„Composites“ Compendium Polyester Resins
advertisement

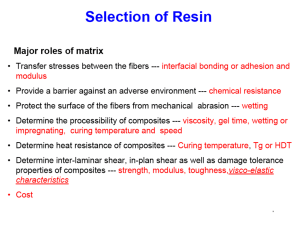
Nur für Unterrichtszwecke !!! Compendium „Composites“ ASM Handbook / extraction Polyester Resins Prof. H. Hansmann Hochschule Wismar FB MVU, Werkstofftechnologien / Kunststofftechnik Oct. 2003 Polyester Resins Tim Pepper, Ashland Chemical Company Introduction UNSATURATED POLYESTER (UPE) RESIN is used for a wide variety of industrial and consumer applications. In fact, more than 0.8 billion kg (1.7 billion lb) was consumed in the United States in 1999. This consumption can be split into two major categories of applications: reinforced and nonreinforced. In reinforced applications, resin and reinforcement, such as fiberglass, are used together to produce a composite with improved physical properties. Typical reinforced applications are boats, cars, shower stalls, building panels, and corrosion- resistant tanks and pipes. Nonfiber reinforced applications generally have a mineral “filler” incorporated into the composite for property modification. Some typical nonfiber reinforced applications are sinks, bowling balls, and coatings. Polyester resin composites are cost effective because they require minimal setup costs and the physical properties can be tailored to specific applications. Another advantage of polyester resin composites is that they can be cured in a variety of ways without altering the physical properties of the finished part. Consequently, polyester resin composites compete favorably in custom markets. Polyester Resin Chemistry Polyesters are macromolecules that are prepared by the condensation polymerization of difunctional acids or anhydrides with difunctional alcohols or epoxy resins. Unsaturated polyester resins, commonly referred to as “polyester resins,” are the group of polyesters in which the acid component part of the ester is partially composed of fumaric acid, a 1,2ethylenically unsaturated material. Maleic anhydride is the predominant source of this fumarate. Maleic anhydride is incorporated into the polyester backbone and then isomerized to provide fumarate esters (commonly referred to as unsaturated polyesters). In most cases, the polymer is dissolved in styrene to provide a solution that will typically have a viscosity in the range of 0.2 to 2 Pa · s (200–2000 cP). Other reactive vinyl monomers, such as vinyl toluene, diallyl phthalate, or methyl methacrylate may be used to obtain specific properties. The resin viscosity is tailored for specific fabrication processes in which the resin is ultimately “cured” via a free-radical process. A final formulation may include resin, inorganic filler, fiberglass reinforcement, and a free-radical initiator, such as an organic peroxide. This final formulation is formed against a mold prior to the cross-linking reaction between the unsaturated polymer and the unsaturated monomer. The “curing” is a cross-linking chain reaction, converting the low-viscosity solution into a three- dimensional thermoset plastic (Ref 1). This is referred to as the cure. Terminology has developed to distinguish between various types of UPEs. General-purpose resins are a group of resins generally used because of their low cost. While processing parameters can affect this, the cost of the raw materials tends to limit the type of unsaturated polyesters selected. The resins used in this area are commonly referred to as PET, DCPD, and ortho resins. While PET (polyethylene terephthalic) resin and orthophthalic anhydride are sources of low-cost saturated acids, DCPD (dicyclopentadiene) is typically coupled with maleic anhydride during an initial step, prior to both the isomerization and the condensation polymerization. Dicyclopentadiene improves the solubility of the polyester resin in styrene. As one drives toward lower-cost resins, solubility in styrene is a serious concern. Dicyclopentadiene offers a low-cost solution. Due to government regulations aimed at minimizing styrene emissions in open molding, a subset of general-purpose resins, “lowstyrene” resins, has been developed. These resins tend to have a higher dependency on DCPD and are cooked to a lower molecular weight. This allows less styrene to be used to achieve the desired viscosity, and performance generally suffers. Isophthalic resins are based on isophthalic acid and maleic anhydride. The incorporation of isophthalic acid creates a high-molecular-weight resin with good chemical and thermal resistance and good mechanical properties. The use of nonpolar glycols contributes to improved aqueous resistance, which is required to protect the fiberglass. Bisphenol A (BPA) fumarate resins are prepared by the reaction of propoxylated BPA with fumaric acid. The result is a