Chemistry 222 Name__________________________________________ Fall 2015 Exam 2: Chapters 5,6,7
advertisement

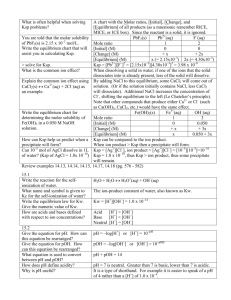
Chemistry 222
Fall 2015
Exam 2: Chapters 5,6,7
80 Points
Name__________________________________________
Complete two (2) of problems 1-3, problem 4, and three (3) of problems 5-8. CLEARLY mark the
problems you do not want graded. You must show your work to receive credit for problems
requiring math. Report your answers with the appropriate number of significant figures.
Do two of problems 1-3. Clearly mark the problem you do not want graded. (10 pts each)
1. A real solution at equilibrium will likely contain several equilibria. The statement “all
equilibrium conditions must be satisfied simultaneously” is often used in describing
these systems. Clearly describe the chemical significance of this statement, especially as it
pertains to an ion or molecule that appears in more than one equilibrium in the same solution.
There can only be one concentration of each species in solution. To reach equilibrium, all
equilibria must adjust to this single equilibrium concentration. The concentration of each
species must satisfy the equilibrium constant expressions for all equilibria. For example, if
ammonium ion is present in three equilibria in a system, the concentration of ammonium that
satisfies the first equilibrium must also satisfy the second and third equilibria.
2. Three primary factors that play a role in determining activity coefficients of ions. Identify each
and briefly describe the impact of each factor on the activity of an ion.
Each affect has an impact on the tendency for an ion to interact with other charged species in
solution. Your description should illustrate this.
1. Ion Size or Hydrated diameter (): The more strongly solvated the ion is (larger ), the
less likely it is to interact with competing ions in solution (larger ).
2. Ionic charge (z): The larger the charge, the greater the electrostatic interaction with
competing ions (smaller ).
3. Ionic strength (): The greater the effective concentration of ions in solution, the more
opportunities for the ion of interest to interact with competing species (smaller ).
3. If I prepare a saturated silver chloride (Ksp = 1.8 x 10-10) solution by putting 100 g of AgCl in
10 mL of water and you prepare a saturated silver chloride solution by putting 100 g of AgCl
in 100 mL of water, what is the relative concentration of Ag+ in your solution compared to
mine? Clearly explain your reasoning.
Since both solutions are saturated, the silver ion concentrations are identical. It doesn’t
matter what mass is introduced into the solvent, once the solution is saturated, no additional
solute can dissolve.
[Ag+] = (Ksp)1/2
You MUST do problem 4. (15 points)
4. Consider a 0.010 M silver nitrate solution that is saturated with silver carbonate AND silver
chloride. Set up the equations necessary to determine the solubility of silver carbonate,
considering the equilibria below. You must write the charge balance expression and at least
one mass balance. Identify all unknowns and write enough explicit, independent mass
balance, charge balance, and equilibrium expressions so that only algebra remains to solve
for the unknowns. A numerical answer is not necessary.
Ag2CO3 Ksp = 8.1 x 10-12
AgCl
Ksp = 1.8 x 10-10
H2CO3 Ka1 = 4.46 x 10-7, Ka2 = 4.69 x 10-11
H2O
Kw = 1.0 x 10-14
8 unknowns, need 8 equations (unknowns are in BOLD and UNDERLINED)
AgCl (s) = Ag+ + ClAg2CO3 (s) = 2 Ag+ + CO32H2CO3 = HCO3- + H+
HCO3- = CO32- + H+
H2O = H+ + OH-
Ksp = [Ag+][Cl-]3
Ksp = [Ag+]2[CO32-]
Ka1 = [HCO3-][H+]/[H2CO3]
Ka2 = [CO32-][H+]/[HCO3-]
Kw = [H+][OH-]
Charge Balance:
[Ag+] + [H+] = [OH-] + [HCO3-] + 2[CO32-] + [Cl-] + [NO3-]
Mass Balance:
[NO3-] = 0.010 M
[Ag]total = 2[CO3]total + [Cl]total + [NO3-]
[Ag ] = 2([H2CO3] + [HCO3-] + [CO32-]) + [Cl-] + 0.010M
+
Do three of problems 5-8. Clearly mark the problem you do not want graded. (15 pts each)
5. Clearly describe the case when it is preferable to use calibration by standard additions, rather
than a traditional calibration curve for an analysis. Include an example of how you would run
the experiment and extract an unknown concentration from your data.
.
Calibration by standard additions is appropriate when the sample composition is unknown or
complex and affects the analytical signal. This matrix effect makes it difficult to prepare
reliable standards.
Here is one procedure for using standard additions:
1. Prepare several solutions, each "spiked" with a different (and known) concentration of
analyte (including "0").
