SCoPE Site Lesson Plan
advertisement

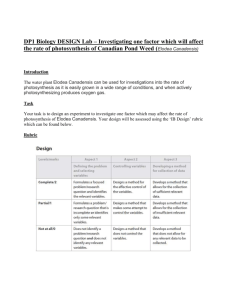
High School Science Biology Analyzing Ecosystems SCoPE Site Lesson Plan Title: Lesson 2— What Goes Around Comes Around Abstract In a previous lesson, students explored the relationships among members of a community by setting up model aquarium ecosystems, and investigated the photosynthetic capacity of Elodea and changes in biomass of producers and consumers. In this lesson students consider the variables that may effect respiration to predict changes in their model ecosystem related to the carbon cycle. With teacher guidance they generate new questions and design and conduct experiments to explore how variations affect the rate of photosynthesis. Students develop models for other cycles in nature. Subject Area: Science Grade Level and Course Title: High school Biology Unit of Study: Analyzing Ecosystems Content Expectations B1.1A - Generate questions for investigations B1.1C - Conduct scientific investigations B1.1f - Predict results of changes in variables B3.1B: Illustrate and describe the energy conversions that occur during photosynthesis and respiration. B3.3b - Describe environmental processes (e.g., the carbon and nitrogen cycles) and their role in processing matter crucial for sustaining life. B2.5f - Relate plant structures and functions to the process of photosynthesis and respiration Key Concepts carrying capacity the carbon cycle food web Instructional Resources Equipment/Manipulative Baking soda (about 2 g per group) Colored pencil (3 colors per student, erasable) Elodea (2 sprigs per group) Erasers External light source (full spectrum) Eye protection Graduated cylinders (250 ml or greater 2 per group) Meter stick Razor blade (single edged, or scalpel, one for teacher only) November 4, 2003 SCoPE SC090402 Page 1 of 5 High School Science Biology Analyzing Ecosystems Water (aged) Student Resource The Microbial World: The Nitrogen cycle and Nitrogen fixation. Ed. Jim Deacon. University of Edinburgh. 4 November 2003 <http://helios.bto.ed.ac.uk/bto/microbes/nitrogen.htm>. Texley, Juliana, and Claudia Douglass. Unit 4 Lesson 2 Student Pages. Teacher-made material. Lansing, MI: Michigan Department of Treasury, 2003. November 4, 2003 SCoPE SC090402 Page 2 of 5 High School Science Biology Analyzing Ecosystems Teacher Resource Texley, Juliana, and Claudia Douglass. Grade 9 Unit 4 Teacher Background. Teacher-made material. Lansing, MI: Michigan Department of Treasury, 2003. Sequence of Activities Advance Preparation: Put a fresh sprig of Elodea in a large graduated cylinder of aged water and baking soda, as described in Lesson 1. Place the graduated cylinder with the Elodea in full light before class time, so that there is a build up of oxygen bubbles on the stem. Safety Precautions: Water can cause a light bulb to explode dangerously. If there is any chance that drops of water could reach the light bulb eye protection is a must. 1. Ask students to recall to their observations of photosynthesis by Elodea (Anacharis) that began in Lesson 1. Explain to them that you added a little baking soda to the water. Ask: “What effect does the baking soda have on the water?” You may want to review the formula for sodium bicarbonate (baking soda) at this time. [It increases the amount of carbon dioxide in the water.] Ask: “What is happening within the Elodea?” [Photosynthesis.] “Where do the bubbles on the cut end of the Elodea come from?” [They are traveling in the stem and out the fresh cut. Note: As cold water from the tap warms, some dissolved oxygen will bubble out and attach to stems and leaves. These bubbles are confusing. That is why the water should be at room temperature before the demonstration begins.] Ask students to describe the process of photosynthesis in their own words on the Student Page. 2. Ask students to explore their own ideas about how carbon cycles in through an aquatic ecosystem. Give them these directions: “Draw a pond ecosystem. Add Elodea and as many other organisms as you can. (You do not have to be an artist!) Next using a colored pencil, draw a small number of carbon atoms in the Elodea plant. Then draw an arrow to show where the carbon atoms go. Draw carbon in at least five other places in your diagram. Connect the carbon atoms by arrows. Your last arrow should return to the Elodea plant.” Give students time to express their preconceptions, and to discuss their drawings in groups. Emphasize that they can erase their arrows and redraw them. Then lead a discussion which helps students clarify the steps in the carbon cycle: a) “Where is the carbon in the Elodea leaf?”[In organic molecules (sugar, starch, and protein), and in carbon dioxide.] b) “Is there carbon in the water?” [Yes, dissolved. This is a tricky question for students. They may not realize that gases can dissolve in water.] c) “When the plant dies, where does the carbon in the leaves go?” [It breaks down and becomes food for animals, snails.] d) “Where does the carbon in your food go?” [It forms tissues and is exhaled as carbon dioxide into the air.] e) “If the carbon becomes parts of animals, what happens next?” [Other animals up the food chain eat them. Some of the carbon is released as a byproduct of metabolism and is released into the air.] f) “Where does the carbon dioxide in the air go?” [Some dissolves in water, some is used by plants during photosynthesis.] November 4, 2003 SCoPE SC090402 Page 3 of 5 High School Science Biology Analyzing Ecosystems As students complete their diagrams, provide them with the chemical formulas for carbon dioxide,CO2, and glucose, C6H12O6. 3. Using another colored pencil to illustrate the role of energy in the carbon cycle. Solar (electromagnetic) energy becomes stored chemical energy. 4. Facilitate a whole group discussion about the carbon cycle. Begin by asking: “Now that you have diagrammed the carbon cycle, do you think that the Earth can get more carbon if we run out?” [This is actually a misconception. While a miniscule amount of carbon could land on Earth in a meteorite, the practical answer is “no.”] Carbon is released into the atmosphere as carbon dioxide or methane gas and pools as oil organic matter. 5. Now that the students are acquainted with the carbon cycle, they are asked to design their own experiment that will show the effect of one variable on the rate of photosynthesis. Write the formula for photosynthesis on the board and review both the reactants and products. Ask: “What factors affect the rate of photosynthesis?” [The amount of sunlight, the wavelength of the light, and the amount of carbon dioxide and water available to the plant.] “We cannot expect this particular plant to live outside of water, but we could vary the amount of carbon dioxide or the amount of light available to the plant to determine their effects on the rate of photosynthesis.” Divide the class into two groups (or more if you want to replicate the experiment). One group will investigate the effect on light on the rate of photosynthesis. The second group will compare the photosynthetic activity of plants with and without extra carbon dioxide provided by adding baking soda (sodium bicarbonate) to the water. Next ask the students to design for their experiment. They need to determine how they will measure the rate of photosynthesis (they already know how to count the bubbles from the stem of an Elodea plant) and they must control for all other variables. Students should write their procedure on their Student Pages. 6. After you check the experimental procedures, give each group two graduated cylinders with water, or more depending upon the supplies you have available. Students should put a sprig of Elodea in each graduated cylinder. In each case, the Elodea sprigs should be about the same length and from the same part of the plant (stem or end, etc.). Note: Do not provide too many directions. This should be a semi-open inquiry. Students already know how to count the bubbles from the stem of the Elodea. They should be able to complete a lab report, to control other variables, and to establish a data collection technique without too much direction. If necessary, you can suggest that students vary the light intensity by placing the graduated cylinders various distances from the light source and vary the carbon dioxide available for photosynthesis by altering the amount of baking soda added to the water. 7. Students can make this experiment more quantitative by including these options: Using an indicator like bromthymol blue, you can visually get a qualitative indication of decrease in carbonic acid in solution, and compare that to observed oxygen bubbles. (It is often useful to review the action of this indicator by using it in fresh and “flat” soda water or Sprite® for comparison.) Using a pH probe, you can trace the rate of photosynthesis indirectly (measuring a decrease in the carbonic acid in solution). This quantitative result can be graphed November 4, 2003 SCoPE SC090402 Page 4 of 5 High School Science Biology Analyzing Ecosystems opposite measures of oxygen released and the correlation (a negative one) calculated. Students can remove most dissolved gases from water (by boiling and then cooling) and then add measured amounts of carbon dioxide for better quantification. Students can compare the effectiveness of various wavelengths of light on photosynthesis. [In most plants, green wavelengths are least effective, but accessory pigments can modify these results.] 7. Ask students to return to their drawing of a pond ecosystem. With another colored pencil, ask them to add a shell. Ask what element the shell might add to the ecosystem. Then ask them to find four other places in the pond where calcium might be found and connect them by arrows. [Shells are calcium carbonate. Eggshells are 94% calcium carbonate, with magnesium carbonate, calcium phosphate, and other organic matter. Calcium hydroxide is sometimes dilute in acidic water. Calcium sediments are dissolved from limestone rocks in chemical weathering and enter the soil and lake water.] Provide the following additional information and allow the students to expand their drawings of the calcium cycle: “Calcium is about 3.4% of the mass of the Earth’s crust, found in igneous rocks as calcium silicates and in sedimentary and metamorphic rocks as calcium carbonates. Concentrations of Ca++ in fresh water range from 0.01 to 0.1 millimoles, but is hundreds of times higher in salt water. When water is warm, calcium carbonate precipitates easily and settles into the lake bottom. Calcium is brought back to land in shells and fish skeletons. It is also moved through geologic processes as sedimentary limestones are raised (like the white cliffs of Dover).” 8. Students may wish to explore the nitrogen cycle at the University of Edinburgh web site shown in the Student Resources. This cycle is sometimes difficult for students because they may not have the background to understand that the diatomic nitrogen which makes up about three-quarters of Earth’s atmosphere is insoluble and almost useless to living things until nitrogen-fixing bacteria or lightning act on it. The web site provides an age-appropriate review. Students can use a third color of pencil to add the nitrogen cycle to their drawings. Assessment Students should be able to draw cycles for all nutrients studied in this lesson. Application Beyond School Students can further their knowledge of nutrient cycles by exploring how commercial fertilizers make heavy use of urea or liquid nitrogen to increase agricultural yields. Connections Mathematics When experimenting with various nutrient cycles, students graph experimental data and summarize their results. November 4, 2003 SCoPE SC090402 Page 5 of 5