Gas Laws 1787 1662 – Charles’s Law
advertisement
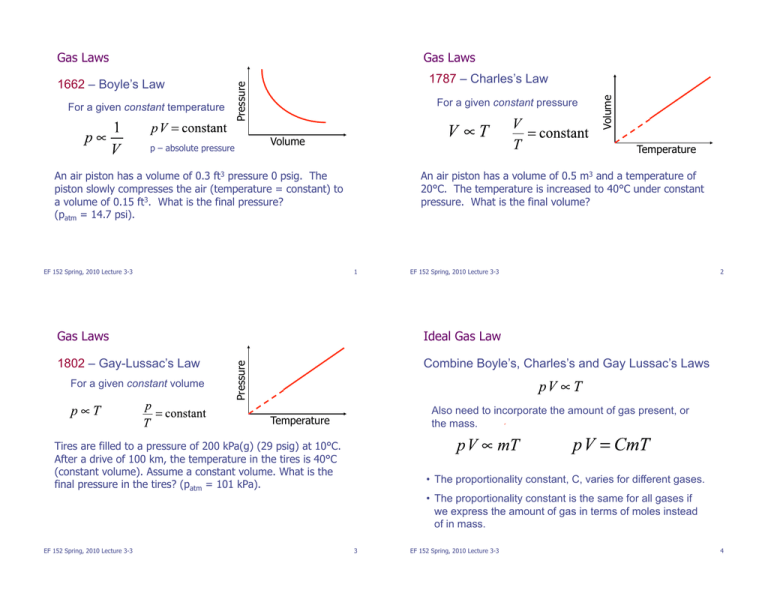
Gas Laws Gas Laws p – absolute pressure For a given constant pressure Volume Temperature An air piston has a volume of 0.3 ft3 pressure 0 psig. The piston slowly compresses the air (temperature = constant) to a volume of 0.15 ft3. What is the final pressure? (patm = 14.7 psi). EF 152 Spring, 2010 Lecture 3-3 Volume For a given constant temperature 1787 – Charles’s Law Pressure 1662 – Boyle’s Law An air piston has a volume of 0.5 m3 and a temperature of 20°C. The temperature is increased to 40°C under constant pressure. What is the final volume? 1 EF 152 Spring, 2010 Lecture 3-3 Ideal Gas Law 1802 – Gay-Lussac’s Law Combine Boyle’s, Charles’s and Gay Lussac’s Laws For a given constant volume Pressure Gas Laws 2 Also need to incorporate the amount of gas present, or the mass. Temperature Tires are filled to a pressure of 200 kPa(g) (29 psig) at 10°C. After a drive of 100 km, the temperature in the tires is 40°C (constant volume). Assume a constant volume. What is the final pressure in the tires? (patm = 101 kPa). • The proportionality constant, C, varies for different gases. • The proportionality constant is the same for all gases if we express the amount of gas in terms of moles instead of in mass. EF 152 Spring, 2010 Lecture 3-3 3 EF 152 Spring, 2010 Lecture 3-3 4 Ideal Gas Law Example: Mass of air in a room • Mole (mol): amount of substance that contains as many atoms or molecules that are in precisely 12 grams of carbon 12. A room measures 30 m x 30 m by 8 m and contains air at 20°C (68°F). What is the mass of air in the room? • Quantity of substance whose mass in grams is numerically equal to the molecular mass. 1. Order of magnitude guesstimate. 2. Ideal gas law estimate. • Molecular mass given in Periodic Table. n = number of moles; mass in grams / molecular mass R = universal gas constant = 8.314 J / (mol-K) Air is about 20% oxygen (O2) and 80% nitrogen (N2). The molecular masses are 2x16=32 and 2x14=28 respectively. An average value is about 29. Laws are only approximations. Accurate if: • Pressure and density not too high • Gas not too close to liquefaction EF 152 Spring, 2010 Lecture 3-3 5 EF 152 Spring, 2010 Lecture 3-3 Ideal Gas Law: Other Forms Examples: • Avogadro’s number: number of molecules in one mole; 6.02x1023 N = number of molecules Estimate how many molecules you breathe in with a 1 L breath of air. k = Boltzmann constant = 1.38x10-23 J/K 6 Closed test tube: V=28 cm3 of air, p=1 atm, T=17 °C. Stopper: diameter=2.0 cm, will pop off with net upward force of 19 N. Find the temperature required to pop off the stopper. • Standard Temperature and Pressure (STP) T = 273K p = 1.00 atm (101.3kPa) Volume of 1 mole of any gas at STP is 22.4 L • van der Walls equation – a ‘better’ ideal gas law a and b are empirical constants that are gas specific. ! an 2 $ p + # & (V ' nb) = nRT V2 % " EF 152 Spring, 2010 Lecture 3-3 7 EF 152 Spring, 2010 Lecture 3-3 8




