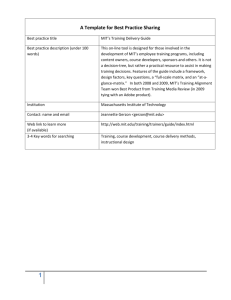
3.23 Electrical, Optical, and Magnetic Properties of Materials MIT OpenCourseWare Fall 2007
advertisement

MIT OpenCourseWare
http://ocw.mit.edu
3.23 Electrical, Optical, and Magnetic Properties of Materials
Fall 2007
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
3.23 Fall 2007 – Lecture 6
VARIATIONS AND VIBRATIONS
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Last time
1.
2.
3.
4.
Orbitals in atoms, nodal surfaces
Good quantum numbers
Spin
Spin-statistics, Pauli principle, auf-bau filling of the periodic table
5. Mean field solutions for non-hydrogenoid atoms
in a central potential
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
1
Study
• “Study
Study 4”
4
posted: Prof Fink’s notes on
lattice dynamics
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
From waves to vector space
A vector space V is a set which is closed under “vector addition” and “scalar multiplication”
We start with an abelian group, with an operation “+” and elements “u, v,…”
1. Commutative: u+v=v+u
2. Associative: (u+v)+w=u+(v+w)
3. Existence of zero: 0+u=u+0=u
4. Existence of inverse –u: u+(-u)=0
We add a scalar multiplication by “α,β…”
5. Associativity of scalar multiplication: α(βu)=
α(βu) (αβ)u
6. Distributivity of scalar sums: (α+β)u=αu+βu
7. Distributivity of vector sums: α(u+v)= αu+ αv
8. Scalar multiplication identity: 1u=u
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
2
Dirac’s <bra|kets> (elements of vector space)
G
ψ = ψ (r ) = ψ
Scalar product induces a metric → Hilbert space
G
G
(= δ
G
*
∫ψ i ((r )ψ j ((r ) ddr = ψ i ψ j
ij
if orthogonal
th
l)
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Expectation values
ψ =
∑c
n =1, k
n
ϕn
{ ϕ } orthogonal
n
ψ Ĥˆ ψ
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
3
Matrix Formulation (I)
Hˆ ψ = E ψ
ψ =
∑
c
n ϕn
n =1, k
{ ϕ } orthogonal
n
ϕm H
Ĥ ψ = E ϕ m ψ
∑
c
n =1, k
n
ϕ m Ĥ ϕ n = Ecm
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Matrix Formulation (II)
∑
H
n =1 k
n=1,k
⎛ H11
⎜
⎜ .
⎜ .
⎜⎜
⎜ .
⎜H
⎝
k1
......
......
c = Ecm
mn n
H
1k ⎞ ⎛ c1 ⎞
⎛ c1 ⎞
⎟ ⎜ ⎟
⎜ ⎟
.
⎟ ⎜ .
⎟
⎜ .
⎟
.
⎟ ⋅
⎜ .
⎟ =
E
⎜ .
⎟
⎟⎟
⎜⎜
⎟⎟
⎜⎜
⎟⎟
.
⎟ ⎜ .
⎟
⎜ .
⎟
⎜ c
⎟
H
kk ⎟⎠
⎜⎝
c
k ⎟⎠
⎝
k ⎠
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
4
Matrix Formulation (III)
⎛ H11 − E
⎜
.
⎜
det ⎜
.
⎜
.
⎜
⎜ H
k1
⎝
H1k ⎞
⎟
.
⎟
⎟=0
.
⎟
.
⎟
H kk − E ⎟⎠
......
H 22 − E
......
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Variational Principle
E [Ψ ] =
E [ Ψ ] ≥ E0
ΨH
Ĥ Ψ
Ψ Ψ
If E [ Ψ ] = E0, then Φ is the ground
state wavefunction, and viceversa…
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
5
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
6
Atomic Units
• me=1
1, ee=1
1, a0 (Bohr radius)
radius)=1
1, = = 1
ε0 =
1
4π
1 Z 2
2 n2
(1 atomic unit of energy=1 Hartree=2 Rydberg=27.21 eV
E
Energy
off 1s
1 elect
l tron= −
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Energy of an Hydrogen Atom
Eα =
Ψα Ĥ Ψα
Ψα Ψα Ψα = C exp ( −α r )
Ψα Ψα = π
C2
α
3
,
C2
1
Ψα − ∇ 2 Ψα = π
2
2α
Ψα −
C2
1
Ψα = −π 2
r
α
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
7
Hydrogen Molecular Ion H2+
• Born-Oppenheimer
Born Oppenheimer approximation: the
electron is always in the ground state
corresponding to the instantaneous ionic
positions
⎡ 1
⎛
1
1
⎢− ∇2 + ⎜ G 1 G −
G −
G
⎜ RH − RH
⎢ 2
−
−
r
R
r
R
H
H2
2
1
⎝ 1
⎣
⎞⎤ G
⎟ ⎥ψ (r ) = Eψ (rG )
⎟⎥
⎟⎥
⎠⎦
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Linear Combination of Atomic Orbitals
• Most common approach to find out the groundstate solution – it allows a meaningful definition
of “hybridization”, “bonding” and “anti-bonding”
orbitals.