relatively nonpolar polyester with a reduced number of ester linkages. The reduced number of ester linkages contributes to excellent corrosion resistance. Bisphenol A fumarates were once the workhorse of the corrosion-resistant composite industry. In recent years, they have been replaced by isophthalic resins for mildly corrosive applications and bisphenol A epoxy based vinyl esters in more aggressive environments. Currently, BPA fumarates are used almost exclusively in applications requiring exceptional corrosion resistance to caustic environments. Chlorendic resins are prepared using either chlorendic anhydride or chlorendic (HET) acid (Ref 2) reacted with maleic anhydride. Composites made from these resins have excellent chemical resistance and some fire retardancy because of the presence of chlorine. Chlorendic resins are used in applications requiring exceptional resistance to acidic or oxidizing environments. In many of these environments, metals are attacked quite aggressively, while chlorendic resin composites generally perform much better. Vinyl ester resin is the common name for a series of unsaturated resins that are prepared by the reaction of a monofunctional unsaturated acid, typically methacrylic acid, with an epoxy resin. The epoxy resin is “end-capped” with an unsaturated ester to form the vinyl ester resin. The resulting polymer, which contains unsaturated sites only in the terminal positions, is mixed with an unsaturated monomer, generally styrene. At this point, the appearance, handling properties, and curing characteristics of vinyl ester resins are the same as conventional polyester resins. However, the corrosion resistance and mechanical properties of vinyl ester composites are much improved over standard polyester resin composites. These improved properties have enabled vinyl ester resins to become the workhorse of the polyester custom corrosion industry. However, the properties of vinyl ester resins are not as easily tailored to a specific application as are standard unsaturated polyester resins. This combined with the use of higher-cost raw materials has somewhat limited the ability of vinyl ester resins to penetrate the unsaturated polyester resin market. Within each of these five resin classifications, specific polyesters can be formulated by varying the starting materials. Specific properties such as flexibility, thermal properties, fire retardancy, and hydrophobicity can be altered (Ref 3) by varying the type of dihydric alcohol/epoxy resin used or by varying the fumaric/saturated acid ratio. Low-profile additives (LPAs) are a class of saturated resins that are used for dimensional stability. Although these materials are not UPEs, they play a very important role in the use of UPEs. One of the problems associated with the use of UPEs is that they shrink volumetrically about 6 to 8% upon curing. This creates challenges in fabricating a high-quality surface and/ or maintaining the dimensional stability of a part. Low-profile additives offer a unique solution to this problem when processing at elevated temperatures. Addition of an LPA to a UPE formulation reduces or eliminates shrinkage. Some formulations using LPAs can actually have expansion greater than the original mold or form size. Since the LPA does not chemically react with the UPE or styrene, care should be taken in determining the type and amount of LPA used. Mechanical Properties Mechanical properties are often the critical factor in selecting a polyester resin for a specific application. Table 1 lists the common test methods of the American Society for Testing and Materials (ASTM) that are used to characterize the mechanical properties of polyester resin composites. Table 1 ASTM test methods for characterizing mechanical properties of polyester resins Properties ASTM Test Method Tensile strength, modulus, and % elongation D 638 Flexural strength and modulus D 790 Compressive strength, modulus, and % compression on break D695 Izod impact D256 Heat distortion D 648 Barcol hardness D 2583 While the physical properties of polyester composites are predominately controlled by reinforcement, the physical properties of the polyester resin do affect the durability and thermal performance. Representative examples of clear- cast polyester resin data are shown in Table 2. It should be noted that within each class of resins, modifications are made to the polymer. These modifications effectively trade off thermal performance for increased toughness (Table 2). Table 2 highlights the differences among the classes of polyesters. Isophthalic resins tend to show higher tensile and flexural properties than orthophthalic resins. This may be because isophthalics usually form more