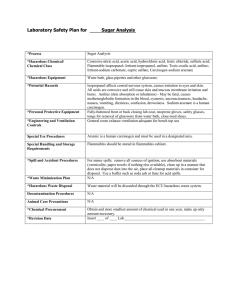
EXPERIMENT: ANILINE POINT AIM: To determine Aniline point of the given oil (Diesel) sample. EQUIPMENTS: Aniline Point apparatus, Thermometer & Stand, Beaker, Test tube, heating bath, cooling bath. CHEMICALS: Diesel, Acetone, Aniline. THEORY: ANILINE POINT The minimum equilibrium temperatures at which equal volume of aniline and oil under test condition are completely miscible. The aniline point (or mixed aniline point) is useful as an aid in the characterization of pure hydrocarbons and in the analysis of hydrocarbon mixtures. Aromatic hydrocarbons exhibit the lowest, and paraffin’s the highest values. Cycloparaffins and olefins exhibit values that lie between those for paraffin’s and aromatics. In homologous series the aniline points increase with increasing molecular weight. Although it occasionally is used in combination with other physical properties in correlative methods for hydrocarbon analysis, the aniline point is most often used to provide an estimate of the aromatic hydrocarbon content of mixtures. The value gives an approximation for the content of aromatic compounds in the oil, since the miscibility of aniline, which is also an aromatic compound, suggests the presence of similar (i.e. aromatic) compounds in the oil. The lower the aniline point, the greater is the content of aromatic compounds in the oil as obviously a lower temperature is needed to ensure miscibility. The approximate method of testing the ignition quality of oils is based measuring the proportion of paraffin hydrocarbons in the fuel. In the case of oils the high paraffin content to better ignition quality. The aromatic hydrocarbons mix readily with aniline at room temperature but mix only at relatively higher temperatures. A high aniline point indicates a high paraffin content and higher ignition quality. Moreover aromatic hydrocarbons dissolve natural rubber and some synthetic rubbers also. This means a higher aromatic content in a lubricating oil otherwise it may damage the oil sealing or packing which are usually made of rubber and used in the machines. ANILINE POINT APPARATUS PROCEDURE: 1. 2. 3. 4. 5. 6. 7. Clean and dry the apparatus. Prepare the 20-ml of aniline & 20 ml of diesel sample in test tube. Insert the thermometer bulb does not touch the side wall of the test Tube. Stir the sample. Heat the mixture of test tube until complete miscibility is obtained. Remove the heat by allowing the mixture to cool. Observe the temperature at which sample will start to separate as an aniline point. RESULT: Aniline point of given sample is ________________℃ CONCLUSION: