STANDARD OPERATING PROCEDURE AND SAFETY GUIDE FOR BOMB CALORIMETER (
advertisement

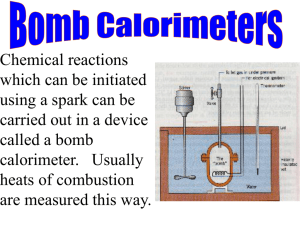
STANDARD OPERATING PROCEDURE AND SAFETY GUIDE FOR BOMB CALORIMETER (Located in Rm. H-17 Head Hall) Prepared May 14, 2010 Table of Contents 1. Scope ........................................................................................................................................4 1.1 Objective ............................................................................................................................4 1.2 Regulations ........................................................................................................................4 2.Apparatus Overview and objective ...........................................................................................4 2.1 Apparatus overview ...........................................................................................................4 3. Hazards and control evaluation ................................................................................................9 3.1 Possible fire event ..............................................................................................................9 3.2 Ventilation........................................................................................................................10 3.3 Kinetic, thermal & accoustic............................................................................................11 3.4 Electrical ..........................................................................................................................13 3.5 Water ................................................................................................................................14 3.6 Benzoic acid & Oxygen ...................................................................................................15 3.7 General, physical & equipment concerns ........................................................................15 3.8 Access ..............................................................................................................................20 3.9 Training ............................................................................................................................20 3.10 Personal equipment ........................................................................................................20 4. Operation................................................................................................................................20 4.1 Qualified personnel ..........................................................................................................20 4.2 Experiment preparation ....................................................................................................20 4.3 Lab instructions ................................................................................................................25 4.4 Operating procedure.........................................................................................................26 5. Inspections .............................................................................................................................27 5.1 Operational & Periodic inspections .................................................................................27 6. Typical test .............................................................................................................................28 Appendix A: Apparatus lab manual ...........................................................................................29 Appendix B: Apparatus field notes sheet...................................................................................30 ii H-17, Head Hall Floor Plan 3 1. Scope 1.1 Objective This standard operating procedure is intended to provide operating instructions and safety information for the Department of Mechanical Engineering’s Bomb calorimeter experiment apparatus located in H-17, Head Hall. This document is intended as a guideline and supplement to proper training that must be provided by qualified personnel before the apparatus is operated. The aim of this document is to ensure that safe work practices have been developed for the bomb calorimeter experimental work. This SOP is primarily concerned with the assembly, procedure, hazards of the experiment and safety precautions that must be taken to avoid injuries. 1.2 Regulations This document has been developed in accordance with the Environmental Health and Safety Office of the University of New Brunswick. 