linear, higher- molecular-weight polymers than orthophthalics. In contrast, the BPA fumarate and chlorendic resins are formulated for service in aggressive corrosive conditions and consequently are much more rigid. This results in clear castings that are brittle and have low tensile elongation and strength. The vinyl ester, because of its bisphenol diepoxide content, exhibits excellent tensile and flexural properties as well as high elongation. Table 2 Mechanical properties of clear-cast (unreinforced) polyester resins Material Barcol hardness Tensile strength MPa Tensile modulus Elongation, % ksi GPa 10 psi 6 Flexural strength MPa ksi Flexural modulus Compressive strength GPa 106 psi MPa Heat-deflection temperature ksi °C °F Orthophthalic … 55 8 3.45 0.50 2.1 80 12 3.45 0.50 … … 80 175 Isophthalic 40 75 11 3.38 0.49 3.3 130 19 3.59 0.52 120 17 90 195 BPA fumarate 34 40 6 2.83 0.41 1.4 110 16 3.38 0.49 100 15 130 265 Chlorendic 40 20 3 3.38 0.49 … 120 17 3.93 0.57 100 15 140 285 Vinyl ester 35 80 12 3.59 0.52 4.0 140 20 3.72 0.54 … … 100 212 In this article, it is impossible to discuss thoroughly the effects that dihydric alcohols, acids, levels of unsaturation, monomer types and amounts, and cure temperatures have on mechanical properties; however, Ref 4 provides an excellent review. In general, increasing the chain length of the dihydric alcohol increases the flexibility of the cross-linked resin. The same occurs with saturated acids. Aromatic groups, in either the dihydric alcohol or acid component, increase stiffness and hardness. Using a reinforcing fiber to produce a polyester composite dramatically improves both the tensile and flexural properties. Table 3 lists the same five samples as Table 2; however, Table 3 shows the mechanical properties of the fiberglass reinforced polyester resin composites. In Tables 4 and 5, the properties obtained were dependent on the amount and type of glass fiber used. The last two entries in Table 5 show the influence of fiberglass orientation. Both contained 70 wt% glass fiber, but the unidirectional composite showed much higher tensile properties and flexural modulus when tested in the glass fiber direction. The properties of unidirectional composites are even more anisotropic than typical polyester laminates. Mechanical properties measured transverse to the glass direction will approach those observed for clear castings. The pultrusion or filament-winding process commercially produces polyester composites employing unidirectional reinforcements. They can be used in structural applications where strength or stiffness is required in only one direction. In Table 6, physical properties of fiberglass reinforced polyester resin composites are compared to those of various metals. Table 3 Mechanical properties of fiberglass-polyester resin composites (glass content, 40 wt%) Tensile Tensile strength modulus Material Flexural Flexural Compressive Izod impact strength modulus strength Barcol 106 106 Elongation, MPa ksi GPa MPa % hardness MPa ksi GPa psi psi ksi J/mm ft · lbf/in. Orthophthalic … 150 22 5.5 0.8 1.7 220 32 6.9 1.0 … … … … Isophthalic 45 190 28 11.7 1.7 2.0 240 35 7.6 1.1 210 30 0.57 10.7 BPA fumarate 40 120 18 11.0 1.6 1.2 160 23 9.0 1.3 180 26 0.64 12 Chlorendic 40 140 20 9.7 1.4 1.4 190 28 9.7 1.4 120 18 0.37 7 Vinyl ester … 160 23 11.0 1.6 … 220 32 9.0 1.3 210 30 … … Table 4 Effect of glass content on mechanical properties of fiberglass reinforced polyester composites Material Glass content, wt% Flexural strength Flexural modulus MPa ksi GPa 106 psi Tensile strength MPa ksi Tensile modulus GPa 106 psi Compressive strength MPa ksi Orthophthalic 30 170 25 5.5 0.80 140 20 4.8 0.70 … … 40 220 32 6.9 1.00 150 22 5.5 0.80 … … 30 190 28 5.5 0.80 150 22 8.27 1.20 … … 40 240 35 7.58 1.10 190 28 11.7 1.70 210 30 25 120 17 5.1 12 7.58 1.10 170 24 35 150 22 8.27 1.20 100 14 10.3 1.50 170 24 40 160 23 8.96 1.30 120 18 11.0 1.60 180 26 24 120 17 5.9 11 7.58 1.10 140 21 34 160 23 6.89 1.00 120 18 9.65 1.40 120 18 40 190 28 9.65 1.40 140 20 9.65 1.40 120 18 25 110 16 5.4 12.5 6.96 1.01 180 26.5 35 260 37.3 9.52 1.38 153.4 22.25 10.8 1.56 230 34 40 220 32 30 Isophthalic BPA fumarate Chlorendic Vinyl ester 0.74 80 0.85 80 0.79 86.2 8.89 1.29 160 23 11.0 1.59 210 Table 5 Effect of glass type and amount on mechanical properties of fiberglass reinforced polyester composites Type of glass fiber reinforcement Glass content, wt% Density, g/cm3 Tensile strength MPa ksi Tensile modulus Elongation, % GPa 10 psi 6 MPa ksi Flexural modulus GPa 106 psi Compressive strength MPa ksi Neat cured resin 0 1.22 59 8.6 0.783 2.0 88 12.8 3.90 0.565 156 22.6 Chopped-strand mat 30 1.50 117 17.0 10.80 1.566 3.5 197 28.6 9.784 1.419 147 21.3 50 1.70 288 41.8 16.70 2.422 