2. Perform analysis using each solution
3. Plot signal vs. added analyte concentration
4. Calculate least-squares line
5. Extrapolate the line to the x-intercept. The unknown concentration in the measured
solution corresponds to the value at the x-intercept.
6. Account for dilutions to back-calculate the original composition of the unknown solution.
It is also possible to run standard additions with only two samples, an adequate description if
this alternate procedure was also acceptable.
6. Using activities, calculate the fluoride concentration in a saturated solution of calcium fluoride
in a solution that contains 0.010 F magnesium nitrate and 0.020 F sodium chloride. The Ksp
for calcium fluoride is 3.2 x 10-11, assume that all other salts are soluble. You may ignore the
autoprotolysis of water and any acid-base character of the ions in solution.
I
C
E
CaF2 =
----
Ca 2+
0
+x
x
+
2F0
+2x
2x
Ksp = ACa2+(AF-)2 = Ca2+[Ca2+](F-[F-])2 = CA2+x(f-2x)2 = Ca2+(F-)24x3
Since Ksp is so small, little dissolution of CaF2 will occur, and the ionic strength will be
determined by the concentrations of Mg(NO3)2 and NaCl.
= ½{[Mg2+](+2)2 + [NO3-](-1)2 + [Na+](+1)2 + [Cl-](-1)2 } ½
= ½ (0.010M(4) + 0.020 M(1) + 0.020 M(1) + 0.020 M(1)) = 0.050 M
Using the table of activity coefficients at this ionic strength, Ca2+ = 0.485, F- = 0.81.
(The Debye-Huckel equation gives similar values.)
Therefore, the expression to solve is: 3.2 x 10-11= (0.485)(0.81)24x3
Given these values, and solving for x, x = 2.93 x 10-4 M, [F-] = 2x = 5.86 x 10-4 M
7. What is the silver ion concentration in a solution prepared by mixing 50.0 mL of 0.496 M
silver nitrate with 50.0 mL of 0.387 M sodium carbonate? The Ksp of silver carbonate is
8.1 × 10-12. You may ignore autoprotolysis and the acid-base behavior of carbonate ion.
Ag2CO3(s) = 2Ag+ + CO32First find the composition of the solution after Na2CO3 and AgNO3 have had the opportunity
to react to form some Ag2CO3. What is the limiting reagent?
50.0 mL x 0.496 mol AgNO3 x 1 mol CO32- = 12.4 mmol CO32- needed
1L
2 mol AgNO3
50.0 mL x 0.387 mol Na2CO3 x
1 mol CO32= 19.35 mmol CO32- present
1L
1 mol Na2CO3
So, Ag is the limiting reagent and (19.35 – 12.4) = 6.95 mmol CO32- will remain after reaction,
producing a carbonate concentration of 6.95 mmol/100. mL = 0.0695 M. Now ICE table!
Ag2CO3
=
2Ag+
+
CO32i
0
0.0695 M
c
+2x
+x
e
2x
0.0695 + x
Ksp = [Ag+]2[CO32-] = (2x)2(0.0695 + x)
We can simplify things if we assume x<<0.0695, then Ksp = (2x)2(0.0695)
1/2
Ksp
= 5.40 x 10-6 M
(0.0695)
[Ag+]=2x =1.08 x 10-5 M
Checking our assumption, to 3 sig figs, 0.000108<0.0695!
8. In an instrumental method for the determination of mercury in a sample, a calibration curve
was prepared to relate the mercury signal (in Volts) to mercury concentration (in ppm).
Least-squares analysis of the data resulted in the relationship:
Signal = (0.0556 V/ppm)(concentration) + 0.0001 V.
Five blank measurements gave the following results: 0.0003 V, 0.0002 V, 0.0002V,
0.0001 V, 0.0000 V. What is the detection limit for this method?
x
=
To find the detection limit, we need to find the concentration that gives a signal 3 times the
standard deviation of the blank signal (sblank) above the average blank signal (yblank).
From the data, yblank = 0.00016 V and sblank = 0.00011
So, the signal at the detection limit is yblank + 3sblank = 0.00016V +3(0.00011V) = 0.00050 V
Now we use the calibration curve to convert this signal into a concentration:
Concentration = (signal – 0.0001V)/(0.0556V/ppm) = (0.0005V – 0.0001V)/(0.0556 V/ppm)
Crunching the numbers, we find the detection limit is 0.0072 ppm.
Blank Space if You Need Extra Room
Possibly Useful Information
KaKb = KW = 1.0 x 10-14
pH = -log [H+]
y = mx + b
SLOD = Sblank + 3sblank
log
0.51z 2
1 305
G = H -TS = -RTlnK
Ix
Is x
(with in pm)
x i
kx i
k sf x f sf x f
x
1
2
c i zi
2 i
b b 2 4ac
2a
Analyte Signal
Standard Signal
F
Analyte Concentration
Standard
Concentrat
ion