MAMMALS OF MISSISSIPPI 5:1–8 Big brown bat ALISHA A. WORKMAN
advertisement

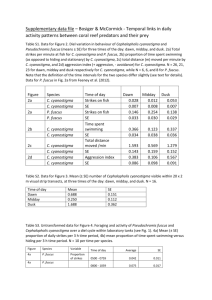
MAMMALS OF MISSISSIPPI 5:1–8 Big brown bat (Eptesicus fuscus) ALISHA A. WORKMAN Department of Wildlife and Fisheries, Mississippi State University, Mississippi State, Mississippi, 39762, USA Abstract—Eptesicus fuscus (Beauvois, 1796) is a vespertilionine commonly called the big brown bat. It is sexually dimorphic with the female being slightly larger than the male. Dorsal color ranges from pinkish tan to rich chocolate and the ventral color ranges from pink to olive buff. It represents one out of twenty four in the genus Eptesicus. Eptesicus fuscus is distributed through all of the United States except Hawaii, most of Central America except the Yucatan Peninsula, and its southern limit is northwestern South America. Eptesicus fuscus generally prefers to live in caves, trees, bridges, and buildings. Eptesicus fuscus is currently not listed as a species of special concern. Published 5 December 2008 by the Department of Wildlife and Fisheries, Mississippi State University Big Brown Bat Eptesicus fuscus (Beauvois, 1796) CONTEXT AND CONTENT. Order Chiroptera, Suborder Microchiroptera, Family Vespertilionidae, Subfamily Vespertilioninae, Tribe Vespertilionini. Figure 1. Big brown bat (Eptesicus fuscus) in Ann Arbor, Michigan. Photo used with permission of the photographer Phil Myers under a Creative Commons Attribution,(http:// animaldiversity.ummz.umich.edu/site/resources/phil_ myers/classic/eptesicus_best.jpg/view.html) GENERAL CHARACTERS The big brown bat, Eptesicus fuscus (Fig. 1), is one of the most common bats in North America (Barbour and Davis 1969). The pelage color varies by geographic location, but ranges from pinkish tan to rich chocolate on the dorsal side and pink to olive buff on the ventral side. The bare-skin areas of the face, wings, and ears are black. The fur, while extremely soft, has a somewhat oily texture (Miller 1907). Figure 2. Geographic distribution of Eptesicus fuscus. Map used with permission of NatureServe, (http://www. natureserve.org/explorer/servlet/NatureServe?searchNa me=Eptesicus+fuscus). DISTRIBUTION Eptesicus fuscus occurs throughout a large portion of North and Central America and as far south as northwestern South America (Fig. 2). It also resides in Cuba, Jamaica, Puerto Rico (Hall 1981), and some of the Bahama Islands (Buden 1985). The big brown bat occurs throughout the United States except Hawaii (Hall 1981) and in Mexico except the Yucatan Peninsula (Buden 1985). Although there are 11 subspecies of E. fuscus (Neubaum et. al 2007), only E. fuscus fuscus occurs in Mississippi (Hall 1981). Figure 3. Dorsal, ventral and lateral views of the skull and a lateral view of the mandible of an adult female Eptesicus fuscus in Lexington, Massachusetts. Greatest length of the skull is 18.2 mm. Drawings by J. Love. Measurements (in mm) of E. fuscus are: total length, 87–138; length of tail vertebrae, 34–57; length of hind foot, 8–14; length of ear from notch, 10–20; length of tragus, 6–10; length of forearm, 39-54; length of third metacarpal, 43-50; length of tibia, 17-21; greatest length of skull, 15.1–23.0 (Fig.3); zygomatic breadth, 11.1–14.2; breadth of braincase, 7.5–9.6; length of maxillary toothrow, 7.0–9.8. Adults typically weigh 11-23g. This species is sexually dimorphic, with females slightly larger than males (Burnett 1983a). The big brown bat and the hoary bat (Lasiurus cinereus) are the only vespertilionids that produce an audible sound during flight. Wing and skull size is positively correlated with the amount of environmental moisture (Burnett 1983b). FORM AND FUNCTION Form.