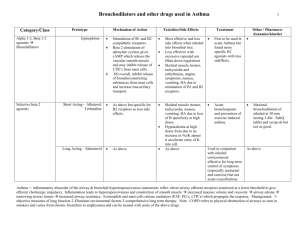
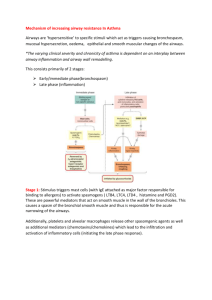
Basic & Clinical Pharmacology 11e Drugs Used for the Management of Asthma
advertisement

Institute for Personalized Respiratory Medicine Department of Medicine (Section of Pulmonary, Critical Care, Sleep and Allergy ) Department of Pharmacology Center for Cardiovascular Research Drugs Used for the Management of Asthma Jason X.-J. Yuan, M.D., Ph.D. Professor of Medicine and Pharmacology University of Illinois at Chicago Reference Katzung BG, Masters SB, Trevor AJ Basic & Clinical Pharmacology 11e Chapter 20: Drugs Used in Asthma (Homer A. Boushey and Bertram G. Katzung) Leaning Objectives Definition and basic pathology of asthma Various cell types and mediators in the pathogenesis of asthma Rationale for the use of β-agonist therapy (bronchodilation) and its side effects Therapeutic actions of cromolyn (inhibiting mast cell degranulation), corticosteroids (anti-inflammation), and theophylline (bronchodilation and anti-inflammation) 1 Definition of Asthma (What is Asthma?) Physiologically characterized a) by increased responsiveness of the trachea and bronchi to various stimuli and b) by widespread narrowing of the airways Pathologically featured by airway smooth muscle contraction, mucosal thickening from edema and cellular infiltration, an inspissation in the airway lumen of abnormally thick, viscid plugs of mucus Definition of Asthma Asthma is a chronic inflammatory disease of the airways Hyper-responsiveness Airway contraction (bronchospasm) Inflammation Airway/bronchial remodeling (thickening) Asthma Therapy Short-term Relievers: Bronchodilators – β-adrenoceptor agonists (e.g., isoproterenol) – Antimuscarinic agents (e.g., theophylline) Long-term Controllers: Anti-inflammatory Agents – Inhaled corticosteroid – Leukotriene antagonists – Inhibitors of mast cell degranulation (e.g., cromolyn or nedocromil) 2 Schematic Diagram of the Deposition of Inhaled Drugs Metered-dose inhaler (MDI) Delivery by inhalation results in the greatest local effect on airway smooth muscle with the least systemic toxicity. Aerosol deposition depends on particle size, breathing pattern, airway geometry. Even with particles in the optimal size range of 2-5 μm, 80-90% of the total dose of aerosol is deposited in the mouth or pharynx. Pathogenesis of Asthma (Immunological Model) 1) IgE antibodies bound to mast cells in airway mucosa 2) On reexposure to antigens, antigen-antibody interaction on the surface of master cells triggers release/synthesis of mediators (e.g., histamine, tryptase, leukotrienes, and PGs) 3) Mediators (also including cytokines, interleukins) cause bronchial contraction (smooth muscle), vascular leakage, cellular infiltration, mucus hyper-secretion 4) Inflammatory response Conceptual Model for the Immunopathogenesis of Asthma 4 Cytokines activate eosinophils/ neutrophils releasing ECP/MBP proteases, PAF, and cause late reaction 3 3 Bronchoconstriction, vascular leakage, cellular infiltration 3 2 2 4 On reexposure to allergens, antigen-antibody interaction causes release of mediators 1 1 Allergen causes synthesis of IgE which binds to mast cells; Allergen activates T-cells 3 Hyperresponsiveness Bronchospasm can be elicited by: Allergens (hypersensitivity to) Non-antigenic stimuli (e.g., distilled water, exercise, cold air, sulfur dioxide, and rapid ventilation) (“nonspecific bronchial hyperreactivity” ) Bronchial hyperreactivity is quantitated by measuring the fall in FEV1 (forced expiratory volume in 1 s) provoked by inhaling aerosolized histamine or methacholine (serially increasing concentration) Mechanisms of Bronchial Hyperreactivity 1) Inflammation of airway mucosa 2) Increased ozone exposure, allergen inhalation, & viral infection (causing airway inflammation) 3) Increased inflammatory cells (eosinophils, neutrophils, lymphocytes and macrophages) and increased products from these cells (causing airway smooth muscle contraction) 4) Sensitization of sensory nerves (afferent and efferent vagal nerves) in the airways 5) Cellular mechanisms in airway smooth muscle cells and epithelial cells Asthmatic Bronchospasm Caused by a combination of: Increased release/synthesis of contractile mediators (mainly from master cells and inflammatory cells) Enhanced responsiveness of airway smooth muscle to these mediators Afferent and efferent vagal nerves (e.g., cholinergic motor fibers innervate M3 receptors on the smooth