CELLS Introduction An Introduction to Cell Structure & Function

CELLS
An Introduction to Cell Structure & Function
Introduction :
In this lab exercise we will be studying three general aspects of cellular structure and function.
First, we will observe the anatomical structures or organelles that are commonly associated with animal cells on an animal cell model. Using your lecture material , you will include a brief description of the molecular composition and general function of these organelles seen on the model.
The second part includes several different experiments where you will observe how cell membranes are semipermeable or selectively permeable. In other words, how the membrane functions to select which molecules may enter and exit the cell’s cytoplasm. These experiments focus on diffusion and osmosis as the major transport mechanisms that cells use to move some substances from one side of the cell membrane to the other.
Lastly, we will study cellular functions that are carried out by specialized proteins (molecules) called enzymes which carry out specific chemical reactions for the cell. We will be performing simulated and in-lab experiments where you will observe enzyme activity and other experiments where you will change the environment and affect how enzymes work under various conditions of pH change.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 1
Activity 1: Observation of the (animal) Cell Model
The cell is the basic unit of life. A single human is composed of trillions of individual cells. Each cell is surrounded by a cell membrane composed of a phospholipid bilayer which functions include providing a boundary and selectively transporting specific molecules into or out of the cell. Within cells, are organelles which are structures that function to isolate or compartmentalize specific sets of chemical reaction(s). The largest organelle is the nucleus which houses the DNA (deoxyribonucleic acid). The cytoplasm includes all the contents within the cell membrane except the nucleus. Cytosol refers to the gel like fluid that fills the space between the organelles of the cytoplasm.
Procedure :
1) Prepare for lab by using your textbook and/or lecture notes to label the organelles and cellular structures common to many human body cells in your notebook. Your labels should include all of the following: cell membrane, cytosol or cytoplasm, nucleus, nuclear membrane, nuclear pore, nucleoli, chromatin, rough endoplasmic reticulum, ribosomes, smooth endoplasmic reticulum, golgi (body), vesicles, mitochondria, lysosomes, cytoskeleton and centriole. Your diagram may include flagella, cilia and/or microvilli.
2) Prepare for lab by completing the table in your lab notebook, using your textbook and/or lecture notes, in your lab notebook by identifying the components (for example a plasma membrane, DNA, RNA, proteins, etc.) and the general functions each of these perform.
3) Prepare for lab by practicing identifying the cellular components using the photograph above or color photo provided on our course site.
4) During Lab, use your diagram in your notebook to aid in identifying the cellular anatomy on the cell model available.
5) Answer the remaining questions in your lab notebook.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 2
Activity 2: Diffusion Experiment
Diffusion is the movement of a substance from an area of high concentration to an area of low concentration.
The movement is due to the inherent kinetic energy of the particles of the substance. Diffusion is a key transport mechanism utilized by cells and it provides an efficient transport mechanism because the cell need not expend
ATP energy in order to transport these substances.
There are several factors that influence the rate of diffusion for any substance.
Chemical Characteristics
The chemical characteristics of the substances determine whether they may cross the membrane.
Substances that have similar chemical characteristics of the plasma membrane may diffuse across the membrane directly or if the solutes have different chemical characteristics then they may diffuse through the carrier proteins located within the plasma membrane.
Medium
The medium through which the solute is traveling affects its speed or rate of diffusion. If the solute is a gas, it moves through empty space at extremely rapid velocities. If the solute is traveling through a liquid, the liquid molecules may contribute to the speed of diffusion.
Temperature
Temperature at which the diffusion occurs can affect the rate. Temperature directly affects the kinetic energy of substances, the higher the temperature, the more kinetic energy a substance has.
In this experiment we will observe the movement of ions from a purple salt crystal, Potassium Permanganate
(KMnO
4
) as it dissolves and diffuses through a liquid and a semisolid. The semisolid is agar (1.5% gelatin) and the liquid will be water. The semisolid is analogous to the gel like composition of the cytoplasm . The water is the solvent common to biological systems which is analogous to the extracellular or interstitial fluid that bathes cells of our body.
**Note: record your results in the corresponding spaces of your lab notebook as you progress through the experiment.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 3
Experimental Procedure: Diffusion of KMnO
4
through a Semisolid
1) You will need the following materials: a. one petri dish with agar b. KMnO
4
crystals with forceps c. one sheet of white paper
2) Locate an area on your bench where the petri dish may be set up and remain undisturbed for approximately 1 hour.
3) Open the petri dish and place each (lid and base) on the white sheet of paper. (Save the lid for the
Experimental Procedure of potassium permanganate through liquid).
4) Obtain the KMnO
4
crystals and forceps, carefully drop several small crystals in the center of the agar.
Record the ‘Start Time’ for the semisolid in your Experimental Results Table.
5) Allow the KMnO
4
crystals to remain undisturbed for a minimum of one hour. (Go on to other activities)
6) Record the ‘End Time’ in your results table.
7) Using a millimeter ruler, measure the distance of diffusion (radius) from where you dropped the crystals on the agar to the outer edge.
8) Divide the distance moved (millimeters) by the time of diffusion (minutes) and record this time in your results table.
9) When you have completed this activity, the agar plate should be disposed of directly in the waste container (no special disposal is necessary).
Experimental Procedure: Diffusion of KMnO
4
through Liquid
1) You should have the lid of your petri dish on the white sheet of paper.
2) Squirt enough water, with the water bottle, to create a thin layer of water in the lid.
3) Wait several minutes for the water to come to a rest. (Do not agitate the water during this experimental procedure).
4) Use the forceps to place several small KMnO
4
crystals in the center of the water. Record the ‘Start Time’ for the liquid in your Experimental Results Table.
5) Allow the KMnO
4
crystals to remain undisturbed for 5-10 minutes. (Go on to other activities).
6) Record the ‘End Time’ in your results table.
7) Using a millimeter ruler, measure the distance of diffusion (radius) from where you dropped the crystals on the liquid to the outer edge.
8) Divide the distance moved (millimeters) by the time of diffusion (minutes) and record this time in your results table.
9) When you have completed this experimental procedure, the purple solution of water and KMnO
4
should be carefully poured into the sink with extra water running and then the lid may be disposed of in the waste container (no special disposal is necessary).
**Note: Answer all questions in your lab notebook.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 4
Activity 3: Osmosis Experiment
The movement of water across a plasma membrane occurs by diffusion, specifically, it is called osmosis — the diffusion of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration.
Approximately 80% of the volume of a cell is composed of water, whereas, the extracellular solutions surrounding cells is also mostly water and so water is the universal solvent . Within the water are dissolved substances or solutes . Water will move by diffusion with its concentration gradient.
We will use terms of tonicity to describe the concentration of the solution outside the cell as it compares to the concentration of the solution inside the cell —hypertonic, hypotonic and isotonic.
When a cell is placed in a solution with more solutes outside the cell as compared to inside the cell the solution is described as hypertonic . The solution outside the cell has more solutes, thus less water. Therefore, water will diffuse from the cytoplasm, across the membrane through special proteins called aquaporins . As a result, the cell will change shape, shrink or crenate .
If a cell is placed in a solution that has fewer solutes outside the cell as compared to the cytoplasm, the solution is described as hypotonic . Because there are more water molecules outside the cell, water will move into the cell.
As the cell takes on water, it will swell and may eventually lyse (burst).
In order to maintain homeostasis, cells are ideally bathed in isotonic solutions, where the solution outside the cell has an equal number of solutes as compared to inside the cell. Because the concentration of water is the same on both sides of the membrane, water moves in and out of the cell equally. Therefore, there is no change in the cells shape.
In the following experimental procedures we will be using potato cells and observing the movement of water in and out of their cytoplasm. Remember, plant cells have a cell wall which is responsible for maintaining the cell’s shape and preventing osmotic lysis. Animal cells lack cell walls, so animal cell shapes will change more dramatically than plant cells.
Before lab, define these terms in your lab notebook. There is a space provided for you to diagram the movement of water in/out of a cell. You should be using the diagrams with their labels provided in your completed lecture notes.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 5
Lab Safety: Use caution when handling glassware. Identify the location of the broken glassware container.
Experimental Procedure: Osmosis in Potato Strips
1) You will need the following Materials: a. test tube rack b. two test tubes c. grease pencil d. water e. 10% sodium chloride solution f. two potato strips (each cut approximately 7 cm long and 1 cm wide)
2) Label the test tubes #1 and #2.
3) Place one potato strip into each test tube.
4) Fill test tube #1 with water covering the potato strip.
5) Fill test tube #2 with 10% Sodium Chloride (NaCl) solution covering the potato strip.
6) Identify the tonicity of each solution covering the potato strips your table of Results: Tonicity of Potato Strips.
7) Allow the potato strips to remain covered in each solution for at least one hour. (Go on to other parts of today’s activities).
8) After at least one hour, pour the solutions off of the potato strips in the sink and observe each potato strip for bendability. The bendability of the potato strip is related to the amount of water that was removed from the potato cells or the amount of water that entered into the potato cells. Record your observations and explanation in your table of Results: Tonicity of Potato Strips.
9) When you have completed recording your results, place the potato strips in the waste container and use the dish soap to wash your test tube, remove the marks and rinse well. Place the clean wet test tubes upside down in the test tube rack with a dry paper towel underneath.
**Answer the related questions in your lab notebook.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 6
Activity 4: Introduction to pH (PreLab Activity)
Many chemical reactions that are involved in a cell’s metabolism produce or consume hydrogen ions (H
+
). The measure of the concentration of H
+
in a solution is the pH of the solution. As pH changes, the H
+
concentration rises or falls, can drastically affect other chemical reactions within the cell and create homeostatic imbalance.
Pure water has neutral pH. In a sample of pure water, there will be some water molecules that form ions; specifically, hydrogen ions and hydroxide ions. Because one water molecule splits creating one of each, it is balanced, and the water is neutral. Since water is the universal solvent and cells require water for chemical reactions to occur, some of these reactions can create more H
+
or more OH
-
and then the system is no longer neutral and we describe the resulting solutions as either acidic or basic.
H
2
O H
+
+ OH
-
The pH scale ranges from 0 to 14. A pH of 7 indicates a neutral solution where the number of H
+
equals the number of hydroxide OH
-
ions. A pH <7 is an acidic solution where there are more H
+
ions than hydroxide ions.
Any solution with a pH>7 is alkaline or basic and there are fewer H
+
than hydroxide ions.
As chemical reactions occur within a cell, Hydrogen ions may be produced or removed changing the pH.
Enzymes (proteins) require a specific pH in order to catalyze a chemical reaction. Changing the pH will directly affect how the enzyme functions.
To accommodate the changes in H
+ ion concentration, cells and fluids of the body contain buffers. Buffers are substances that can take up or give up H
+ ions in order to maintain a relatively constant pH. The most common buffers include blood proteins and bicarbonate ions.
Procedure: pH and Cells
1) To demonstrate how a solution’s pH will change, there is a table in your lab notebook that identifies three different solutions within three different test tubes. The pH was measured initially and then again after several drops of an hydrochloric acid were added to each test tube . From the pH measurements provided in the table, identify each solution as either Yes (buffered) or No (not buffered).
2) The last column of the table requires an explanation of your answer, explain (sentence) how the pH measurements led you to your Yes or No answer.
3) Answer the Experimental Analysis Questions.
.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 7
Activity 5: (PreLab Activity) Buffering Capacity
As hydrogen ion concentrations increases or decreases, there are buffers which help to maintain homeostasis. If the hydrogen ion concentration changes too much or too fast, proteins may be denatured (rendered inactive) which directly affect cell function.
Albumin and bicarbonate ions are buffers found in blood. The chemical reaction below shows that bicarbonate
(HCO
3
-
) can take up hydrogen ions and produce the products water and carbon dioxide (which is excreted as waste by the lungs) which would raise the pH as hydrogen ion concentration decrease. The reverse reaction occurs as bicarbonate is formed, producing hydrogen ions which raise the pH.
HCO
3
-
+ H
+
H
2
CO
3
H
2
O + CO
2
Note: The chemical reaction above is reversible, , demonstrating how H
+
can be used up/removed and formed/created.
HCO
3
-
+ H
+
H
2
CO
3
H
2
O + CO
2
Hydrogen ions are used up/removed to make water and carbon dioxide.
HCO
3
-
+ H
+
H
2
CO
3
H
2
O + CO
2
Hydrogen ions are formed/created in the formation of bicarbonate from carbon dioxide and water.
In the following simulated experiment, you will prepare a graph to demonstrate how pH changes when acid is added to three different solutions: pure water, simulated cytoplasm and an inorganic buffer. The experiment consisted of adding 5 drops of HCl (hydrochloric acid) to a beaker containing a solution and measuring the resulting pH and repeating the addition of another 5 drops and pH measurement until a total number of 50 drops of acid was added to each beaker.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 8
Organize the following information as a graph in your lab notebook on the grid provided.
1) Include a Title on your graph.
2) Use the number of drops of acid as a label on the x-axis (horizontal).
