Cell Therapy Process and Cost Model Simulations
Defining Process Improvement Strategy
Matthieu Egloff, ATMI (megloff@atmi.com) Guillaume De Viron, NORIVED
Addressing Cost of Goods
According to Dawn Driscoll1 and ISCT experts, defining a good business model for cell therapy cannot be brought to fruition without taking into consideration every relevant aspect of a product
lifecycle. Success is not only built on therapeutic effects, it is generated by dealing with each and all innovation aspects. Being able to reach the last clinical phases requires investment. Healthcare
agencies are increasingly looking for cost-effective solutions. Besides, regulatory approval and sale license are not necessarily the key to commercial success. Well-defined strategies for reimbursement, prices and commercialization are indispensable. The economic aspects of a product need to be addressed from the very beginning – i.e., the early phase of development – to enable
a viable lifecycle.
Cell Therapy Production: Challenges
Efficient manufacturing strategies are the key to tackle commercialization challenges. Within the framework of cell therapy
products, we can easily claim that the Product is the Process.
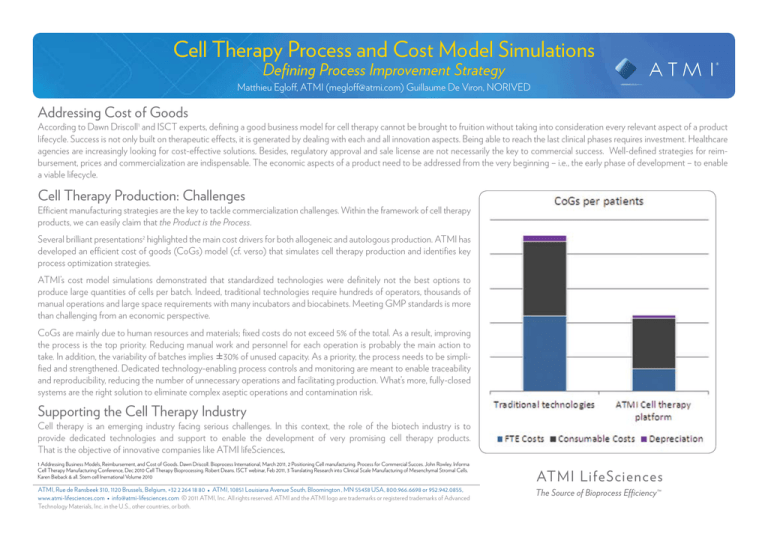
Several brilliant presentations2 highlighted the main cost drivers for both allogeneic and autologous production. ATMI has
developed an efficient cost of goods (CoGs) model (cf. verso) that simulates cell therapy production and identifies key
process optimization strategies.
ATMI’s cost model simulations demonstrated that standardized technologies were definitely not the best options to
produce large quantities of cells per batch. Indeed, traditional technologies require hundreds of operators, thousands of
manual operations and large space requirements with many incubators and biocabinets. Meeting GMP standards is more
than challenging from an economic perspective.
CoGs are mainly due to human resources and materials; fixed costs do not exceed 5% of the total. As a result, improving
the process is the top priority. Reducing manual work and personnel for each operation is probably the main action to
take. In addition, the variability of batches implies ±30% of unused capacity. As a priority, the process needs to be simplified and strengthened. Dedicated technology-enabling process controls and monitoring are meant to enable traceability
and reproducibility, reducing the number of unnecessary operations and facilitating production. What’s more, fully-closed
systems are the right solution to eliminate complex aseptic operations and contamination risk.
Supporting the Cell Therapy Industry
Cell therapy is an emerging industry facing serious challenges. In this context, the role of the biotech industry is to
provide dedicated technologies and support to enable the development of very promising cell therapy products.
That is the objective of innovative companies like ATMI lifeSciences.
1 Addressing Business Models, Reimbursement, and Cost of Goods. Dawn Driscoll. Bioprocess International, March 2011, 2 Positioning Cell manufacturing. Process for Commercial Succes. John Rowley. Informa
Cell Therapy Manufacturing Conference, Dec 2010 Cell Therapy Bioprocessing. Robert Deans. ISCT webinar, Feb 2011, 3 Translating Research into Clinical Scale Manufacturing of Mesenchymal Stromal Cells.
Karen Bieback & all. Stem cell Inernational Volume 2010
ATMI, Rue de Ransbeek 310, 1120 Brussels, Belgium, +32 2 264 18 80 • ATMI, 10851 Louisiana Avenue South, Bloomington , MN 55438 USA, 800.966.6698 or 952.942.0855,
www.atmi-lifesciences.com • info@atmi-lifesciences.com © 2011 ATMI, Inc. All rights reserved. ATMI and the ATMI logo are trademarks or registered trademarks of Advanced
Technology Materials, Inc. in the U.S., other countries, or both.
ATMI LifeSciences
The Source of Bioprocess Efficiency™
SimStem™: Cost Model For Cell Therapy Production
The SimStem cost model is based on a bottom-up simulation. The model enables
to integrate the variability of each batch. On the basis of a specific process and key
variables – e.g., cell doubling time – the model is able to calculate the right production capacity. Such method is particulary adequate for autologous therapy as each
batch can be different.
Based on these inputs, the model determines for each batch:
The number of operations
The duration of culture
The required capacities (FTE, material, consumables)
By compiling the results of each batch, the program simulates the total capacity
required per day.
Resources required per year are then calculated to guarantee optimal production.
CASE STUDY
Based on Karen Bieback3 article, a standard autologous cell therapy production has been simulated: 1 billion cells per batch—3000 patients per year. CoGs have been calculated
for a standard process: multitray stacks technologies vs. ATMI’s technological platform.








