Fischer Projection
advertisement
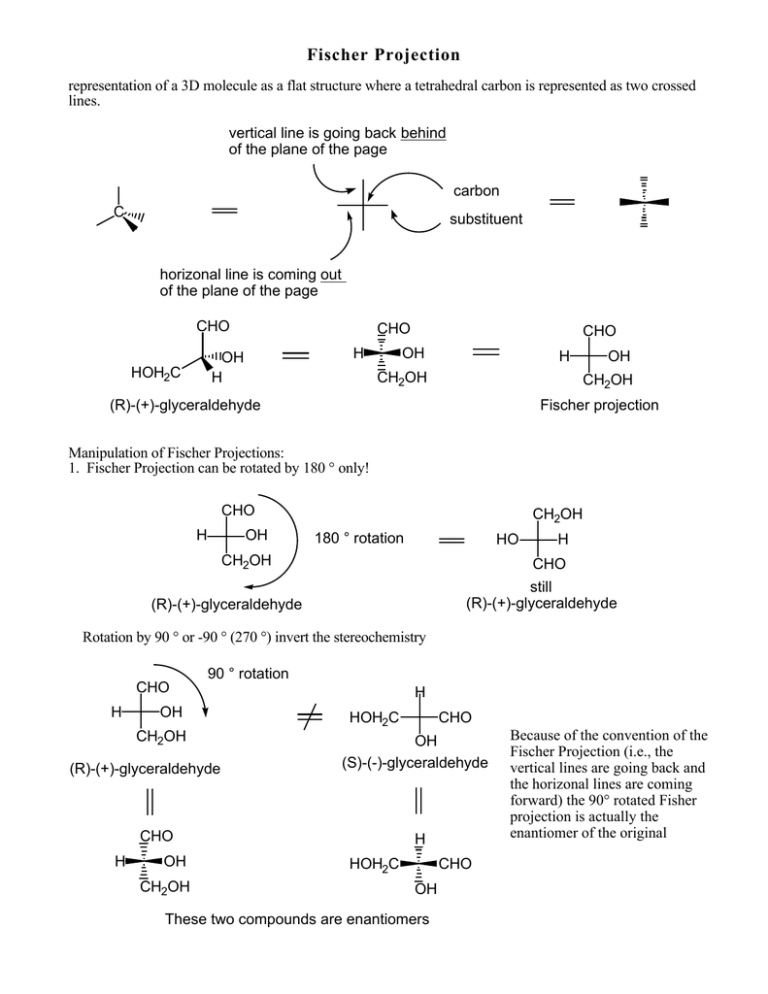
Fischer Projection representation of a 3D molecule as a flat structure where a tetrahedral carbon is represented as two crossed lines. vertical line is going back behind of the plane of the page carbon C substituent horizonal line is coming out of the plane of the page CHO CHO H OH H HOH2C CHO OH H CH2OH OH CH2OH (R)-(+)-glyceraldehyde Fischer projection Manipulation of Fischer Projections: 1. Fischer Projection can be rotated by 180 ° only! CHO H OH CH2OH 180 ° rotation HO CH2OH H CHO still (R)-(+)-glyceraldehyde (R)-(+)-glyceraldehyde Rotation by 90 ° or -90 ° (270 °) invert the stereochemistry CHO H 90 ° rotation OH CH2OH (R)-(+)-glyceraldehyde H HOH2C OH (S)-(-)-glyceraldehyde CHO H OH CH2OH CHO H HOH2C CHO OH These two compounds are enantiomers Because of the convention of the Fischer Projection (i.e., the vertical lines are going back and the horizonal lines are coming forward) the 90° rotated Fisher projection is actually the enantiomer of the original 2. If one group of the Fischer projection is held steady, the other groups can be rotated either clockwise or counter clockwise. hold this group steady CHO H OH CHO HOH2C CH2OH H OH still (R)-(+)-glyceraldehyde (R)-(+)-glyceraldehyde Assigning R and S-configurations to Fischer projections 1. Assign priorities to the four substituents according to the Cahn-Ingold-Prelog convention. 2. Perform the two allowed manipulations of the Fischer projection to place the lowest priority group on one of the vertical positions (either top or bottom) 3. If the priorities of the other three groups (1-2-3) proceed clockwise, the stereogenic center is assigned as R. If the priorities of the other three groups (1-2-3) proceed counter clockwise, the stereogenic center is assigned as S. 4 H 2 CHO OH 1 4 H CH2OH lowest priority group. Move to a vertical position hold one group steady and rotate the other three CHO 2 1 HO CH2OH 3 3 hold steady The priorities of the other three groups (1-2-3) is clockwise. The stereogenic center is assign as R For more structures with more than one stereogenic center such as carbohydrates, the tetrahedral carbons are "stacked" on top of one another. OH CHO O HO HO H H H HO OH CH2OH Threose For carbohydrates, the convention is to put the carbonyl group at the top for aldoses and closest to the top for ketoses. The carbons are numbered from top to bottom. 1 CHO H 1 CH2OH OH HO O 2 H HO H H OH H OH H OH H OH CH2OH CH2OH D-Glucose D-Fructose Carbohydrates and amino acids are designated as D- or L- according to the stereochemistry of the highest numbered carbon in the Fischer projection. If the hydroxyl group (or amino group for amino acids) is pointing to the right in the Fischer Projection, the sugar (or amino acid) is designated as D. If the hydroxyl group (or amino group for amino acids) is pointing to the left in the Fischer projection, the sugar (or amino acid) is designated as L. Most naturally occurring carbohydrates are of the D-configuration while most naturally occurring amino acids are L. 1 CHO H 2 OH 3 HO H 4 H OH highest numbered stereogenic carbon H 5 OH 1 CHO H 2 OH HO 3 HO 4 H H 6 CH2OH 5 CH2OH D-Glucose L-Arabinose highest numbered stereogenic carbon Converting Fischer Projections into Haworth Projections. 1. Identify the hydroxyl group which is cyclizing onto the carbonyl group. This hydroxy will become the ring oxygen in the hemiacetal or hemiketal form of the carbohydrate. For D-glucose, it is the C5 hydroxyl in the pyranose form; for D-ribose, it is the C4-hydroxyl for the furanose form. 2. Manipulate the Fischer projection so this hydroxyl group is on the bottom. 3. Draw the Haworth projection so that the ring oxygen is on the top. For pyranoses, draw the sixmembered ring laying on it side with a oxygen at the upper right. 4. Substituents on the right side of the Fischer projection will be on the bottom face of the Haworth projection. Substituents on the left side of the Fischer projection will be on the top face of the Haworth projection. 5. Remember that in the cyclic form, the C1-hydroxyl group (the anomeric center) can usually adopt either the up or down configuration. T T T O T T B B B B O T B T B B 1 CHO OH H H OH H H OH HOH2C D-glucose H OH H OH H OH H OH H OH H OH HOH2C CH2OH D-Ribose Ring oxygen H OH OH 1 2 OH H 1 CHO O 5 OH 4 3 OH Ring oxygen CHO 6 CH2OH OH HO H CH2OH B Pyranose CHO HO T= top B= bottom T B Furanose H T OH β-D-glucopyranose HO 4 6 OH O 3 OH 2 OH β-D-ribofuranose 1