3) Use the pH value as a label on the y-axis (vertical).
4) Use three different colors to represent each of the three different solutions to create three lines on your graph. For example , use a red pen for Beaker #1 Water only, blue pen for Beaker #2 Inorganic Solution and a green pen for Beaker #3 Simulated Cytoplasm. So you will have one graph with three different colored lines on the graph (overlapping) demonstrating each solution’s pH change as the acid was added.
5) Be sure to add a legend to your graph (a legend is where you identify the color of the pen to the beaker it represents). Graph the data for each beaker and connect the points.
6) Once you have completed your graph, answer the experimental data analysis questions. pH
Number of Drops
Of 0.1 M
Hydrochloric Acid*
Beaker #1
Water
Beaker #2
Inorganic Solution
Beaker #3
Simulated Cytoplasm
0
5
7.0
6.3
6.8
6.8
7.1
7.0
10
15
20
25
5.5
4.8
4.1
3.3
6.8
6.8
6.8
6.8
7.0
7.0
7.0
6.8
30
35
40
45
2.8
2.2
1.8
1.5
6.8
6.8
6.8
6.0
6.5
5.0
4.2
4.0
50 1.5 4.8 3.2
*M (molarity) is a unit of concentration of solutions. 0.1M HCl represents a dilute solution of hydrochloric acid and water.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 9
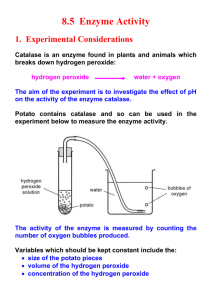
Procedure 6: Enzyme Activity
Enzymes are proteins which function as biological catalysts. They speed up chemical reactions. Many function to synthesize larger molecules from atoms or smaller molecules or they break larger molecules down into smaller molecules or atoms. Since enzymes are proteins, each protein has its own unique three-dimensional shape.
Shape always dictates function.
Enzymes interact or fit with reactant (s) ( substrates ). The place on the enzyme that the substrate fits is called the active site . Once the enzyme interacts with the substrate, the enzyme changes shape slightly which helps the substrate to rearrange, break or create new chemical bonds leading to the formation of a new product .
We will be observing the formation of oxygen gas (O
2
), a product , which is produced by the degradation of the enzyme catalase which acts on its substrate / reactant hydrogen peroxide (H
2
O
2
). Catalase is an enzyme located within the cytoplasm of many different types of cells, including potato cells. If the catalase protein can interact with its substrate hydrogen peroxide, then it will break the bonds in hydrogen peroxide forming the products oxygen gas, observed as bubbles, and water.
H
2
O
2
—
catalase
--> H
2
O + O
2
Hydrogen Peroxide
Catalase
Oxygen gas and Water
Catalase
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 10
Lab Safety: Caution—Protective Eyewear should be worn!
Caution—if any hydrogen peroxide comes in contact with your skin, go to the sink and wash with soap and water.
centimeter ruler you will find the hydrogen peroxide, sand and potato at the counter
Materials: protective eyewear test tube rack three clean test tubes grease pencil
Experimental Procedure: Enzyme Activity
1) Prepare for this lab activity by completing the definitions and descriptions in your lab notebook. (Complete the test results and experimental analysis question upon completion of the following experiment)
2) Label the three test tubes, #1, #2 and #3 with the grease pencil.
3) From the bottom of the test tube, use the centimeter ruler to identify where a liquid would fill the test tube to a depth of 3 cm.
4) Place the following in test tube #1: a) pinch of sand b) fill the test tube to the 3 cm line with Hydrogen peroxide. c) Record the contents of test tube #1 in your results table.
5) Place the following in test tube #2: a) fill the test tube to the 3 cm line with Hydrogen peroxide. b) Add one (cubic centimeter) potato to the test tube. c) Record the contents of test tube #2 in your results table.
6) Place the following in test tube #3 a) Fill the test tube to the 3 cm line with Hydrogen peroxide. b) Add approximately one cubic centimeter macerated potato to the test tube. (It should be about the same amount of potato as test tube #2 just macerated or mashed). c) Record the contents of test tube #3 in your results table.
7) Gently agitate or swirl the contents of the test tubes. You may allow them to sit for a couple of minutes (no more than 5 minutes) and diagram the bubbling and record the amount of bubbling.
