Document 10288465
advertisement

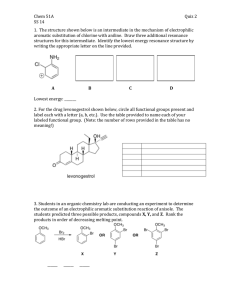
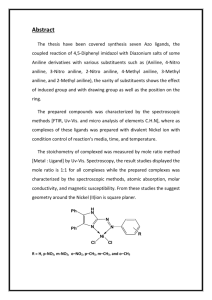
Experimental Organic Chemistry: A Miniscale and Microscale Approach Section 21.2 SYNTHESIS OF SULFANILAMIDE. Part A. PREPARATION OF ANILINE NAME (print): DATE: INSTRUCTOR: 1. LABORATORY SECTION: Mechanistically, the reaction to be performed (underline all answers that apply) is (a) an elimination reaction, (b) a nucleophilic substitution reaction, (c) a nucleophilic addition-elimination reaction, (d) an electrophilic substitution reaction, (e) a free-radical substitution reaction, (f) an oxidation of the aromatic substrate, (g) a reduction of the aromatic substrate, (h) none of these. 2. Determine the limiting reagent in this reaction. Show your work. 3. Why is the reduction of nitrobenzene more efficient with tin powder that is free of surface oxides? 4. What changes in the reaction mixture do you expect to see as the reduction progresses? 5. Determine whether adding the specified amount of 12 M aqueous sodium hydroxide to the reaction mixture prior to steam distillation is sufficient to make the mixture basic. Show your calculation. PL. 141 ©2006 Thomson Brooks/Cole Experimental Organic Chemistry: A Miniscale and Microscale Approach 6. How is aniline to be separated from residual nitrobenzene in this procedure? 7. Why is the reaction mixture to be cooled after being saturated with salt but before extraction with diethyl ether? 8. What color is pure aniline, and why are undistilled samples of it often brown in color? 9. What are possible effects if aniline gets in your eyes? 10. Is nitrobenzene listed as a possible carcinogen? 11. Is aniline listed as a possible mutagen? PL. 142 ©2006 Thomson Brooks/Cole