• Also knows as LCAO, LCAO-MO (for molecular orbitals), or tight-binding (for solids)
• Trial wavefunction is a linear combination of
atomic orbitals – the variational parameters are
the coefficients:
Ψ trial = c1Ψ1s
(
G G
G G
r − RH + c2 Ψ1s r − RH 2
1
)
(
)
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
8
Bonding and Antibonding (I)
z
z
z
1sA
+
y
y
y
_
Overlap
region
1sB
∗
(a)
∗
(b)
(c)
The orbital region for the σg1s and σ 1s LCAO molecular orbitals. (a) The overlapping orbital regions of
∗ orbital region of the σ 1s LCAO-MO. (c) The orbital Region of
the 1sA and 1sB atomic orbitals. (b) The
g
u
the σ 1s LCAO-MO. The orbital regions of the LCAO molecular orbitals have the same general features
as the "exact" Born Oppenheimer orbitals whose orbital regions were depicted in Figure 18.4.
u
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Formation of a Bonding Orbital
Image removed due to copyright restrictions. Please see the animation of hydrogen bonding orbitals at
http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma/index.html
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
9
Formation of an Antibonding Orbital
Image removed due to copyright restrictions. Please see the animation of hydrogen antibonding orbitals at
http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma-star/index.html
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Bonding and Antibonding (II)
8
σ*1s
u energy
6
4
EBO/eV
2
0
σg1s energy
1
Born-Oppenheimer energy
of exact orbitals
2
3
4
-2
-4
-6
R/10-10m
Orbital Energies for the σg 1s and σ*u 1s LCAO Molecular Orbitals.
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
10
The Quantization of Vibrations
• Electrons are much lighter than nuclei
(mproton/melectron~1800)
• Electronic wave-functions always rearrange
themselves to be in the ground state (lowest
energy possible for the electrons), even if the ions
are moving around
• Born-Oppenheimer approximation: electrons in the instantaneous potential of the ions (so, electrons can not be excited – FALSE in general)
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Nuclei have some quantum action…
• Go back to Lecture 1 – remember the h
i oscillator
ill t
harmonic
8
4
2
Mass
EBO/eV
Spring
Equilibrium
position of
mass
σ*1s
u energy
6
Stationary
object
z
0
z
0
σg1s energy
1
Born-Oppenheimer energy
of exact orbitals
2
3
4
-2
-4
A mass on a spring. This system can be
represented by a harmonic oscillator.
-6
R/10-10m
Orbital Energies for the σg 1s and σ*u 1s LCAO Molecular Orbitals.
Figures by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
11
The quantum harmonic oscillator (I)
⎛ =2 d 2 1 2 ⎞
+ kz ⎟ ϕ ( z ) = E ϕ ( z )
⎜−
2
2
⎝ 2M dz
⎠
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
The quantum harmonic oscillator (I)
⎛ =2 d 2 1 2 ⎞
+ kz ⎟ ϕ ( z ) = E ϕ ( z )
⎜−
2
2M
dz
2
⎝
⎠
ω=
k
m
a=
km
=
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
12
The quantum harmonic oscillator (II)
E (in units of hνo)
5
V(x)
n=4
4
n=3
3
n=2
2
n=1
1
n=0
0
x
Figure by MIT OpenCourseWare.
1⎞
⎛
E = =ω ⎜ n + ⎟
2⎠
⎝
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Quantized atomic vibrations
Figure by MIT OpenCourseWare.
Courtesy of Dr. Klaus Hermann. Used with permission.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
13
Specific Heat of Graphite (Dulong and Petit)
2500
CP (J.K-1.kg-1)
2000
1500
1000
500
0
0
500
1000
1500
2000
2500
Temperature (K)
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
14