Result Record Amount of Bubbling
No bubbling
Moderate bubbling
Good bubbling
Profuse bubbling
0
+
++
+++
8) Experiments are designed to be reproducible. Confirm that you have typical results by comparing your results with some of the other lab partners and your lab instructor. Record your results in your lab notebook.
9) Once your lab results have been confirmed by your lab instructor, the liquid portion of each test tube may be rinsed down the sink with water, the potato and sand should be placed in a waste container. Wash your test tubes with soap and rinse with water before returning to your test tube rack.
10) Answer the experimental analysis questions as time permits during lab.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 11
Activity 7: Effect of pH on Enzyme Activity
All proteins have a unique 3-dimensional shape . This shape can be changed by a variety of factors including temperature and pH. Once the shape is changed, the protein, in this case an enzyme may have an altered active site. By changing the shape of the active site, the enzyme loses its ability to catalyze a chemical reaction. This is called denaturation or the enzyme is denatured .
By changing the pH, either raising or lowering, a decrease the productivity of the enzyme results. So each enzyme has an optimum (best) pH at which it can carry out its function.
In this experiment, we will be adding acid or a base and observing how well the enzyme catalase can produce its product.
Lab Safety: Caution—Protective Eyewear should be worn!
Caution—if any hydrogen peroxide, HCl (acid) or NaOH( base) comes in contact with your skin, go to the sink and wash with soap and water.
Materials : protective eyewear test tube rack three clean test tubes grease pencil centimeter ruler distilled water hydrogen peroxide hydrochloric acid (HCl sodium hydroxide
(NaOH) potato
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 12
Experimental Procedure: Effect of pH on Enzyme Activity
1) Prepare for this lab activity by defining the terms in your lab notebook.
2) Label the three test tubes, #1, #2 and #3 with the grease pencil.
3) From the bottom of the test tube, use the centimeter ruler to identify where a liquid would fill the test tube to a depth of 2 cm and 6 cm.
4) Place the following in test tube #1: a) fill the test tube to the 2 cm mark with distilled water. b) add 1 cubic centimeter of macerated potato c) agitate or mix the contents of the tube. d) Wait 3 minutes before continuing to ‘e’. e) add hydrogen peroxide to the 6 cm mark.
5) Place the following in test tube #2: a) fill the test tube to the 2 cm mark with Hydrochloric Acid (HCl). Be careful to use only the pipette provided
(never mix up pipettes) b) add 1 cubic centimeter of macerated potato c) agitate or mix the contents of the tube. d) Wait 3 minutes before continuing to ‘e’. e) add hydrogen peroxide to the 6 cm mark.
6) Place the following in test tube #3 a) fill the test tube to the 2 cm mark with Sodium Hydroxide (NaOH). Be careful to use only the pipette provided (never mix up pipettes) b) add 1 cubic centimeter of macerated potato c) agitate or mix the contents of the tube. d) Wait 3 minutes before continuing to ‘e’. e) add hydrogen peroxide to the 6 cm mark.
7) You may allow them to sit for a couple of minutes (no more than 5 minutes) and record the amount of bubbling.
Result
No bubbling
Moderate bubbling
Good bubbling
Record Amount of Bubbling
0
+
++
Profuse bubbling +++
8) Experiments are designed to be reproducible. Confirm that you have typical results by comparing your results with some of the other lab partners and with your lab instructor. Record your results in your lab notebook.
9) Once you have confirmed your results with your lab instructor, pour the liquid into the sink with running water.
The potato should be placed in the waste containers. Wash your test tubes with soap and rinse with water before returning to your test tube rack.
10) Answer the experimental analysis questions during lab as time permits.
Note : After you have completed all lab activities (results, answered questions, etc.) write the conclusion of the entire Lab Exercise.
Lab Exercise: Cells , An Introduction to Cell Structure and Function (Revised Spring, 2012) page 13
Filename:
Directory:
Template:
Title:
Subject:
Author:
Keywords:
RevSpr2012_Cells
E:\CopyUDrive\Lab_Manual_Rev_Spr2012
C:\Users\karen\AppData\Roaming\Microsoft\Templates\Normal.dotm
CELLS klchambe
Comments:
Creation Date:
Change Number:
Last Saved On:
5/13/2012 7:14:00 AM
2
5/13/2012 7:14:00 AM
Last Saved By:
Total Editing Time: karen
0 Minutes
Last Printed On: 5/13/2012 7:14:00 AM
As of Last Complete Printing
Number of Pages: 13
Number of Words: 3,478 (approx.)
Number of Characters: 19,826 (approx.)