—Each June, Eptesicus fuscus molts by shedding its winter coat (Phillips 1966). The dental formula is i 2/3, c 1/1, p 1/2, m 3/3, total 32. The crowns on M1 and M2 are narrower than those of M3. The two pectoral mammae contain milk that contains 2.5% lactose, 6.2% protein, and 16.4% fat (Kunz et. al 1983). This species displays pararhinal glands on the sides of the nose that consist of apocrine tubules (for producing sweat), which set atop sebaceous units (Dapson et. al 1977). The red blood cell count is 11.96 x 106/mL (Dunaway and Lewis 1965). The bones that make up the right and left appendages weigh the same, which indicates that E. fuscus is ambidextrous (Dawson 1975). Polydactyly, deformed vertebrae, and underdeveloped radii are a few of the skeletal anomalies that can occur in this species (Kunz and Chase 1983). Over time, this species has lost the ceratohyal of the hyoid (Griffiths 1983). The baculum is about 0.8 mm long (Hamilton 1949). E. fuscus will defecate within 90–130 minutes of eating. It will completely pass a meal within 24 hours of eating (Luckens et. al 1971). Function.—The big brown bat has a large heart that can represent 0.9% of its fat-free body mass. The resting heart rate is about 450 beats/min. During flight, the heart rate more than doubles to about 1,022 beats/min (Studier and Howell 1969). During torpor, the heart rate dramatically drops between 4–62 beats/ min. After arousal from hibernation, the heart rate increases from 12–800 beats/min (Rauch 1973). Rhodopsin and an unidentified molecule are the two photopigments in the retina (Kurta and Baker 1990). A few ocular abnormalities are known to occur, such as an unpigmented choroid, undifferentiated retina, and underdeveloped lenses (Kunz and Chase 1983). When the ambient temperature is <30ºC, the bat will go into a torpid state. During torpor, the body temperature is between 32 and 36 C (Herreid and Schmidt-Nielsen 1966). If overheating occurs, E. fuscus is able to lower its core body temperature by dilating blood vessels that lead to the wings (Kluger and Heath 1970). Some bats in this species have been observed panting and spreading saliva on their bodies when the ambient temperature exceeds 40ºC (Kurta and Baker 1990). Eptesicus fuscus uses echolocation by sending out sonar signals to detect prey and avoid obstacles. Echolocation is split into three phases: search, approach, and terminal. When the bat begins hunting it sends out narrow signals at a low rate of 2-5 Hz. Once it detects something of interest, it begins the approach phase where signals increase progressively from 5–10 to 30 Hz as the bat moves closer to the target. During the terminal phase, the bat is almost to its target and emits signals between 100–150 Hz. The sounds in this phase can be heard on a bat detector and are commonly called the “terminal buzz.” The bat decodes this acoustic information to determine how close the prey is (Sanderson and Simmons 2005). ONTOGENY AND REPRODUCTION Eptesicus fuscus copulates between September and March (Phillips 1966). However, females delay ovulation and fertilization until arousal from hibernation occurs (Kurta and Baker 1990). Males are sexually mature by the end of their first autumn (Kurta and Baker 1990). During early stages of pregnancy, females accumulate extra fat. During pregnancy, females require about 48.9 kJ of assimilated energy/day to sustain her and her developing young. During the 32–40 lactation period, females need 105.1 kJ/day (Kurta and Baker 1990). Female big brown bats usually give birth to one pup in the western United States and two pups in the eastern United States and in Cuba (Barbour and Davis 1969). One theory on why this occurs is that several biogeographic events resulted in regional separation of ancestral bats. This separation led to several different mitochondrial DNA (mtDNA) lineages, which likely resulted in the observed regional difference in litter sizes (Neubaum et al. 2007). Females can release up to five eggs/ovary (Birney and Baird 1985). Gestation lasts for two months. Young are born from May to July, with earlier births occurring in lower latitudes (Barbour and Davis 1969). The newborns are altricial at birth and fledge at 18–35 days (Gould 1971). The fledgling’s body mass is about 75% of adult body mass (Burnett and Kunz 1982). ECOLOGY Population characteristics.—Eptesicus fuscus abundance decreases from the deciduous forest biome to the coniferous forest biome (Kurta and Baker 1990). Big brown bats often live >10 years; the oldest recorded age is 19 years (Paradiso and Greenhall 1967). Males live longer than females. Males occur at higher elevations in mountainous regions than females (Kurta and Baker 1990). Postnatal mortality before weaning is 7–10%. In adults, common mortality factors are predation, failure to store enough fat for hibernation, accidents, and inclement weather. Space use.