3.5 197 28.6 14.49 2.102 160 23.2 Roving fabric 60 1.76 314 45.5 19.50 2.828 3.6 317 46.0 15.00 2.175 192 27.8 Woven glass fabric 70 1.88 331 48.0 25.86 3.750 3.4 403 58.4 17.38 2.520 280 40.6 70 1.96 611 88.6 32.54 4.720 2.8 403 58.4 29.44 4.270 216 31.3 Unidirectional roving fabric Source: Ref 1 5.40 Flexural strength Table 6 Comparative properties of fiberglass reinforced polyester composites and various metals Flexural strength Glass content, wt% Material Flexural modulus Tensile strength Tensile modulus Density, 106 106 3 MPa ksi GPa MPa ksi GPa g/cm psi psi Unidirectional glass 70 roving, rod and bar 2.0 690 100 40 6 690 100 40 6 Unidirectional glass 50 roving, sheet 1.6 205 30 2 140 20 12 1.8 Chopped-strand mat 30 1.5–1.7 110– 16– 6.9– 1.0– 60– 190 28 8.3 1.2 120 9– 18 6–12 0.8– 1.8 Aluminum sheet … 2.8 140 10 40– 190 6– 27 70 10 Stainless steel … 8.0 210– 30– 190 240 35 28 210– 30– 190 28 240 35 Low-carbon steel … 8.0 190 30 200– 29– 210 30 230 33 Compressive strength Material Elongation, MPa % 20 28 15 70 210 Impact strength Thermal Specific heat Coefficient conductivity of thermal Btu · expansion, ft · W/m kJ/kg Btu/lb ksi J/mm in./h · 10–6/K lbf/in. · K ·K ·°F 2 ft ·°F Unidirectional glass roving, 1.5 rod and bar 410 60 2.6 49 0.70 5 1.0 0.24 5 Unidirectional glass roving, 1.5 sheet 140 20 0.96 18 0.60 4 1.2 0.28 9 Choppedstrand mat 1–1.2 100– 170 15– 25 0.2– 0.64 4–12 0.20– 1.2– 0.25 1.6 1.3– 1.4 0.31– 20–35 0.34 Aluminum sheet 30–40 … … … … 120– 810– 0.92– 0.22– 20–25 130 1620 0.96 0.23 Stainless steel 40–50 210 30 0.45– 8.5– 0.59 11 14– 25 96– 185 Low-carbon steel 190 28 … 35– 65 260– 0.42– 0.10– 10–15 460 0.46 0.11 38–50 … 0.50 0.12 15–20 As mentioned earlier, different types of reinforcement affect mechanical properties. While Eglass is the most commonly used reinforcement in polyester resins, S-glass, aramid, and carbon fibers can also be used. Table 7 compares a variety of reinforcements in both orthophthalic polyester and vinyl ester resins. While the tensile strength of the orthophthalic polyester was much improved with aramid, no such improvement was seen with the vinyl ester. Table 7 Effect of reinforcement on mechanical properties of polyester-matrix composites Tensile strength Tensile modulus Material MPa ksi GPa 106 psi Elongation, % E-glass-ortho polyester Ambient temperature 157 22.8 11.0 1.59 1.7 50 °C (125 °F) 148 21.5 8.41 1.22 2.4 65 °C (150 °F) 140 20.3 7.31 1.06 2.6 Ambient temperature 159 23.0 10.3 1.49 1.9 50 °C (125 °F) 165 24.0 8.55 1.24 2.6 65 °C (150 °F) 157 22.8 7.38 1.07 2.6 Ambient temperature 212 30.7 12.5 1.82 2.0 50 °C (125 °F) 208 30.2 11.5 1.67 2.1 65 °C (150 °F) 200 29.0 9.52 1.38 2.4 Ambient temperature 206 29.9 12.6 1.83 2.1 50 °C (125 °F) 192 27.9 11.4 1.66 2.2 65 °C (150 °F) 201 29.2 11.6 1.68 2.4 Ambient temperature 198 28.7 11.4 1.66 2.2 50 °C (125 °F) 172 25.0 9.6 1.39 2.3 65 °C (150 °F) 199 28.8 10.3 1.49 2.5 Ambient temperature 189 27.4 12.1 1.76 1.8 50 °C (125 °F) 221 32.0 11.7 1.70 2.2 65 °C (150 °F) 218 31.6 11.7 1.69 2.2 S-glass-ortho polyester Aramid-ortho polyester E-glass-vinyl ester S-glass-vinyl ester Aramid-vinyl ester Inorganic fillers are commonly used in polyester resin composites. While they do improve stiffness, as shown by an increase in modulus (see Table 8), they have little effect on other strength characteristics. They are used primarily to reduce cost. Table 8 Effect of filler and glass fiber reinforcement on mechanical properties of polyester resins Flexural strength Flexural modulus Tensile strength Tensile modulus Reinforcement 106 MPa ksi GPa MPa ksi GPa material psi Neat resin casting (A material) 106 psi Heat deflection Barcol temperature(a) Elongation,% hardness °C °F 129 18.7 3.60 0.522 70.0 10.1 3.50 0.507 3.0 80 pph A; 20 pph CaCO3 (B 109 15.8 4.30 0.623 52 material) 7.5 5.60 0.812 1.26 74 pph B; 26 pph 2.5 cm long chopped 183 26.5 6.10 0.884 116 16.8 9.694 1.406 1.72 strand (C material) pph, parts per hundred. (a) For 250 µm (10 mil) deflection at 1.82 MPa (0.264 ksi). Source: Ref 1 30 58 135 45 67 150 45 >260 >500 As expected, mechanical properties at elevated temperatures vary significantly among the general classifications of polyester resins (Fig. 1). The extreme rigidity and high glass transition temperature (Tg), of BPA fumarate and chlorendic polyesters result in high flexural strength retention up to 120 °C (250 °F). Vinyl esters also show performance advantages over isophthalic polyesters in fatigue studies (Fig. 2, Ref 5). The advantage of vinyl ester was evident at elevated temperatures (Ref 6). At 105 °C (220 °F), vinyl ester and isophthalic polyester composites (60% glass) were cycled to a stress level of 60 to 70 MPa (9–10 ksi). After 200,000 cycles, the