HCl + H + H O
advertisement
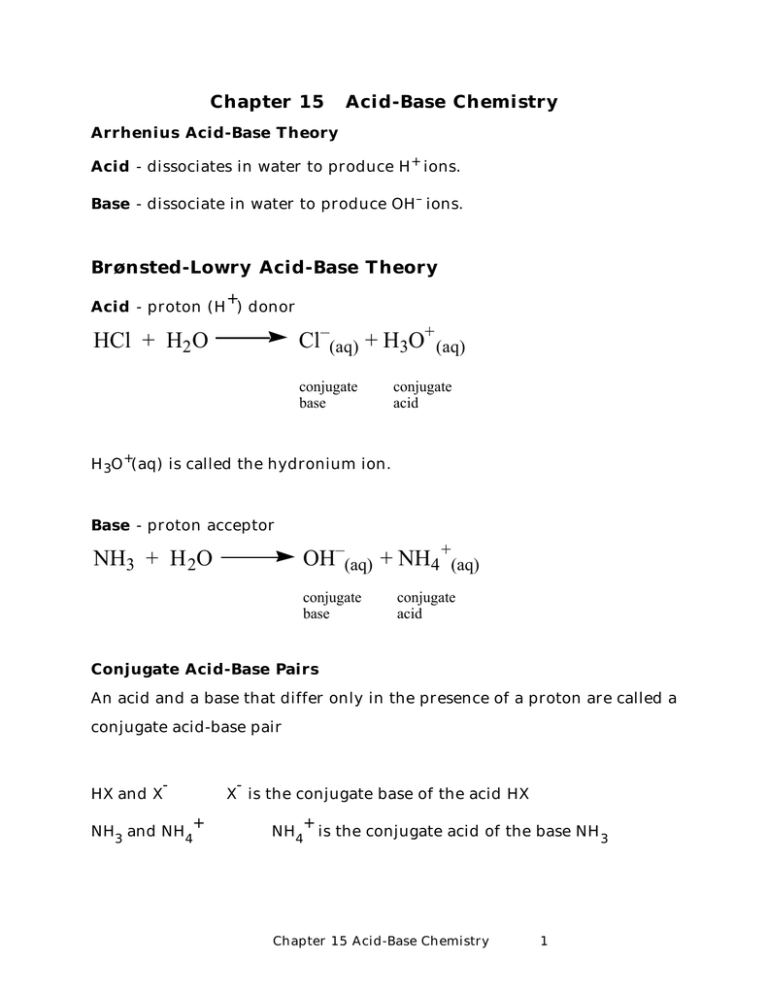
Chapter 15 Acid-Base Chemistry Arrhenius Acid-Base Theory Acid - dissociates in water to produce H+ ions. Base - dissociate in water to produce OH– ions. Brønsted-Lowry Acid-Base Theory + Acid - proton (H ) donor Cl–(aq) + H3O+ (aq) HCl + H2 O conjugate base conjugate acid H 3O +(aq) is called the hydronium ion. Base - proton acceptor OH–(aq) + NH4 +(aq) NH3 + H 2O conjugate base conjugate acid Conjugate Acid-Base Pairs An acid and a base that differ only in the presence of a proton are called a conjugate acid-base pair HX and X - + NH3 and NH4 X is the conjugate base of the acid HX + NH4 is the conjugate acid of the base NH 3 Chapter 15 Acid-Base Chemistry 1 Amphiprotic substances can behave as either acids or bases H+ + H2O acid H 3 O+ base OH– + NH4+ H2O + NH 3 acid base Autoionization of Water 2 H2 O H O + H H OH– + H3 O+ H O H O H – + O + H H [ H + ][OH − ] Kc = [ H2 O] Kc [ H2 O] = K w = [ H + ][OH − ] = 1.0 ×10 −14 at 25°C In pure water [H+] = [OH - ] = 1.0X10 –7 M [ OH − ] = [HKw+ ] other examples of ampiprotic substances: H2PO4 , HCO 3 Chapter 15 Acid-Base Chemistry 2 Measuring the Concentration of H + or OH - pH and pOH p ≡ − log [ ] pH = − log H + = log [ 1 H+ ] pOH = − log OH − = log [ ] Acidic Solutions: [H+] > 1.0X10 -7 M, pH < 7.00 Basic Solutions: [H+] < 1.0X10 -7 M, pH > 7.00 Neutral Solutions: [H +] = 1.0X10 -7 M, pH = 7.00 + [H ] = [OH ] in pure water + -14 [H ][OH ] = 1.0 X 10 x 2 = 1.0 X 10 x = 1.0 X 10 -7 -14 + = [H ] = [OH ] + pH = -log[H ] = -log 1.0 X 10 pOH = -log[OH ] = -log 1.0 X 10 + pKw = -log([H ][OH ]) = -7 -7 -log 1.0 X 10 = 7.0 = 7.0 -14 =14.0 + + Kw = [H ][OH ] → -logKw =(-log[H ]) + (-log[OH ]) * pKw = pH + pOH * 14.0 = pH + pOH pH = 14.0 - pOH pOH = 14.0 - pH Chapter 15 Acid-Base Chemistry 3 1 OH − [ ] Strong acids - completely ionize in water HClO4, H 2SO 4, HNO 3, HI, HBr, HCl HI + H2O I– + H 3O+ • Water "levels" the acids to the level of H 3O + (H 3O + is the strongest acid that can exist in aqueous solition.) • I- , Cl- , Br - , NO3- , HSO4- , ClO4- are weaker bases than H2O. Weak acids - partially dissociate, exists in solution as acid molecules and component ions. CH3COOH CH3 COO– + H+ [H + ][CH3COO − ] Ka = = 1.8× 10 −5 [CH3COOH] Ka, equilibrium constant of the acid, subscript a indicates it is an acid (K b for bases) • The smaller the Ka, the weaker the acid. 10-5 > 10-10. Polyprotic Acids - more than one acidic proton. H 2SO 4, H 3PO4, Chapter 15 Acid-Base Chemistry 4 Polyprotic weak acids have a Ka for each proton – H3PO4 – 2– H2PO4 HPO4 2– 3– HPO4 K for: + H2PO4 + H PO4 H 3PO4 Ka1 = 7.5 x 10 + +H Ka2 = 6.3 x 10 + +H Ka3 = 3.6 x 10 3 H + + PO43– -3 -8 -13 K = Ka1 X Ka2 X Ka3 Strong Base OH–(aq) + Na+(aq) NaOH + H2 O LiOH, NaOH, KOH, RbOH, CsOH, Ba(OH)2 Weak Base OH– + NH4+ NH3 + H2O [NH4 + ][OH − ] Kb = = 1.8 × 10 −5 [ NH3 ] Ethylamine, methylamine, pyridine, aniline B + H 2O BH + + OH - Chapter 15 Acid-Base Chemistry 5 Percent Ionization percent ionization = ionized acid concentration at equilibrium ×100% initial concentration of acid H+ ] [ percent ionization = ×100% [HA ]0 • Increases with dilution 0.50 M HF 3.8% ionized 0.050 M HF 11.2% ionized Acid Strength - extent of dissociation H-X If H and X form a strong bond, the weaker the acid H ( 1s) covalent bond strength increases B C N O Al Si P S F 2s 2p Cl 3s Br 4s I 5s Chapter 15 Acid-Base Chemistry 6 H-O-X The strength of the O-X bond influences the strength of the acid or whether it has basic character. X O H bond b bond a Increasing Electronegativity increases acid strength Increasing Oxidation Number of non-metals i) H 2CO3 < HNO3 (N more electroneg.) ii) HNO2 < (N is HNO3 +5 in HNO3) Acid Strength and pK a pKa = -log Ka The greater the pKa, the weaker the acid Acid pKa 40-50 Acid HF pKa 3.17 34 HCl -7 H 20 15.74 HBr -9 HF 3.17 HI -10 CH4 NH3 Chapter 15 Acid-Base Chemistry 7 Acid H 2SO 3 pKa 1.9, 7.21 H 2SO 4 -3.0, 1.99 HNO2 3.29 HNO3 -1.3 H 2CO3 6.37, 10.3 B(OH) 3 9.23 H 2O 15.7 H 2S 7.00 Many values were taken from: http://daecr1.harvard.edu/pKa/pka.html Weak Acids and Their Conjugate Bases H + ( aq) + OAc − ( aq) HOAc( aq) H + ][OAc − ] [ Ka = [ HOAc ] OAc − (aq ) + H2O(l ) OH − ( aq ) + HOAc( aq) OH − ][ HOAc] [ Kb = [OAc− ] HOAc( aq) H + ( aq) + OAc − ( aq) OAc− (aq) + H 2O(l) H 2O(l) Ka OH − ( aq) + HOAc( aq ) Kb H + ( aq) + OH − ( aq ) Kw Ka K b = Kw Ka = Kw Kb Kb = Kw Ka Chapter 15 Acid-Base Chemistry 8 Now to salts of weak acids and weak bases H + ][ A – ] [ HA][OH − ] [ + − Kw = KaKb = = H OH [ ] [ ] − [ HA] A [ ] Ka = Kw/Kb Kb = Kw/Ka Hydrolysis - reaction of salts of weak acids and bases with water to produce acidic or basic solutions. Hydrolysis reaction: X– + H 2O → HX + OH– [H + ][ X − ] Ka = [ HX] [OH − ][HX ] [OH − ][HX ] Kh = = = Kb [X − ][H2O] [X − ] A salt resulting from a strong acid and a strong base is a neutral salt. Ex: NaCl, KBr. A salt resulting from a weak acid and strong base is a basic salt. Ex: NaCH3CO2, KCN A salt resulting from a strong acid and a weak base is an acidic salt. Ex: NH4Cl Chapter 15 Acid-Base Chemistry 9 (OAc– = acetate ion, CH 3CO2–) NaOAc – OAc + H 2O = HOAc + OH – Kh OH ][ HAOc ] [ = [OAc ] Kh [OH ][ HAOc ] = K = K [OAc ] − − − w − a 1.0 ×10 −14 −10 = = 5.6 × 10 1.8 × 10 −5 NH4CN Acidic or Basic? NH4+ + H 2O = NH3 + H 3O + acidic CN- + H 2O = HCN + OH - basic Ka = Kw/Kb = (1.0x10 -14 )/(1.8x10 ) = 5.6x10 -5 Kb = Kw/Ka = (1.0x10 -14 )/(4x10 -10 ) = 2.5x10 -10 -5 Kb > Ka , so solution is basic! Chapter 15 Acid-Base Chemistry 10 Preparation of Acids and Bases acids from oxides of non-metals: SO 3 + H 2O → H 2SO 4 bases: from oxides of active metals: CaO + H2O → Ca(OH)2 Lewis Acids and Bases Lewis Acid - electron pair acceptor Lewis Base - electron pair donor H+ + Acid Cl Cl Al X H X Base Cl Cl + Acid electron pair acceptor Fe2+ + C O H N Al H H N Cl Cl Base electron pair donor Fe2+ C H H H O Chapter 15 Acid-Base Chemistry 11 Ag+ + 2 NH3 H3N Ag(NH3)2 + Ag+ NH3 Donor atom Donor atom Central metal ion Coordination sphere: [Ag(NH3) 2] Coordination number = number of donor atoms Lewis Acids: Group 3A halides, Nonmetal oxides, transition metal ions Lewis Bases: Amines, water, halide ions, CO, CN- , OH–. Amphoterism metal oxides and metal hydroxides that dissolve in strongly acidic and strongly basic solutions because they behave as either acids or bases. They are amphoteric. Al(OH)3, Sn(OH)2, Zn(OH)2 H Al +3 O H Al(H 2O) 3+ 6 ( aq) + H2 O(l ) + Al (OH)( H2 O)2+ 5 ( aq) + H3O (aq) Chapter 15 Acid-Base Chemistry 12 Overview A. Calculating pH of systems (solutions) I. Strong acids-bases II. Weak acids-bases III. Salt of a weak acid or base (Hydrolysis) IV. Buffers: combinations of a weak acid or base with it salt B. Titrations Combination of strong (or weak) acid with strong (or weak) base (4 combinations to consider) Chapter 15 Acid-Base Chemistry 13 The pH of Strong Acids and Strong Bases Assume 100% dissociation. Strong Acids : HCl, HBr, HI, HNO3, H 2SO 4, HClO 4 [H 3O +] = [A–] = initial concentration of the acid. pH = –log[H 3O +] Example: What is the pH of at 0.650 M solution of nitric acid? Strong Bases - alkali metal hydroxides [OH –] = initial concentration of base pOH = –log[OH–] pH = 14 – pOH Example: What is the pH of a 0.0250 M solution of KOH? Chapter 15 Acid-Base Chemistry 14 Equilibrium in Solutions of Weak Acids HA(aq) + H2O(l) → H 3O +(aq) + A–(aq) [H3O+ ][A− ] Ka = [ HA] [H 30+] = [A–] = x If [HA] > 100•Ka, then use the approximation, [HA – x] ≈ [HA] [H3O+ ][A− ] Ka = [ HA] x2 Ka = [HA] x = Ka [ HA] Example: What is the pH of a 0.100 M solution of hypobromous acid, Ka = 2.0 x 10 -9 ? Chapter 15 Acid-Base Chemistry 15 Percent Dissociation %dissociation = [ HA]dissociated × 100 [ HA]undissociated Determining acid concentration and Ka from pH. Since pH = -log[H+], then [H +] = antilog(pH) [ HA] = [ ] H+ 2 Ka Example: A 0.10 M aqueous solution of lactic acid has a pH of 2.43. What is the value of Ka for lactic acid? Chapter 15 Acid-Base Chemistry 16 Polyprotic Acids For diprotic acids, H 2A → 2 H + + A2– Calculate the first ionization as usual. Use this for the pH of the solution. The second ionization is much smaller. It has little effect on pH. The concentration of the dianion, A2- , is approximately equal to the second dissociation constant, Ka2. H + ][ HA − ] [ Ka1 = [ H 2 A] [ H + ] ≈ [ HA− ] H + ][ A 2− ] [ 2− Ka2 = ≈ A [ ] − HA [ ] What is the pH of 0.10 M solution of carbonic acid? What is the concentration of carbonate ion, CO32- at equilibrium? Chapter 15 Acid-Base Chemistry 17 Weak Bases B(aq) + H2O(l) BH +(aq) + OH–(aq) Weak bases are commonly amines. Amines are derivatives of ammonia, NH3, where one or more hydrogen is replaced by another group. Ex: Determine the pH of a 0.075 M trimethylamine, (CH 3) 3N, solution. Kb = 6.5 x 10 –5. Salt of a weak acid What is the pH of a 0.015 M solution of NaOCl? HOCl, Ka = 3.5x10 -8 . Chapter 15 Acid-Base Chemistry 18