—Bat-habitat relationships can be very complicated due to their high mobility. Therefore, studies have failed to quantify habitat requirements of Eptesicus fuscus. However, this species occurs in both urban and rural areas. Night roosts are frequently in locations that allow it to forage and engage in social interactions. Big brown bats frequently use buildings as roosts, often near a light where insect densities are likely higher. They will also roost in caves, tunnels, mines, tree cavities, rock crevices, and under bridges (Agosta, 2002). They seldom move farther than 80 km between their summer and winter roosts (Kurta and Baker 1990). Diet.—Eptesicus fuscus is insectivorous (Agosta 2002). Big brown bats are also generalists and do not have a preference for over-water versus over-land foraging sites. They begin foraging within an hour after sunset and spend an average of 100 min/night foraging. Prey varies by geographic location but generally consists of beetles, moths, mosquitoes and dragonflies (Kurta and Baker 1990). Diseases and parasites.—There are many ectoparasites known to affect Eptesicus fuscus including Cimex (bedbugs), Basilia (bat flies), Ornithodoros (soft ticks), Leptotrombidium (chigger mites), and Myodopsylla (bat fleas) (Kurta and Baker 1990). One species of rosensteiniid mite,Nycteriglyphus fuscus, lives in the guano of E. fuscus (Dood and Rockett 1985). To avoid ectoparasites, female big brown bats may frequently change roost sites (Agosta 2002). Many endoparasites are also known to affect E. fuscus, including nematodes from the genera Allintoshius, Cyrnea, Physocephalus, and Capillaria. Maseria vespertilionis is a nematode that only infects the subcutaneous tissue in the plantar surface of the bat’s feet. Females that roost in colonies are frequently infected whereas males are not. Big brown bats are also infected with cestodes such as Hymenolepis (tapeworms). Parasitic trematodes include Dicrocoelium (liver flukes), Limatulum, and Glyptoporus (Kurta and Baker 1990). The big brown bat is a vector for St. Louis encephalitis. A mosquito will bite an infected bat and then bite a human, which is how the virus is transmitted (Herbold et. al 1983). Histoplasma capsulatum, a fungus, is found in the big brown bat’s tissues and guano. This fungus causes histoplasmosis (Darling’s disease) in humans, cats, and dogs, which affects the lungs (Bartlett et. al 1982). Eptesicus fuscus is known to carry rabies throughout the United States (Trimarchi and Debbie 1977). However, local epizootics are rare (Kurta 1979; Pybus 1986). In this species, rabies infects the brain, brown fat, and salivary glands (Kurta and Baker 1990) but is not transmitted across the placenta (Constantine 1986). Incubation of the disease has been observed to last up to 209 days (Moore and Raymond 1970). Interspecific interactions.—Common predators include common grackles, longtailed weasels, various owl species, house cats, and bullfrogs. Interspecific competition in foraging areas is known to occur between Eptesicus fuscus and Lasionycteris noctivagans (silver-haired bat), as well as Chordeiles minor (common night hawk). However, the extent to which these interactions occur in Mississippi is not known (Reith 1980). Intraspecific competition occurs at highly aggressive levels but the significance of these events is unknown (Kurta and Baker 1990). BEHAVIOR Infant big brown bats will sound an “isolation call” that can be heard from about 10 m away when they are separated from their mothers or fall from the nest. Females respond with an ultrasonic chirping noise (Gould 1971). Eptesicus fuscus has demonstrates a 24-hr behavior rhythm that is persistent but inexact (Twente and Twente 1987). Big brown bats can use olfactory cues to distinguish among individual colony members as well as between young in a maternity colony, which may reduce agonistic interactions (Boss et. al 2002). Decreasing ambient temperature appears to trigger hibernation in