drop in flexural modulus was only 5% for the vinyl ester, compared to 12% for the isophthalic polyester. The thermal performance of vinyl esters, combined with their excellent mechanical properties and toughness, explains why they are often chosen for structural resin composites. Fig. 1 Flexural strength versus temperature, glass-polyester composites of 40% glass Fig. 2 Flexural fatigue strength References cited in this section 1. M. Grayson and D. Eckroth, Ed., Encyclopedia of Chemical Technology, 3rd ed., Vol 18, John Wiley & Sons, 1982, p 575 4. H.V. Boenig, Unsaturated Polyesters: Structure and Properties, Elsevier, 1964 5. B. Das, H.S. Loveless, and S.J. Morris, Effects of Structural Resins and Chopped Fiber Lengths on the Mechanical and Surface Properties of SMC Composites, 36th Annual Conference of the Reinforced Plastics Composites Institute, The Society of the Plastics Industry, 1981 6. P.K. Mallick, Fatigue Characteristics of High Glass Content SMC Materials, 37th Annual Technical Conference, Society of Plastics Engineers, 1979, p 589 Thermal and Oxidative Stability Polyester resins are commonly used in elevated-temperature applications, especially in the electrical and corrosion-resistance areas. At temperatures above 150 °C (302 °F), the polymer begins to slowly dissociate chemically. The temperature at which this decomposition occurs depends on the structure of the polyester used. Regardless of the polymer composition, at temperatures near 300 °C (570 °F), the cured polyester resin will undergo spontaneous decomposition. This is characteristic of vinyl polymers and is caused by their depolymerization to form monomeric species. Among polyesters, high-molecular-weight UPEs and epoxy vinyl esters show better stability above 150 °C (302 °F) than low-molecular- weight UPEs. High-molecular-weight isophthalic, PET, DCPD, and BPA fumarate resins, when formulated properly, can perform very well in thermal applications (Fig. 3). In thermal stability, nonhalogenated resins outperform halogenated resins. While some chlorinated resins have respectable thermal stability, brominated resins universally have poor thermal stability with aromatic bromides performing somewhat better than aliphatic bromides. Low-styrene (general-purpose) resins also exhibit poor thermal stability. The same is true for orthophthalic resins, as they tend to be lowmolecular-weight resins. In spite of their poor thermal stability, orthophthalic resins perform well in moderate- to low-temperature applications and are often preferred in such applications because of their low cost. Fig. 3 Thermal stability of glass-polyester composites at 180 °C (355 °F) Monomer type also plays an important role in the thermal stability of a polyester resin. For example, a polyester resin in vinyl toluene shows superior thermal stability when compared to the same resin in styrene. This is attributed to the strong copolymer bonds formed with the fumarate unsaturation. Even after aging at 200 °C (390 °F), an isophthalic resin in vinyl toluene has better flexural strength retention than an isophthalic resin in styrene (Fig. 4). While vinyl toluene versions of an isophthalic polyester and a BPA fumarate showed comparable performance at 200 °C (390 °F), the BPA fumarate had much better flexural strength retention at 220 °C (430 °F) (Fig. 5) and 240 °C (465 °F) (Fig. 6). Fig. 4 Flexural strength retention of glass-polyester composite when aged at 200 °C (390 °F), tested at room temperature Fig. 5 Flexural strength retention of glass-polyester composite when aged at 220 °C (430 °F), tested at room temperature Fig. 6 Flexural strength retention of glass-polyester composite when aged at 240 °C (465 °F), tested at room temperature Chemical Resistance Polyester resins have been used for many years in applications requiring resistance to chemical attack. Parts made from UPEs have corrosion-resistant properties that complement most metals. As an environment increases in polarity, it becomes less aggressive toward parts made from UPEs. Numerous applications for corrosion-resistant tanks, pipes, ducts, and liners can be found in the chemical process and pulp and paper industries. Resin selection depends on the specific chemical environment to be contained. Table 9 shows how four different classes of polyester resin perform in several environments. Isophthalic resins perform well in both mild aqueous and mild organic environments. Generally, they are the most economic resin choice. Vinyl ester resins are typically used in more aggressive environments. A properly selected vinyl ester resin will perform well in many applications. When the environment becomes more aggressive, premium polyesters are preferred. Premium polyester