muscle) Airway smooth muscle cells Airway epithelial cells 4 Mechanisms of Inhaled Irritant-mediated Bronchial Constriction CNS Inhaled irritants can cause bronchoconstriction by: (1) Triggering release of chemical mediators from response cells (e.g., mast cells, eosinophils, neutrophils) (2) Stimulating afferent receptors to initiate reflex bronchoconstriction (via acetylcholine, ACh) or to release tachykinins (e.g., substance P) that directly stimulate smooth muscle contraction 1 2 1 ACh Asthmatic Bronchospasm Treated by drugs that: Reduce the amount of IgE bound to mast cells (antiIgE antibody) Prevent mast cell degranulation (cromolyn, βagonists, calcium channel blockers) Block the action of released mediators (antihistamine, leukotriene receptor blockers) Inhibit the effect of acetylcholine (ACh) released from vagal motor nerves (muscarinic antagonists) Directly relax airway smooth muscle (theophylline, β-agonists) Basic Pharmacology of Agents for Treatment of Asthma The drugs mostly used for management of asthma are: β-Adrenoceptor agonists – Used as “short-term relievers” or bronchodilators Inhaled corticosteroids – Used as “long-term controllers” or antiinflammatory agents 5 Basic Pharmacology of Agents for Treatment of Asthma Symathomimetic Agents (β-adrenoceptor agonists) Epinephrine, isoproterenol, salmeterol, formoterol Corticosteroids Beclomethasone, flunisolide, fluticasone, triamcinolone Methylxanthine Drugs Theophylline, theobromine, caffeine Antimuscarinic Agents Ipratropium, atropine Cromolyn and Nedocromil (inhibitors of mast cell degranulation) Leukotriene Inhibitors Zileuton, montelukast, zafirlukast Other Drugs in the Treatment of Asthma: Anti-IgE monoclonal antibodies (omalizumab), calcium channel blockers (nifedipine, verapamil), Nitric oxide donors (sodium nitroprusside) Basic Pharmacology (Sympathomimetic Agents) Adrenergic Receptors (adrenoceptors): – α-receptors (α1, α2) – β-receptors β1, heart muscle (causing increased heart rate/contractility); kidney (causing renin release) β2, airway smooth muscle (causing bronchodilation); GI smooth muscle, cardiac muscle, skeletal muscle, vascular smooth muscle β3, adipose tissue (causing lipolysis, increasing fatty acids in the blood) Bronchodilation is Promoted by Increased cAMP Bronchodilation β-agonists AC, adenylyl cyclase cAMP Bronchial tone Acetylcholine Muscarinic antagonists Theophylline Adenosine Theophylline Bronchoconstriction + Activate or increase _ Inhibit or decrease 6 Basic Pharmacology (Sympathomimetic Agents) Mechanisms of Action – – Activation of β-adrenergic receptor β1 and β2 receptors G protein-coupled receptor Stimulation of adenylyl cyclase (AC) Ten known ACs (AC1-AC10) AC1, AC3 and AC8 are activated by Ca2+/CaM AC5 and AC6 are inhibited by Ca2+/CaM – Increase in the formation of cAMP – Relaxation of airway smooth muscle Molecular Action of β2agonists to Induce Airway Smooth Muscle Relaxation Basic Pharmacology (Sympathomimetic Agents) “Non-selective” β-Adrenoceptor Agonists (β1 and β2) – Epinephrine – Ingredient in non-prescription inhalants Ephedrine – Injected subcutaneously or inhaled as a microaerosol, rapid action (15 min) Oral intake, long-lasting action, obvious central effects (used less frequently now) Isoproterenol Inhaled as a microaerosol, rapid action (5 min) 7 Basic Pharmacology (Sympathomimetic Agents) Selective β2-Adrenoceptor Agonists (most widely used β-agonists for the treatment of asthma) – Terbutaline, Metaproterenol, Albuterol, Pirbuterol, Levalbuterol, Bitolterol – Inhalation from a metered-dose inhaler Bronchodilation is maximal by 30 min and persists for 3-4 hrs Salmeterol, Formoterol Long-acting β2 agonists (12 hrs or more) High lipid solubility (into smooth muscle cells) Interact with inhaled corticosteroids to improve asthma control Basic Pharmacology (β-adrenoceptor Agonists) Administration – – Inhalation (by aerosol) Available orally and for injection Side Effects – – – – Muscle tremor Tachycardia and palpitations Increased free fatty acid, glucose, lactate V/Q mismatch due to pulmonary vasodilation Basic Pharmacology (Corticosteroids) Mechanism of Action – Anti-inflammatory effect mediated by inhibiting production of inflammatory cytokines – – – – Inhibition of the lymphocytic, eosinophic airway mucosal inflammation of asthmatic airways Reduce bronchial reactivity Reduce the frequency of asthma exacerbations if taken regularly No relaxant effect on airway smooth