big brown bats. While both the males and females will deposit fat in anticipation of hibernation, females deposit fat about one month earlier than males (Pistole 1988). Although females begin fat deposition earlier, males enter hibernation first (Phillips 1966). Big brown bats typically hibernate at ambient temperatures below freezing (Barbour and Davis 1969), using rock crevices, caves, old buildings, and mines as hibernacula (Mills et. al 1975). Most big brown bats do not hibernate in colonies, but small groups are common (Mumford 1958). Female big brown bats will usually form large maternity colonies in trees, caves, and buildings to give birth and raise their young. By doing so, thermoregulation costs go down which is important as stable temperatures are vital for proper development of young (Boss et. al 2002). They also take advantage of cooperative foraging to reduce energy spent searching for prey. Also, individual predation risks may be lower in the colonies (Willis and Brigham 2004). CONSERVATION The IUCN lists the big brown bat as a species of least concern which means it is not in danger of becoming threatened or endangered (IUCN 2007). They are, however, ‘critically imperiled’ in Louisiana. Eptesicus fuscus are important members of the ecosystems in which they live. They are primary insect consumers, which fills an important ecological niche. Also, pesticides and other man-made chemicals increasingly pose threats to E. fuscus (Agosta 2002). ACKNOWLEDGMENTS I wish to thank my professor Dr. J. Belant for his help with this project, as well as my fellow peers who served as editors. I also wish to thank the staff of the Mitchell Memorial Library and the College of Forest Resources at Mississippi State University for assistance in gaining access to valuable information and materials. LITERATURE CITED Agosta, S. J. 2002. Habitat use, diet and roost selection by the big brown bat (Eptesicus fuscus). Mammal Review 32(2):179–198. Barbour, R. W., and W. H. Davis. 1969. Bats of America. University Press Kentucky, Lexington 286 pages. Bartlett, P. C., L. A. Vonbehren, R. P. Tewari, R. J. Martin, L. Eagleton, M. J. Isaac, and P. S. Kulkarni. 1982. Bats in the belfry: an outbreak of histoplasmosis. American Journal of Public Health 72:1369–1372. Birney, E. C. and D. D. Baird. 1985. Why do some mammals polyovulate to produce a litter of two? The American Naturalist 126:136–140. Boss, J., T. E. Acree, J. M. Bloss, W. R. Hood, and T. H. Kunz. 2002. Potential use of chemical cues for colony-mate recognition in the big brown bat, Eptesicus fuscus. Journal of Chemical Ecology 28(4):819–834. Buden, D. W. 1985. Additional records of bats from the Bahama Islands. Caribbean Journal of Science 21:19–25. Burnett, C. D. 1983a. Geographic variation and sexual dimorphism in the morphology of Eptesicus fuscus. Annals of Carnegie Museum 52:139–162. Burnett, C. D. 1983b. Geographic and climate correlates of morphological variation in Eptesicus fuscus. Journal of Mammalogy 64:437–444. Burnett, C. D., and T. H. Kunz. 1982. Growth rates and age estimation in Eptesicus fuscus and comparison with Myotis lucifugus. Journal of Mammalogy 63:33–41. Constantine, D. G. 1986. Absence of prenatal infection of bats with rabies virus. Journal of Wildlife Diseases 22:249–250. Dapson, R. W., E. H. Studier, J. Buckingham, and A. L. Studier. 1977. Histology of odoriferous secretions from integumentary glands in three species of bats. Journal of Mammalogy 58: 531– 535. Dawson, D. L. 1975. Ambedexterity in bat wings as evidenced by bone weight. Journal of Anatomy 120: 289–293. Dood, S. B., and C. L. Rockett. 1985. Nycteriglyphus fuscus (Acari: Rosensteiniidae), a new species associated with big brown bats in Ohio. International Journal of Acarology 11:31– 35. Dunaway, P. B. and L. L. Lewis. 1965. Taxonomic relation of erythrocyte count, mean corpuscular volume, and body weight in mammals. Nature 205:481484. Gould, E. 1971. Studies of maternalinfant communication and development of vocalizations in the bats Myotis and Eptesicus. Communications in Behavioral Biology 5:263–313. Griffiths, T. A. 1983. Comparative laryngeal anatomy of the big brown bat, Eptesicus fuscus, and the mustached bat, Pteronotus parnellii. Mammalia 47:375– 394. Hall, E. R. 1981. The mammals of North America. Second ed. John Wiley and Sons, New York, 1:1–600. Hamilton, W. J., Jr. 1949. The bacula of some North American vespertilionid bats. Journal of Mammalogy 30:97–102. Herbold, J. R., W. P. Heuschele, R. L. Berry, and M. A. Parsons. 