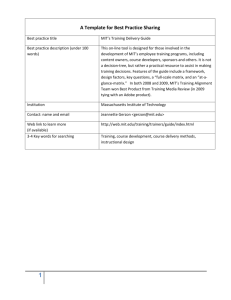
3.23 Electrical, Optical, and Magnetic Properties of Materials MIT OpenCourseWare Fall 2007
advertisement

MIT OpenCourseWare
http://ocw.mit.edu
3.23 Electrical, Optical, and Magnetic Properties of Materials
Fall 2007
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
3.23 Fall 2007 – Lecture 6
VARIATIONS AND
VIBRATIONS
3.23 Electronic, Optical and Magnetic Properties of Materials ‐ Nicola Marzari (MIT, Fall 2007)
Last time
1.
2.
3.
4.
Orbitals in atoms, nodal surfaces
Good quantum numbers
Spin
Spin-statistics, Pauli principle, auf-bau filling of
the periodic table
5. Mean field solutions for non-hydrogenoid atoms
in a central potential
3.23 Electronic, Optical and Magnetic Properties of Materials ‐ Nicola Marzari (MIT, Fall 2007)
Study
• “Study 4” posted: Prof Fink’s notes on
lattice dynamics
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
From waves to vector space
A vector space V is a set which is closed under “vector addition” and “scalar multiplication”
We start with an abelian group, with an operation “+” and elements “u, v,…”
1. Commutative: u+v=v+u
2. Associative: (u+v)+w=u+(v+w)
3. Existence of zero: 0+u=u+0=u
4. Existence of inverse –u: u+(-u)=0
We add a scalar multiplication by “α,β…”
5. Associativity of scalar multiplication: α(βu)= (αβ)u
6. Distributivity of scalar sums: (α+β)u=αu+βu
7. Distributivity of vector sums: α(u+v)= αu+ αv
8. Scalar multiplication identity: 1u=u
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Dirac’s <bra|kets> (elements of vector
space)
G
ψ = ψ (r ) = ψ
Scalar product induces a metric → Hilbert space
G
G G
∫ψ (r )ψ j (r ) dr = ψ i ψ j
*
i
(= δ
ij
if orthogonal)
3.23 Electronic, Optical and Magnetic Properties of Materials ‐ Nicola Marzari (MIT, Fall 2007)
Expectation values
ψ =
∑c
n =1, k
n
ϕn
{ ϕ } orthogonal
n
ψ Ĥ ψ
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Matrix Formulation (I)
Ĥ ψ = E ψ
ψ =
∑c
n =1, k
n
ϕn
{ ϕ } orthogonal
n
ϕ m Hˆ ψ = E ϕ m ψ
ˆ
c
ϕ
H
∑ n m ϕn = Ecm
n =1, k
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Matrix Formulation (II)
∑H
n =1, k
⎛ H11
⎜
⎜ .
⎜ .
⎜
⎜ .
⎜H
⎝ k1
......
......
c = Ecm
mn n
H1k ⎞ ⎛ c1 ⎞
⎛ c1 ⎞
⎜ ⎟
⎟ ⎜ ⎟
. ⎟ ⎜ .⎟
⎜ .⎟
. ⎟⋅⎜ . ⎟ = E ⎜ . ⎟
⎜ ⎟
⎟ ⎜ ⎟
. ⎟ ⎜ .⎟
⎜ .⎟
⎟
⎜
⎟
⎜
⎟
H kk ⎠ ⎝ ck ⎠
⎝ ck ⎠
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Matrix Formulation (III)
⎛ H11 − E
⎜
.
⎜
det ⎜
.
⎜
.
⎜
⎜ H
k1
⎝
......
H 22 − E
......
⎞
⎟
⎟
⎟=0
⎟
⎟
H kk − E ⎟⎠
H1k
.
.