2. Apparatus Overview and Objective: 2.1 Apparatus overview The experimental apparatus is used as a tool to develop practical understanding in students of how a bomb calorimeter works and to calculate the heat of combustion of a specimen (Benzoic acid). The apparatus needs to be assembled before it is operated by the user. Please refer to Appendix ‘A’ (Instructions for the 1341 plain oxygen bomb calorimeter: Assemble the calorimeter) to see the description and details on the assembly of bomb calorimeter. The bomb calorimeter consists of a jacket, which has three supporting pins at its bottom to firmly hold the calorimeter bucket. The bucket has three dimples at its bottom to rest on the supporting pins of the jacket. The thermometer support rod is screwed into the support plate on the jacket cover/calorimeter cover and the stirrer drive motor is attached on the side of jacket with two screws. There is a 3003 thermometer installed on the calorimeter cover, and the stirrer along with its shaft is also attached with the calorimeter cover. To see how to install the thermometer, please refer to Appendix ‘A’ (Instructions for the 1341 plain oxygen bomb calorimeter: Assemble the calorimeter). The stirrer stirs the water in calorimeter bucket to maintain the surrounding temperature of bomb at room temperature 4 (21°C). The Parr 2901 ignition unit is used to ignite the fuse wire in the oxygen bomb. This ignition unit operates from the standard electrical outlet and provides the low voltage firing current. The unit consists of a convenient push button, indicating lamp and two connecting terminals. The push button is pressed to ignite the fuse wire loop and the indicating lamp illuminates upon pressing the button. There is a Parr 1108 oxygen bomb furnished with the 1341 calorimeter. The oxygen bomb is a 342 ml pressure vessel with a removable head and closure that can be sealed by simply turning the screw cap/knurled cap by hand until it is tight. There are two valves installed in the bomb head. On the inlet side of bomb there is a check valve which opens when pressurized oxygen is supplied by the oxygen tank via oxygen filling connection/oxygen hose to the bomb. It closes automatically when the pressurized oxygen supply is shut off. On the outlet side of the bomb there is an vent needle valve, passing through a longitudinal hole in the valve stem and discharging from a short hose nipple at the top. The gases from the bomb are released and vented through this adjustment needle valve. The gas flow through this outlet valve is controlled by turning knurled adjusting knob of the vent valve. The incoming pressurized oxygen to the bomb is diverted by a deflector nut on the inlet passage so that it does not disturb the sample (benzoic acid) in the bomb calorimeter. There is another deflector nut on the outlet side which reduces liquid entrapment when the gases are released and vented from the bomb’s adjustment needle valve/vent valve. The inlet side of the bomb head consists of a looped electrode and straight electrode. A pair of forceps/tweezers is used to bind the fuse wire (Nickel alloy wire) to the two electrodes. A pellet/capsule of benzoic acid is made by pressing 1 gram sample of benzoic acid in the pellet press and forcing it through the die into the sample pan. The pellet is then weighed on the digital balance to be equal to 1 gram with the weight of the sample pan previously tared out. There is a 2500 ml calibrated beaker with the apparatus to fill the calorimeter bucket with 2000 ml of water. There are two syringes 5 ml & 60 ml, provided with the apparatus, which are used to adjust the small amount of water in the calorimeter bucket or bomb. Figures 2, 3, 4 & 5 give an overview of the apparatus and its components. 5 Figure 2: apparatus components overview 6 Figure 3: Apparatus components overview 7 Figure 4: Apparatus components overview 8 Figure 5: Apparatus components overview 3. Hazards Evaluation and Controls:3.1 Possible fire event The bomb calorimeter apparatus is associated with exothermic reaction and high heat energy release on firing the bomb, but the high density bomb made of thick stainless steel prevents any fire event from taking place. There may be a fire event if the apparatus is not handled carefully and instructions are not followed. In the event of fire, evacuate the room immediately. Pull the nearest fire alarm (located outside room). Should you return to attempt to extinguish the fire, do not do so alone and make only one attempt. If unsuccessful, leave immediately. If successful, stay at the scene and have someone alert Security (ph. # 4830) and the Environmental Health and Safety office (ph. # 5075). Please refer to Figure 1 (H-17 Head hall floor 9 plan) to see the locations of fire extinguishers, fire alarm pull station and phone. 