resins offer improved corrosion resistance for specific applications. Chlorendic resins are chosen for strong acid and oxidizing environments, especially at elevated temperatures, while BPA fumarate resins are better in caustic environments. Using glass fiber does not improve the corrosion resistance of polyester resins and, in some cases, actually reduces performance. This is especially true in hydrofluoric acid or strong caustic environments where the chemicals actually attack and dissolve glass. In this and other special cases, reinforcing materials, such as carbon fibers, may be preferred (Ref 7). Table 9 Corrosion resistance of glass fiber polyester resin composites Resin 75% H2SO4 80 °C (175 °F) 15% 5.25% Xylene NaOH 65 NaOCl 65 Ambient °C (150 °C (150 °F) °F) Deionized Seawater 80 water 100 °C °C (180 °F) (212 °F) Isophthalic – – – + – – Chlorendic + – – + + + BPA fumarate – + – + – + Vinyl ester – – + – – + Reference cited in this section 7. H.S. Kliger and E.R. Barker, A Comparative Study of the Corrosion Resistance of Carbon and Glass Fibers, 39th Annual Conference of the Reinforced Plastics/Composites Institute, The Society of the Plastics Industry, 1984 Ultraviolet (UV) Resistance Polyester resins are used in many outdoor applications. They can survive exposure to the elements for periods exceeding 30 years, although some discoloration and loss of strength will occur. The onset of surface degradation is marked by a yellow discoloration that becomes progressively darker as erosion and surface stress crazing occur. In translucent systems, this UV radiation causes yellowing of the composite as a whole, although the color is usually more intense on the surface. The negative effects of UV exposure can be effectively eliminated with the addition of UV stabilizers to the outermost resin layer. Monomer selection also affects UV stability. Styrene and other aromatic vinyl monomer derivatives are more susceptible to oxidative degradation and are usually supplemented with more resistant acrylate or methacrylate monomers. Of the acrylate monomers, methyl methacrylate (MMA) is the most common. When MMA is copolymerized with styrene, the cured polyesters have superior durability, color retention, and resistance to fiber erosion (Ref 8). The improved UV resistance with MMA is evident in Table 10, which compares four polyester resins composed of identical polymers and four different monomer systems. These resins were used to prepare glass fiber reinforced panels that were exposed to outdoor weathering for five years. The gloss retention for styrene/MMA monomer blends was much greater than for either monomer alone. The refractive index of MMA is also lower than that of styrene, allowing the formulation of polyester resins with a refractive index matched to the glass fibers. This, combined with improved UV resistance, has resulted in the use of MMA polyesters to fabricate glass reinforced transparent building panels that can be used in greenhouses, skylights, and other applications. Using UV-absorbing chemical additives, such as the substituted benzophenones or benzotriazoles, can further reduce the effect of radiation. Table 10 Effect of methyl methacrylate on the gloss retention of weathered polyester panels Panel number Components 3 4 5 75 75 60 60 60 Methyl methacrylate, % 25 … 20 10 0 Styrene, % 0 30 40 Gloss retention, % Source: Ref 8 5.2 48 83.5 56.5 11.8 Polyester, % 1 2 25 20 Reference cited in this section 8. A.L. Smith and J.R. Lowry, Long Term Durability of Acrylic Polyesters versus 100% Acrylic Resins in Glass Reinforced Constructions, 15th Annual Conference of the Reinforced Plastics/Composites Institute, The Society of the Plastics Industry, 1960 Electrical Properties Most organic polymers have medium to excellent electrical properties. Wide ranges of thermoset and thermoplastic materials are used in the electrical and electronics industries. Applications in which polyester resins have been used include the insulation of motor windings, encapsulation of electrical components, fabrication of printed circuit boards, highvoltage standoff insulators, switch boxes, and miscellaneous equipment for high-voltage line work. Typical electrical properties of polyester resins are shown in Table 11. Tables 12 and 13 show specific data on an isophthalic polyester and a BPA fumarate as well as the influence of filler type. Table 11 Electrical properties of glass- polyester composites Volume resistivity, 50% relative humidity, Ω· m 1010–1012 Dielectric strength, kV/mm (kV/in.) Short-time, 3.2 mm (⅛ in.) 13.6–16.5 (345–420) Step-by-step, 3.2 mm (⅛ in.) increments 10.8–15.4 (275–390) Dielectric constant 60 Hz 5.3–7.3 1 kHz 4.68 1 MHz 5.2–6.4 Dissipation factor 60 