1983. Reservoir of St. Louis encephalitis virus in Ohio bats. American Journal of Veterinary Research 44:1889–1893. Herreid, C. F., II, and K. Schmidt-Nielsen. 1966. Oxygen consumption, temperature, and water loss in bats from different environments. American Journal of Physiology 211:1108–1112. International Union for Conservation of Nature and Natural Resources. 2007. 2007 IUCN Red list of threatened species. www.iucnredlist.org, accessed 27 September 2008. Kluger, M. J., and J. E. Heath. 1970. Vasomotion in the bat wing: a thermoregulatory response to internal heating. Comparative Biochemistry and Physiology 32:219–226. Kunz, T. H., and J. Chase. 1983. Osteological and ocular anomalies in juvenile big brown bats (Eptesicus fuscus). Canadian Journal of Zoology 61:365– 369. Kunz, T. H., M. H. Stack, and R. Jenness. 1983. A comparison of milk composition of Myotis lucifugus and Eptesicus fuscus. Biology of Reproduction 28:229–234. Kurta, A. 1979. Bat rabies in Michigan. Michigan Academician 12:221–230. Kurta, A. and R. H. Baker. 1990. Eptesicus fuscus. Mammalian Species No. 356. pp 1–10. Luckens, M. M., J. Van Eps, and W. H. Davis. 1971. Transit time of food through the digestive tract of the bat, Eptesicus fuscus. Experimental Medicine and Surgery 29:25–28. Miller, G. S., Jr. 1907. The families and genera of bats. Bulletin of the United States National Museum 57:1–282. Mills, R. S., G. W. Barrett, and M. P. Farrell. 1975. Population dynamics of the big brown bat (Eptesicus fuscus) in southwestern Ohio. Journal of Mammalogy 56:591–604. Moore, G. J., and H. Raymond. 1970. Prolonged incubation period of rabies in a naturally infected bat Eptesicus fuscus (Beauvois). The Journal of Wildlife Diseases 6:167–168. Mumford, R. E. 1958. Population turnover in wintering bats in Indiana. Journal of Mammalogy 39:253–261. Neubaum, M. A., M. R. Douglas, M. E. Douglas, and T. J. O’Shea. 2007. Molecular ecology of the big brown bat (Eptesicus fuscus): Genetic and natural history variation in a hybrid zone. Journal of Mammalogy 88(5):1230–1238. Paradiso, J. L., and A. M. Greenhall. 1967. Longevity records for American bats. The American Midland Naturalist 78:251–252. Phillips, G. L. 1966. Ecology of the big brown bat in Northeastern Kansas. The American Midland Naturalist 75:168– 198. Pistole, D. H. 1988. Sexual differences in the annual lipid cycle of the big brown bat, Eptesicus fuscus. Canadian Journal of Zoology 67:1891–1894. Pybus, M. J. 1986. Rabies in insectivorous bats of western Canada, 1979–1983. The Journal of Wildlife Diseases 22:307– 313. Rauch, J. C. 1973. Sequential changes in regional distribution of blood in Eptesicus fuscus (big brown bat) during arousal from hibernation. Canadian Journal of Zoology 51:973–981. Reith, C. C. 1980. Shifts in times of activity by Lasionycteris noctivagans. Journal of Mammalogy 61:104–108. Sanderson, M. I., and J. A. Simmons. 2005. Target representation of naturalistic echolocation sequences in single unit responses from the inferior colliculus of big brown bats. Journal of the Acoustical Society of America 118(5):3352–3361. Studier, E. H. and D. J. Howell. 1969. Heart rate of female big brown bats in flight. Journal of Mammalogy 50: 842–845. Trimarchi, C. V., and J. G. Debbie. 1977. Naturally occurring rabies virus and neutralizing antibody in two species of insectivorous bats of New York State. The Journal of Wildlife Diseases 3:366– 369. Twente, J. W., and J. Twente. 1987. Biological alarm clock arouses hibernating big brown bats, Eptesicus fuscus. Canadian Journal of Zoology 65:1668–1674. Willis, C. K., and R. M. Brigham. 2004. Roost switching, roost sharing and social cohesion: forest-dwelling big brown bats, Eptesicus fuscus, conform to the fission– fusion model. Animal Behavior 68:495– 505. Contributing editor of this account was Clinton Smith.