3.2 Ventilation Ventilation fans and ductwork are installed such that there shall be fresh air introduced into the room (green duct) and ambient air exhausted from the room (teal duct) at all times the room is occupied. These fans are controlled by wall switches locally (see Figure 6) but are on over-ride controls controlled by Facilities Management (FM). If at any time the fresh air supply or room exhaust fans are not working contact FM immediately (ph # 4889). There is a household CO monitor mounted on the north pillar. Ensure that it is operational by observing the moving LCD display. Figure 6 displays the ventilation controls of H-17 lab. 10 Figure 6: Ventilation controls of room H-17 3.3 Kinetic, Thermal and Acoustic There is a stirrer attached to the calorimeter cover that is driven by the stirrer drive motor via drive belt on the motor and stirrer pulleys. It doesn’t pose any kinetic threat since it rotates at a low RPM of 150 rpm. Ensure that the stirrer shaft runs freely before operating the apparatus to avoid any device failure. The pellet press also consists of a lever that is used to press the sample to produce pellets, but it also does not pose any kinetic threat if handled carefully. However on moving the lever, an external die moves down to press the sample down through the internal die. Keep your fingers away from the dies, while pressing the lever down. Figure below shows the stirrer, lever and dies of the apparatus. 11 Figure 7: Moving parts of the apparatus There are no thermal hazards associated with any component of the apparatus. But in case, if any of the two electrical components (stirrer drive motor/ ignition unit) of the apparatus heat up and cause the apparatus to become hot or produce smoke then turn off the electrical components by removing plugs from the electric supply and notify the Lab technician/instructor. All the wires and cables of the apparatus are well insulated so there is no threat associated with wires getting hot, however the apparatus should be checked regularly for bare wires. 12 Figure 8: Thermal hazards associated components The apparatus does not pose any acoustic threat but if there is any other equipment being operated in H-17 room while using the bomb calorimeter apparatus and it requires hearing protection then disposable earplugs are to be worn by students, instructors and all other room occupants. Instructors are responsible for relaying this information onto all involved. The see the location of earplugs in room H-17, please refer to Figure 1 (H-17, Head hall floor plan). 3.4 Electrical 13 3.4.1 Parr 2901 Ignition Unit The Parr 2901 ignition unit is used to ignite the fuse in oxygen bomb. This unit operates from any standard electrical outlet (see figure 4 for unit description). The unit does not pose any electrical hazard if operating instructions are followed. Be careful while operating this equipment. When the two lead wires on outside of calorimeter jacket are connected to the terminals on ignition unit and the power cable of ignition unit is also plugged in the electrical outlet, then connect the lead wires on the inside of calorimeter jacket to the terminals provided on bomb. Do not press the fire button on ignition unit unless the lead wires on the inside of jacket are connected to the terminals provided on bomb. If this circuit is closed (fire button is pressed) and the bare/unconnected lead wire (lead wires on the inside of cover) happen to be in contact with each other or with a metal object, the resulting short circuit may cause serious damage to the ignition unit. Don’t cause undue stress to the Ignition unit cable while it is energized and also examine the device for bare wires regularly before it is operated. Figure 9 shows the lead wires on the inside and outside of calorimeter jacket, the connecting terminals provided on the bomb and ignition unit and the power cable of ignition unit. Please also refer to Appendix ‘A’ (Instructions for the 1341 plain oxygen bomb calorimeter: Assemble the calorimeter; Connect the ignition unit, page 4) for more details. 3.4.2 Stirrer drive motor The stirrer drive motor is screwed to the calorimeter jacket and the component does not pose any threat if handled carefully. Don’t cause undue stress to the stirrer drive motor cable while it is energized and also examine the device for bare wires regularly before it is operated. 3.4.3 Digital balance The digital balance is used for weighing. The component does not pose any threat if handled carefully. Don’t cause undue stress to the device cable while it is energized and also examine the device for bare wires regularly before it is operated (see figure 4) 14 Figure 9: Parr 2901 Ignition unit and stirrer drive motor 3.5 Water The calorimeter bucket is filled with 2000 ml of water to maintain the surrounding of bomb at room temperature during the actual experiment and 1 ml of water is also added with the help of syringe at the bottom of bomb as a sequestering agent and absorbent for the combustion procedure of the experiment. There is no hazard associated with calorimeter bucket filled with water, but once the bucket is in the jacket and the bomb is lowered in the calorimeter bucket with the two lead wires connected to the bomb head terminals, do not touch the water in bucket or try to remove water or make small adjustments in water level by hands. Always use a syringe to do so. 15 3.6 Benzoic acid and Oxygen Be careful while handling the benzoic acid sample. Spillage of benzoic acid can cause eye, skin and