Hz 0.011–0.041 1 MHz 0.008–0.022 Arc resistance 120–200 Table 12 Electrical properties of isophthalic polyester 3.2 mm (⅛ in.) laminates with various fillers Filler Dielectric Volume Dielectric Dissipation Dielectric Dissipation Dielectric Dissipatio strength short resistivity, constant, factor, 1 constant, factor, 1 constant, factor, 6 time 60 Hz kHz 1 kHz MHz 10–13Ω· m 1 MHz Hz kV/mm kV/in. Calcium 15.0 carbonate 380 7.8 4.10 0.007 4.18 0.005 4.19 0.003 Gypsum CaSO4 14.4 365 2.1 3.69 0.011 4.04 0.023 4.19 0.027 Aluminum 15.4 trihydrate 390 2.6 3.67 0.009 3.81 0.010 3.89 0.011 Clay 14.4 365 6.4 4.08 0.018 4.61 Note: Isophthalic polyester resin based on a vinyl toluene monomer 0.040 5.10 0.057 Table 13 Electrical properties of BPA fumarate polyester 3.2 mm (⅛ in.) laminates with various fillers Filler Dielectric Volume Dielectric Dissipation Dielectric Dissipation Dielectric Dissipatio strength short resistivity, constant, factor ,1 constant, factor, 1 constant, factor, 6 time 60 Hz kHz 1 kHz MHz 10–13Ω· m 1 MHz Hz kV/mm kV/in. Calcium 6.1 carbonate 155 1.6 3.94 0.005 4.00 0.004 4.03 0.004 Gypsum CaSO4 150 3.3 3.72 0.009 4.03 0.024 4.24 0.029 300 3.3 3.64 0.008 3.81 0.015 3.93 0.025 0.043 5.11 0.053 5.9 Aluminum 11.8 trihydrate Clay 12.6 320 3.5 4.08 0.023 4.68 Note: BPA fumarate polyester resin based on a vinyl toluene monomer Because many electrical applications require performance at elevated temperatures, polyester resin composites must have good thermal stability. Thermal stability and electrical performance at elevated temperatures are directly related, as can be seen by comparing the retention of dielectric strength at 200 °C (390 °F), shown in Fig. 7, with the retention of flexural strength at 200 °C (390 °F), shown in Fig. 4. As with flexural strength, a vinyltoluene-based polyester outperforms a styrene-based polyester. At temperatures above 200 °C (390 °F), vinyl-toluene- based BPA fumarates outperform vinyl-toluene- based isophthalic polyesters (Fig. 8, 9). Fig. 7 Dielectric strength retention of glass-polyester composite when aged at 200 °C (390 °F), tested at room temperature Fig. 8 Dielectric strength retention of glass-polyester composite when aged at 220 °C (430 °F), tested at room temperature Fig. 9 Dielectric strength retention of glass-polyester composite when aged at 240 °C (465 °F), tested at room temperature Large electrical equipment, such as high-voltage motors or generators, often operate at elevated temperatures. In such applications, the electrical property of greatest concern is the dissipation factor, especially the dissipation factor versus the temperature. Polyester resins can be formulated for a low dissipation factor at elevated temperatures (Fig. 10). They are used as electrical varnishes at continuous-use temperatures up to 180 °C (355 °F). Fig. 10 Isophthalic polyester at 2.4 kV/mm (60 kV/in.) Flame-Retardant Polyester Resins All organic materials, including polyesters, will burn in the presence of a flame. In many applications, polyester resins are required to have some degree of resistance to burning. This can be accomplished by using either a filler or a specially formulated flame-retardant polyester resin, depending on the degree of resistance required. The addition of filler is the more economical route to achieving flame retardancy in parts made from UPEs. However, the addition of filler increases weight and compromises tensile properties. Incorporating halogen into a polyester resin is an effective way of improving flame retardance. This can be accomplished using a halogenated dibasic acid, such as chlorendic anhydride or tetrabromophthalic anhydride, or a halogenated dihydric alcohol, such as dibromoneopentyl glycol or tetrabromobisphenol A. At equivalent concentrations, bromine is much more effective than chlorine. Additives such as antimony oxides and ferrous oxide act as synergists with halogenated polyesters and improve their flame-retardancy properties (Ref 9). Burning rate and smoke generation are measured using the Steiner Tunnel Test (ASTM E 84). In this test, a gas burner is placed at one end of a 53 cm by 7.6 m (21 in. by 25 ft) section. The distance the flame travels and the amount of smoke generated are measured (by the obscuration of a photoelectric beam). These are compared to two standards: red oak board, which is given a rating of 100 for flame spread and smoke generation, and asbestos cement, which is given a rating of 0 for both flame spread and smoke generation. Smoke generation is also measured with an NBS smoke chamber, which uses a photoelectric cell to measure smoke buildup in a closed chamber. The sample is burned either with or without a direct flame. When NBS smoke chamber testing is used, it