muscle Potentiate the effect of β-agonists 8 Basic Pharmacology (Corticosteroids) Administration – – Inhaled (aerosol treatment is the most effective way to decrease the systemic adverse effects, e.g., lipid-soluble beclomethasone, budesonide, flunisolide, fluticasone, triamcinolone) Oral and parenteral (e.g., intravenous infusion) use is reserved for patients who require urgent treatment (“nonresponders” to bronchodilators) Clinical Pharmacology (Corticosteroids) Side Effects – – Dysphonia Oropharyngeal candidiasis (an opportunistic mucosal infection caused by the fungus ) Both can be reduced by mouth rinsing with water after inhalation vocal cords Effect of Corticosteroids on Inflammatory and Structural Cells in the Airway 1) Anti-inflammation 2) Reducing bronchial reactivity 9 Cellular Mechanism of antiinflammatory Action of Corticosteroids in Asthma GR, glucocorticoid receptor Basic Pharmacology (Methylxanthine Drugs) Major methylxanthines – Theophylline – Aminophylline (a theophylline-ethylenediamine complex) Dyphylline (a synthetic analog of theophylline) Theobromine – 1,3-dimethylxanthine 3,7-dimethylxanthine Caffeine 1,3,7-trimethylxanthine Inexpensive and can be taken orally Basic Pharmacology (Methylxanthine Drugs) Mechanisms of Action – Bronchodilation – Inhibition of phosphodiesterases (PDEs; e.g. PDE4), which results in an increased level of cAMP (and cGMP) causing airway smooth muscle relaxation Inhibition of adenosine receptor on the surface membrane (adenosine causes airway smooth muscle contraction and provokes histamine release from master cells) Anti-inflammation Inhibition of antigen-induced release of histamine from lung tissue 10 Theophylline Affects Multiple Cell Types in the Airway Mechanisms of Theophyllinemediated Bronchodilation ATP/GTP Bronchodilation AC GC β-agonists cAMP cGMP Bronchial tone PDE, phosphodiesterase PDE4 PDE5 Theophylline Theophylline AMP/GMP Acetylcholine Muscarinic antagonists Adenosine Theophylline + Activate or increase _ Bronchoconstriction Inhibit or decrease Basic Pharmacology (Antimuscarinic Agents) Mechanism of Action – Inhibits the effect of acetylcholine (ACh) at muscarinic (M) receptors Block airway smooth muscle contraction Decrease mucus secretion by blocking vagal activity Major Antimuscarinic Agents – – – Atropine Ipratropium bromide (a selective quaternary ammonium derivative of atropine) Tiotropium (for COPD) 11 Antimuscarinic Agentmediated Bronchodilation CNS Atropine and Ipratropium blocks bronchoconstriction induced by vagal activity 1 ACh Basic Pharmacology (Cromolyn & Nedocromil) Mechanism of Action – Blockade of chloride channels and calcium channels in mast cells (and airway smooth muscle cells), and inhibition of cellular activation – Inhibition of mast cell degranulation (inhibiting inflammatory response to allergens, exercise, cold air. Inhibition of eosinophils/neutrophils to release inflammatory mediators – Inhibition of bronchial responsiveness (with long-term treatment) – No bronchodilator or antihistamine activity Basic Pharmacology (Leukotriene Inhibitors) Mechanism of Action – Leukotriene causes bronchoconstriction, increased bronchial reactivity, mucosal edema, and mucus hypersecretion – Inhibition of 5-lipoxygenase on arachidonic acid leads to decreased synthesis of leukotriene (zileuton) – Blockade of leukotriene D4 receptors leads to decreased action of leukotriene (zafirlukast, montelukast) – Both inhibitors (used orally) decrease airway responses to allergens and exercise 12 Effects of Leukotrienes on the Airways and Their Inhibition by Anti-leukotriene Drugs LT Synthesis Inhibitors LTC4 Receptor Blockers Basic Pharmacology (Other Drugs) Anti-IgE Monoclonal Antibodies – – Nifedipine, verapamil Nitric Oxide Donors – Omalizumab (anti-IgE Mab) Calcium channel blockers Sodium nitroprusside (SNP) Possible Future Therapies – Monoclonal antibody against to cytokines (e.g., IL-4/-5/-8), antagonists of cell adhesion molecules, protease inhibitors, etc. Leaning Objectives Definition and basic pathology of asthma Various cell types and mediators in the pathogenesis of asthma Rationale for the use of β-agonist therapy (bronchodilation) and its side effects Therapeutic actions of cromolyn (inhibiting mast cell degranulation), corticosteroids (anti-inflammation), and theophylline (bronchodilation and anti-inflammation) 13 Questions Jason Yuan – 312-355-5911 (office phone) – jxyuan@uic.edu (email) – COMRB 3131 14