respiratory tract irritation. It is harmful if swallowed and it can cause sensitization by inhalation. Flush eyes with water for 15 minutes lifting the upper and lower eyelid in case of contact of acid with eye. Flush skin with plenty of water for 15 minutes in case of acid contact with skin. If swallowed, do not induce vomiting and drink 2-4 cups of milk and water. If inhaled then remove the victim to fresh air immediately. Highly pressurized oxygen can be dangerous if not handled carefully. In this experiment bomb is pressurized with oxygen at 30 atm. Do not pressurize the oxygen in bomb more than 40 atm pressure. The bomb inlet check valve should be working properly. Ensure that the filling connection valve is closed. On closing the filling connection valve, oxygen supply is shut off to the bomb and the bomb inlet check valve will close automatically when this happens. The residual pressure in the filling hose is released upon pushing downward on the pressure relief valve lever. The gauge on oxygen tank should now return to zero, but if the pressure drops slowly and a large amount of oxygen gas is released when the pressure relief valve is opened, then the check valve in the bomb head inlet is not working properly. This problem has to be corrected before using the bomb in calorimeter. If too much of the oxygen is introduced in the bomb accidently, then do not proceed with the experiment. Detach the filling connection, exhaust the bomb and remove the bomb head. Then repeat the filling operation. Be careful while exhausting the bomb to open the vent valve/adjustable needle valve (see figure 5) on the bomb head top so that the oxygen escapes slowly The gas release operation should take at least a minute. 3.7 General, physical and equipment concerns The bomb in the apparatus is continuously subjected to high pressures and temperatures which apply heavy stresses on the bomb sealing mechanism. The mechanical condition of the bomb must be therefore monitored carefully. If any part of the bomb shows deterioration it must be replaced prior to the experiment or mechanical failure could result. The bomb should be properly sealed each time prior to the experiment. There is an O ring and a Contact ring that rests on the bomb head. The O ring is placed on the bomb head first and the contact ring rests on top of O ring. The bomb head is then placed in the bomb cylinder. The bomb can then be sealed by simply 16 turning the screw cap/knurled cap by hand until it is tight. Figure 11 & 12 show how to fit the O ring and Contact ring on bomb head. The bomb head parts (head gasket, sealing ring/O ring & seat in the adjustment needle valve) require close attention and frequent replacement. The valve seat deteriorates with use mainly due to corrosive stresses. Leakage or a serious burn out can result from damaged seat if it is not replaced promptly. Similarly the sealing ring/O ring should be replaced depending upon its usage as aging of this part may produce unwanted heat effects in the apparatus. This apparatus is used only once in a year so the existing condition of bomb head parts is most likely good. If the user observes that the O ring is hardening, cracking or that there is leakage from the vent valve/adjustable needle valve, then notify the lab technician/ instructor immediately. 17 Figure 10: Bomb pressurizing description The ignition unit indicator light should be checked. If the indicator light does not come on when the fire button is pressed then notify the lab technician/instructor about this problem immediately. If the indicator light comes on but the fuse wire on electrodes does not ignite, then check for a voltage leak in the system. Most likely the insulated electrodes/lead wires connecting terminals on bomb head are at fault. Notify the lab technician/ instructor about this problem immediately. 18 Figure 11: Sealing the bomb The calorimeter bucket has a highly polished chrome finish to minimize the heat transfer. This allows the room temperature to be maintained in the bucket during the actual experiment. The calorimeter bucket should be replaced if the operator observes that this chrome finish is dull. Similarly if the stirrer within the calorimeter bucket is not rotating freely, it should be repaired or replaced. There should be no additional drag or friction on the stirrer. 19 Figure 12: Sealing the bomb Care should be taken when sealing the bomb. If the O ring/sealing ring and the contact ring are not placed properly on the bomb head by user then the bomb will not be sealed properly. This will result in an oxygen leak and the experiment will fail. 20 3.8 Access All personal in the H-17 laboratory should be preauthorized by the lab faculty supervisor or be under the supervision of authorized personal (lab technician or teacher assistant). No person other than the faculty member in charge or specifically authorized personal are permitted to modify or conduct experiments with the bomb calorimeter apparatus. 