is often common to use ASTM E 162, Flame Spread Index, to measure this variable. Table 14 compares the performance of several resins using the above-mentioned fire and smoke tests. In each of these tests, the halogenated resin clearly outperforms the orthophthalic resin. Ferrous oxide also reduces smoke generation in the NBS chamber when compared to antimony oxide. Table 14 Performance of selected polyester composites in fire tests System Material or test I II III Material Resin 100(a) 100(b) 100(c) Alumina trihydrate 100 Antimony oxide Ferrous oxide 100 100 … 57 … … … 5 Test method and property ASTM E 162 Flame spread index 75 7 7 ASTM E 84 Flame spread 120 23 25 Smoke emission 608 270 268 NBS chamber: flaming mode System Material or test I II III Maximum density 203 433 264 90 s density 2.5 18 11 240 s density 162 245 128 NBS chamber: nonflaming mode Maximum density 481 400 350 90 s density 1 5 1 240 s density 16 45 50 (a) Orthophthalic resin. (b) HET acid resin A, 26% Cl. (c) HET acid resin B, 26% Cl. Source: Ref 10 Table 15 compares various filled and unfilled polyester resin composites. The improvement of an orthophthalic resin by the incorporation of alumina trihydrate (ATH) is dramatic, the flame spread is reduced from 350 to 64. However, halogenated resins reach this level of performance without the incorporation of any filler. Halogenated resins with a synergist show only a slight reduction in flame spread when ATH is added, but smoke emission is greatly reduced. When comparing brominated and chlorinated resins, 26% Cl gave comparable flame and smoke results as 18% Br. Table 15 Flame spread and smoke emission characteristics of unfilled and filled polyester systems Glass reinforced laminates 3.2 mm (⅛ in.) thick, containing 30% glass, tested in accordance with ASTM E 84 System formulation and property value Component or property 100(a) 100(b) 100(b) 100(c) 100(d) 100(d) 100(e) 100(e) 100(f) 100(f) Unfilled system Antimony oxide … … 5 … … 5 … 5 … 5 Flame spread 350 60 25 … 60 15 67 20 69 18 Smoke emission 1100 780 450 … 747 731 980 1043 837 838 100 100 100 100 100 100 100 100 Filled system Alumina trihydrate, phr Antimony oxide … Ferrous oxide … Flame spread 120 100 5 … … 23 … 5 5 5 20 25 … … 10 Smoke emission 608 270 242 168 364 (a) Orthophthalic resin. (b) HET acid resin A, 26% Cl. (c) HET acid resin B, 26% Cl. (d) Dibromotetrahydrophthalic resin, 18% Br. (e) Dibromotetrahydrophthalic glycol resin, 18% Br. (f) Tetrabromophthalic resin, 18% Br. 5 5 100 … 5 … 5 … … 5 25 18 10 10 12 260 450 400 761 620 Source: Adapted from Ref 10 References cited in this section 9. E. Dorfman, W.T. Schwartz, Jr., and R.R. Hindersinn, “Fire-Retardant Unsaturated Polyesters,” U.S. Patent 4,013,815, 1977 10. J.E. Selley and P.W. Vaccarella, Controlling Flammability and Smoke Emissions in Reinforced Polyesters, Plast. Eng., Vol 35, 1979, p 43 Acknowledgment This revised article is largely built around the original article “Polyester Resins” by Charles D. Dudgeon, Ashland Chemical Company, from Composites, Vol 1 of the Engineered Materials Handbook. References 1. M. Grayson and D. Eckroth, Ed., Encyclopedia of Chemical Technology, 3rd ed., Vol 18, John Wiley & Sons, 1982, p 575 2. P. Robitschek and C.T. Bean, Flame Resistant Polyesters from Hexochlorocyclopentadiene, Ind. Eng. Chem., Vol 46, 1954, p 1628 3. E.N. Doyle, The Development and Use of Polyester Products, McGraw-Hill, 1969, p 258 4. H.V. Boenig, Unsaturated Polyesters: Structure and Properties, Elsevier, 1964 5. B. Das, H.S. Loveless, and S.J. Morris, Effects of Structural Resins and Chopped Fiber Lengths on the Mechanical and Surface Properties of SMC Composites, 36th Annual Conference of the Reinforced Plastics Composites Institute, The Society of the Plastics Industry, 1981 6. P.K. Mallick, Fatigue Characteristics of High Glass Content SMC Materials, 37th Annual Technical Conference, Society of Plastics Engineers, 1979, p 589 7. H.S. Kliger and E.R. Barker, A Comparative Study of the Corrosion Resistance of Carbon and Glass Fibers, 39th Annual Conference of the Reinforced Plastics/Composites Institute, The Society of the Plastics Industry, 1984 8. A.L. Smith and J.R. Lowry, Long Term Durability of Acrylic Polyesters versus 100% Acrylic Resins in Glass Reinforced Constructions, 15th Annual Conference of the Reinforced Plastics/Composites Institute, The Society of the Plastics Industry, 1960 9. E. Dorfman, W.T. Schwartz, Jr., and R.R. Hindersinn, “Fire-Retardant Unsaturated Polyesters,” U.S. Patent 4,013,815, 1977 10. J.E. Selley and P.W. Vaccarella, Controlling Flammability and Smoke Emissions in Reinforced Polyesters, Plast. Eng., Vol 35, 1979, p 43 Copyright © 2003 ASM International®. All Rights Reserved.