Homework E PHY4523 Due: April 21, 2010
advertisement
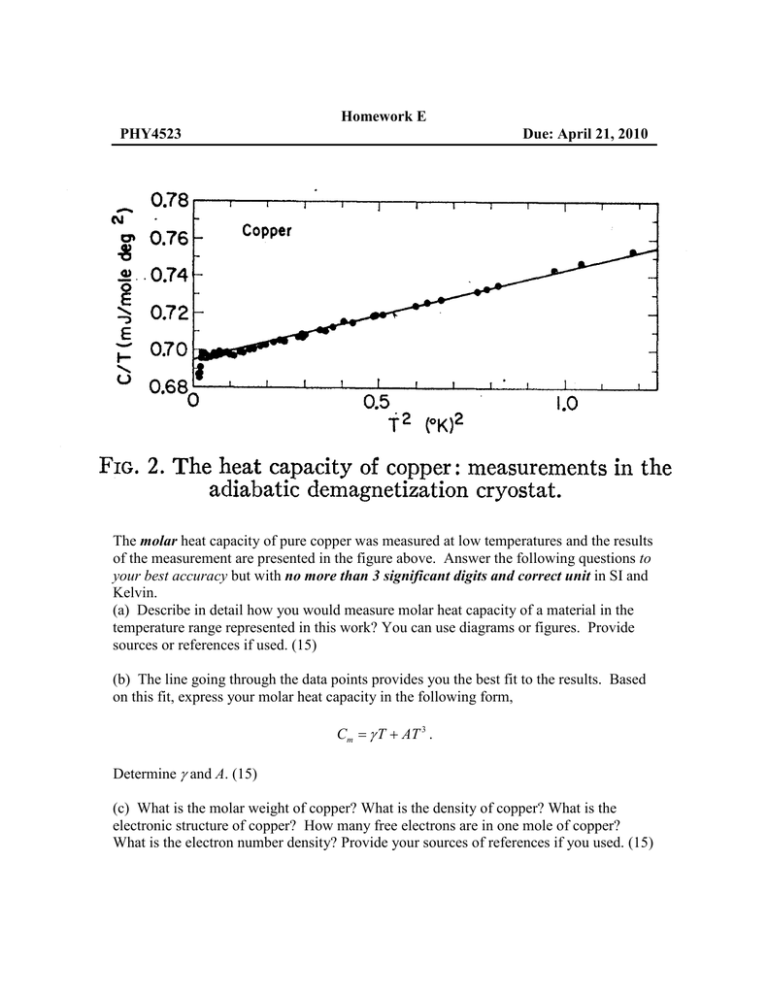
Homework E PHY4523 Due: April 21, 2010 The molar heat capacity of pure copper was measured at low temperatures and the results of the measurement are presented in the figure above. Answer the following questions to your best accuracy but with no more than 3 significant digits and correct unit in SI and Kelvin. (a) Describe in detail how you would measure molar heat capacity of a material in the temperature range represented in this work? You can use diagrams or figures. Provide sources or references if used. (15) (b) The line going through the data points provides you the best fit to the results. Based on this fit, express your molar heat capacity in the following form, Cm = γ T + AT 3 . Determine γ and A. (15) (c) What is the molar weight of copper? What is the density of copper? What is the electronic structure of copper? How many free electrons are in one mole of copper? What is the electron number density? Provide your sources of references if you used. (15) (d) Based on your numbers in (c) and the electron mass, calculate the Fermi temperature (TF), energy (EF), and wavenumber (kF) of copper. (10) (e) Identifying the first term of the heat capacity is the contribution from free electrons as a Fermi gas, calculate the Fermi energy from the experiment. Is this consistent with the value you got in (d)? (15) (f) Since kF depends on the electron number density only, we need a different value for the electron mass to explain the experimentally determined Fermi energy. What should be the effective mass of electron in copper? Why is it different from the bare electron mass? (10) (g) Identifying the second term of the heat capacity comes from lattice vibration, estimate the Debye temperature and sound velocity in copper. (10) (h) Calculate the molar entropy of copper at 1 K from the heat capacity measurement. (10)