3.9 Training All individuals operating the bomb calorimeter apparatus shall be required to receive training in the proper operation and maintenance of the apparatus and its controls. Training will include such topics as the complete assembly, operation, disassembly, and controls for the apparatus. Training programs shall be administered only by qualified personnel at UNB. 3.10 Personal Protective Equipment Following personal protective equipment is recommended while doing the bomb calorimeter experiment: • safety glasses (impact resistant) Other personal protective equipment should be used in the experiment if required to avoid any injury. 4. Operation 4.1 Qualified Personnel These notes in the operation section will provide a guideline to the individual who has been trained by qualified personnel to operate the bomb calorimeter apparatus. Only after the individual has been trained and is confident with the apparatus set up, operation and shutdown procedures should he attempt to operate the apparatus using these notes. Do not proceed if you are not properly trained or are unsure of the safe operation of this apparatus. 21 4.2 Experiment preparation The following procedures should be followed in preparation for this experiment, so as to avoid any accidents while performing the experiment: • Ensure that there is a convenient access to running water, a sink drain and electrical power. About 8 square feet of work space is required for this apparatus. • There should be access to a digital balance for weighing benzoic acid and water. • To prepare the sample, take benzoic acid in magazine paper and tare the magazine paper on digital balance. Weigh out 1 gram of the benzoic acid powder. Do not smell or inhale the benzoic acid powder. Tare the sample pan weight. Make a 1 gram sample in the pellet press by forcing the sample powder down through an internal die (see figure 7). After the pellet is formed, remove it from the die by pushing it into the previously tared sample pan placed below it. Weigh the sample pan with the pellet to determine the pellet weight. See the figure below for an illustrative description. 22 Figure 13: preparing the sample • Use the pair of forceps to bind the nickel alloy fuse wire to the two electrodes (looped and straight electrode) located on the inlet side of the bomb. Please refer to Appendix ‘A’ (Instructions for the 1341 plain oxygen bomb calorimeter: Attaching the fuse) to see the details on how to attach the nickel alloy fuse wire on electrodes. • Add 1 ml of water in the bottom of bomb with the help of syringe. The water will act as sequestering agent and an absorbent for gases in bomb. • Place the sample pan on the looped electrode on the inlet side of bomb head. The loop of fuse wire should be set slightly above the surface of benzoic acid or it can touch the sample itself to get better combustion rates. This contact method also helps hold the sample in place. It is not necessary to submerge the fuse wire in the benzoic acid sample which is placed in sample pan on the looped electrode. Be careful not to disturb the sample when moving the bomb head to the bomb 23 cylinder. Ensure that the contact ring is in place above the O ring/sealing ring (see figure 11& 12) and that the O ring/sealing ring is in good condition, then slide the bomb head gently into the bomb cylinder and push it down until it seats. The bomb is then ready for the oxygen filling operation. Before the oxygen filling, set the screw/knurled cap on bomb cylinder and turn it down firmly by hand. Do not use a wrench or spanner on the screw cap/knurled cap: just hand tighten it. • Charge the bomb with oxygen. Press the fitting on the end of oxygen hose into the inlet valve socket of bomb and turn the knurled union nut finger tight. There are two valves on the oxygen tank, one is filling connection valve and the other one is main oxygen tank valve (see figure 4 & 10). First ensure that the filling connection valve is closed. Open the main oxygen tank valve not more than one quarter of a turn in a counter clockwise direction. Observe the main tank pressure gauge. If it approaches 30 atm pressure then notify the lab technician immediately. This signifies that the tank will soon be empty. Now open the filling connection valve slowly, and observe the pressure rise in pressure gauge until it reaches 30 atm pressure. When the desired pressure is obtained in bomb, close the filling connection valve (turn in clockwise direction). Try to achieve as close to 30 atm as possible. Never pressurize the bomb with more than 40 atm pressure. On closing the filling connection valve on the oxygen tank, the oxygen supply is shut off to the bomb and the bomb inlet check valve will close automatically. Shut off the main tank valve. The residual pressure in the filling/oxygen hose is released when pushing downward on the lever of the pressure relief valve. The bomb pressure gauge should now return to zero, but if the pressure drops slowly and