120. Problem 1 required, attempt 2 of remaining 3 problems; circle which ones you want graded. Possibly helpful formulae and constants q L̂+ ψ`m = h̄ `(` + 1) − m(m + 1)ψ`m+1 q L̂− ψ`m = h̄ `(` + 1) − m(m − 1)ψ`m−1 En = me4 1 2h̄2µn2 ¶ 1 2 L̂+ L̂− + L̂2z − h̄L̂z L̂− L̂+ + L̂2z + h̄L̂z Eψ ∂ψ ih̄ ∂t h̄2 2 − ∇ + V (r) 2m En = h̄ω n + L̂2 = = Hψ = Hψ = H = Z dx xn e−x = n! Z a 0 a2 − cos(a2 ) sin(a2 ) 2a ³ ´2 − 12 xx 1 0 ψ0 (x) = q e 1/2 π x0 sin2 ax dx = ψ1 (x) = q 1 2π 1/2 x0 ψ2 (x) = q 1 µ Ã µ x 4 x0 8π 1/2 x0 Ã µ 1 ψ3 (x) = q ¶ 2x − 12 e x0 48π 1/2 x0 1 ³ x x0 ! ¶2 x 8 x0 ´2 ³ − 12 −2 e ¶3 ! x x0 ´2 −1 x − 12 e 2 x0 ³ x x0 ´2 1. Short answer. Must attempt (only) 4 of 6. (a) Explain what is meant by a 2p state of an atomic electron. (b) What is the degeneracy of the 1st excited state (E = (5/2)h̄ω) of the isotropic 3D simple harmonic oscillator? (c) Sketch the first 3 eigenfunctions of the 1D infinite square well with V = 0 for −a ≤ x ≤ a and V = ∞ otherwise. Label them according to their parity. 2 (d) State whether the following operators are self-adjoint, anti-self-adjoint, unitary, or “none of the above” and why. i. L̂x ii. xp̂x iii. d dx iv. a + a† (a, a† are raising & lowering operators for the 1D simple harmonic oscillator.) (e) If a particle is in the state |ψi, and |ni is the nth eigenvector of Q̂ corresponding to eigenvalue qn , what is the probability of measuring q3 ? (f) Identify: i. ii. iii. iv. Photoelectric effect Davisson-Germer effect Ehrenfest theorem Stefan-Boltzmann law 3 2. Hydrogenic orbitals. An electron moving in the Coulomb field of a proton is in a state described by the wave function (ignoring spin) 1 Ψ(r, θ, φ) = √ [ψ100 (r) + 3ψ311 (r, θ, φ)] 10 (1) (a) What is the expectation value of the energy? (b) What is the expectation value of L̂2 ? (c) Is the wavefunction an eigenstate of parity? Yes or no? Explain either answer. (d) What is the expectation value of the operator 4 ∂ ∂φ in this state? 3. Electron in hydrogenic state. The electron in a hydrogen atom occupies a state s 1 0 ψ = R21 (r) Y + 3 1 s 2 1 Y 3 1 (2) where s R21 (r) = s Y10 = 3 cos θ 4π µ 1 1 3 2a0 ¶3/2 µ Y11 = − , s ¶ r e−r/2a0 a0 3 sin θeiφ 8π , (3) (4) (a) What value(s) could a measurement of the z-component of the orbital angular momentum, L̂z yield, and what is the probability of each? What is the expectation value of L̂z in this state? (b) Calculate the average distance of the electron from the nucleus in this state. (c) What is the expectation value of L̂x in this state? (d) If you measured the z-component of the angular momentum and the distance of the electron from the origin r simultaneously, what is the probability density for finding L̂z with eigenvalue zero at a distance r? 5 4. Scattering potential. For a 1D potential as shown in the figure and E < V0 , assuming the particle is incident from the left, (a) write down the Schrödinger equation and its general solution in the regions I,II, and III. (b) Write down the matching conditions at the boundaries x = a, b. (c) Do not solve for all coefficients, but reduce the problem to a single equation determining the eigenvalues. (d) Sketch the probability of finding the electron in all three regions. 6