.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Variational Principle
E [Ψ ] =
E [ Ψ ] ≥ E0
Ψ Ĥ Ψ
Ψ Ψ
If E [ Ψ ] = E0 , then Φ is the ground
state wavefunction, and
viceversa…
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Atomic Units
• me=1, e=1, a0 (Bohr radius)=1, = = 1
1
ε0 =
4π
1 Z2
Energy of 1s electron= −
2 n2
(1 atomic unit of energy=1 Hartree=2 Rydberg=27.21 eV
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Energy of an Hydrogen Atom
Eα =
Ψα Ĥ Ψα
Ψα Ψα
Ψα = C exp ( −α r )
Ψα Ψα = π
C2
α
3
,
1 2
C2
Ψα − ∇ Ψα = π
2
2α
1
C2
Ψ α − Ψ α = −π 2
r
α
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Hydrogen Molecular Ion H2+
• Born-Oppenheimer approximation: the
electron is always in the ground state
corresponding to the instantaneous ionic
positions
⎡
⎛
1
1
1
⎢− ∇2 + ⎜ G 1 G −
G −
G
⎜ RH − RH
⎢ 2
r − RH1
r − RH 2
1
2
⎝
⎣
⎞⎤ G
⎟ ⎥ψ (r ) = Eψ (rG )
⎟⎥
⎠⎦
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Linear Combination of Atomic
Orbitals
• Most common approach to find out the
ground-state solution – it allows a meaningful
definition of “hybridization”, “bonding” and
“anti-bonding” orbitals.
• Also knows as LCAO, LCAO-MO (for
molecular orbitals), or tight-binding (for solids)
• Trial wavefunction is a linear combination of
atomic orbitals – the variational parameters
are the coefficients:
Ψ trial = c1Ψ1s
(
G G
G G
r − RH + c2 Ψ1s r − RH 2
1
)
(
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
)
Bonding and Antibonding (I)
z
z
z
1sA
+
y
y
y
_
Overlap
region
1sB
∗
(a)
(b)
(c)
The orbital region for the σg1s and σ 1s LCAO molecular orbitals. (a) The overlapping orbital regions of
the 1sA and 1sB atomic orbitals. (b) The orbital region of the σg1s LCAO-MO. (c) The orbital Region of
the σ 1s LCAO-MO. The orbital regions of the LCAO molecular orbitals have the same general features
as the "exact" Born Oppenheimer orbitals whose orbital regions were depicted in Figure 18.4.
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Formation of a Bonding Orbital
Image removed due to copyright restrictions. Please see
the animation of hydrogen bonding orbitals at http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma/index.html
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Formation of an Antibonding
Orbital
Image removed due to copyright restrictions. Please see the animation of hydrogen
antibonding orbitals at http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma-star/index.html
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Bonding and Antibonding (II)
8
σ*1s
u energy
6
4
EBO/eV
2
0
σg1s energy
1
Born-Oppenheimer energy
of exact orbitals
2
3
4
-2
-4
-6
R/10-10m
Orbital Energies for the σg 1s and σ*u 1s LCAO Molecular Orbitals.
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
The Quantization of Vibrations
• Electrons are much lighter than nuclei
(mproton/melectron~1800)
• Electronic wave-functions always rearrange
themselves to be in the ground state (lowest
energy possible for the electrons), even if the
ions are moving around
• Born-Oppenheimer approximation: electrons in
the instantaneous potential of the ions (so,
electrons can not be excited – FALSE in
general)
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Nuclei have some quantum
action…
• Go back to Lecture 1 – remember the
harmonic oscillator
8
4
2
Equilibrium
position of
mass
EBO/eV
Spring
Mass
σ*1s
u energy
6
Stationary
object
z
0
z
0
1
2
3
4
-2
-4
-6
A mass on a spring. This system can be
represented by a harmonic oscillator.
σg1s energy
Born-Oppenheimer energy
of exact orbitals
R/10-10m
Orbital Energies for the σg 1s and σ*u 1s LCAO Molecular Orbitals.
Figures by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
The quantum harmonic
oscillator (I)
⎛ =2 d 2 1 2 ⎞
+ kz ⎟ ϕ ( z ) = E ϕ ( z )
⎜−
2
2
⎝ 2 M dz
⎠
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
The quantum harmonic
oscillator (I)
⎛ =2 d 2 1 2 ⎞
+ kz ⎟ ϕ ( z ) = E ϕ ( z )
⎜−
2
2
⎝ 2 M dz
⎠
k
ω=
m
km
a=
=
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
The quantum harmonic
oscillator (II)
E (in units of hνo)
5
V(x)
n=4
4
n=3
3
n=2
2
n=1
1
0
1⎞
⎛
E = =ω ⎜ n + ⎟
2⎠
⎝
n=0
x
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Quantized atomic vibrations
Figure by MIT OpenCourseWare.
Courtesy of Dr. Klaus Hermann. Used with permission.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)
Specific Heat of Graphite (Dulong and
Petit)
2500
CP (J.K-1.kg-1)
2000
1500
1000
500
0
0
500
1000
1500
2000
2500
Temperature (K)
Figure by MIT OpenCourseWare.
3.23 Electronic, Optical and Magnetic Properties of Materials - Nicola Marzari (MIT, Fall 2007)