a large amount of oxygen gas is released when the pressure relief valve is opened, then the check valve in the bomb head inlet is not working properly. This problem must be corrected before using the bomb in calorimeter. If too much of the oxygen is introduced in the bomb accidently, then do not proceed with the experiment. Detach the filling connection, exhaust the bomb and remove the bomb head. At this point repeat the filling operation until the bomb is filled with desired oxygen pressure. Be careful while exhausting the bomb. Slowly open the vent valve/adjustable needle valve (see figure 5) on the bomb head top. The gas release operation should take at least a minute. • As a safety precaution, the bomb should not be charged with an amount of sample that will release more than 8000 calories of energy when burned in oxygen. The initial oxygen pressure in the bomb should not 24 exceed 40 atm. This also limits the mass of combustible sample (benzoic acid) to be not more than 1.1 gr. If the experiment is being carried out with a sample other than benzoic acid then the caloric value should be determined prior to the experiment. The proper mass used should not exceed 8000 cal. This experiment should be carried out with benzoic acid sample only. • Fill the calorimeter bucket with water. First tare the empty calorimeter bucket on digital display balance. Use hot and cold water to fill the large 2500 ml beaker. Use the thermometer to stir and fill the beaker to the correct temperature (21°C) while adjusting and alternating the flow from hot and cold faucets. Add 2 liters of tap water from the beaker to the bucket. Weigh the bucket to be equal to 2000 gr. The weight should not be necessarily 2000 gram, but an error of ±0.5 gram is acceptable. Use the syringe to add/remove water from the calorimeter bucket until the scale reads 2000 grams. • Now the bucket is ready to be set in calorimeter jacket. Align the bucket dimples with the jacket’s supporting pins. Attach the lifting handles (see figure 3 for lifting handles) to the two holes on the side of knurled cap/screw cap of bomb and lower the bomb into the calorimeter bucket filled with 2 litres of water, spanning the circular boss in the bottom of bucket. Handle the bomb carefully while moving it into the bucket so that the sample within it is not disturbed. Remove the lifting handles and shake the water droplets back if any carried with the handles. This small loss in water droplets can reduce the water level in bucket and can significantly affect the caloric value of sample. Please refer to Appendix ‘A’ (Instructions for the 1341 plain oxygen bomb calorimeter: Operating suggestions, Magnitude of errors (page 11)) to see the significant affect on caloric value of sample due to error in weighing the sample, water and any other general errors in the experiment. Insert the two lead wires on the inside of jacket into the bomb head (see figure 9 for description). • Before placing the calorimeter cover on the jacket, check the stirrer shaft attached on the calorimeter cover to ensure that it turns freely. Place the calorimeter cover on jacket and then slip the drive belt into the pulleys. Now the stirrer is ready to be used in the experiment. Always keep the 3003 thermometer attached on the calorimeter cover in upright position when the calorimeter cover is removed from the jacket and is not in use. Do not place the cover on table top. Always set the cover in a position to protect the thermometer bulb and stirrer shaft of the calorimeter cover (see figure 14). Please refer to Appendix ‘A’ (Instructions for the 1341 25 plain oxygen bomb calorimeter: Install the thermometer) to find details on installation of 3003 thermometer on the cover. • Turn the knob on top of stirrer drive motor until it clicks. The stirrer will start stirring the water in the bomb calorimeter assembly. Let the stirrer run for at least five minutes before performing the actual experiment so that the system attains equilibrium. Figure 14: Calorimeter cover upright position 4.3 Lab Instructions 26 4.3.1).Instructor Responsibilities: The apparatus script/manual provided with the apparatus and this document (SOP) should be read and understood. These documents provide all the necessary information regarding the apparatus components specifications, startup procedure, operation, hazards involved & safety precautions to be taken while using this apparatus. The lab instructor shall remain in the room while the experiment is in progress. After the student group has assembled and before any explanation has begun the instructor shall relay all safety precautions and hazards as outlined in section 3. Inform the students that they must contact the instructor should any problems or concerns arise during the experiment. Make the group aware of the fire extinguisher locations, fire alarm pull stations, phone and exits. Any student from the group missing his any personal protective equipment shall not be allowed by the instructor to enter the laboratory H-17 and proceed with the experiment. 4.3.2). Data/Instrument Locations and Functions - Pressure gauge. The pressure gauge is used to measure and display the pressure of oxygen leaving the tank (see figure 10). - Digital display balance. The balance is used to measure & display the mass of sample/water (see figure 13). - 3003 thermometer. The thermometer is used to measure the temperature of water in bomb calorimeter assembly (see figure 4). The readings of mass and temperature should be accurate and within a small error range of ± 0.5. A small error in this experiment can cause a significant difference in the caloric value of the sample. 4.4 Operating Procedure 4.4.1 Startup and procedure After preparing for the experiment and following all the steps mentioned in section 4.2 to prepare for this experiment, the following steps are required to perform the experiment: 27 • After running the stirrer for 5 minutes, start the timer and record the temperatures to one tenth of the smallest scale division. • Read and record the temperatures at a one minute interval for next five minutes. • At the start of 6th minute, stand back from the bomb calorimeter and fire the bomb by pressing the ignition button and holding it down until the indicator light goes out. Do not have your hands or any other part of the body on bomb while firing. Standing back from the bomb for next 30 seconds. • After firing the bomb the bucket temperature will rise. This rise will be rapid at the start and will decrease after a few seconds. Read and record temperatures from the thermometer at a 15 second interval for the next 3 minutes. • Record the temperature readings at the 20 second interval for rest of the time until the thermometer shows a constant temperature reading. Please refer to Appendix ‘A’ (Instructions for the 1341 plain oxygen bomb calorimeter: Calculating the heat of combustion (page 8)) to see the details of calculations of this experiment • Once the temperature is constant then shut off the stirrer drive motor and apparatus. 4.4.2 Shutdown After the stirrer drive motor is shut off, do the following: Remove the drive belt from calorimeter cover, remove the cover, wipe the thermometer and stirrer with a clean cloth and place the cover in upright position. Be certain that the electrical supply is cut off to the ignition. Lift the bomb from bucket and remove the leads from bomb. Clean the bomb with a clean cloth. • To avoid entrainment losses, the release of gases from the bomb should be gradual and should take at least a minute. This is done by turning the vent valve/ adjustable needle valve on the bomb head. After releasing all the pressure and gases out of the bomb, remove the bomb head and place it in upright position. Wash and clean the interior surface of the bomb. Look for any remaining, unconsumed fuse wire pieces. • Measure the unconsumed fuse wire length if any is found. • Wash and clean the bomb, the washers and the bucket. 28 5.0 Inspections Inspections at regular intervals will be performed on the apparatus to ensure that the apparatus is kept in a safe and well maintained condition. The inspection includes the checking of bomb assembly (bomb head, sealing ring, contact ring & vent valve), the calorimeter cover (stirrer turning freely), the calorimeter bucket for polished chrome and regularly check for bare & cut electrical wires in the apparatus. 5.1 Periodic & Operational Inspections Visual inspections are the responsibility of the person who is conducting experiments on a regular basis with the bomb calorimeter apparatus and should be carried out each time before operating apparatus. A complete periodic inspection of the apparatus shall be performed by the person who is conducting experiments on a regular basis with this apparatus. This will alert the lab faculty supervisor of any deficiencies in the apparatus. The deficiencies such as those listed below shall be examined during both the periodic & operational inspection. The lab faculty supervisor will determine if the deficiencies will affect the safe operation of the apparatus. a. Deformation, corrosion, worn or crack in bomb, calorimeter bucket and jacket b. Function, gauges and warning labels c. Worn, cut or bare electrical wires d. Mercury leakage from the thermometer e. crack and leak from the calibrated beakers f. oxygen leak from the hose 6.0 Typical Tests The actual test procedure will be outlined in the lab script as issued by the professor. A typical test will include the temperature readings for the first five minutes of experiment with one minute interval before the bomb is fired. After the bomb is fired, the temperature is recorded after 15 seconds interval for next 3 minutes and similarly for rest of the time temperature is recorded after 20 seconds interval until it becomes constant temperature. A sample data sheet used during the experiment can be found in Appendix’B’. 29 Appendix ‘A’ 30 31 Appendix ‘B’ 32 33