Cengel and Boles, 7 Ed. ME 305Thermodynamics II Woodbury
advertisement
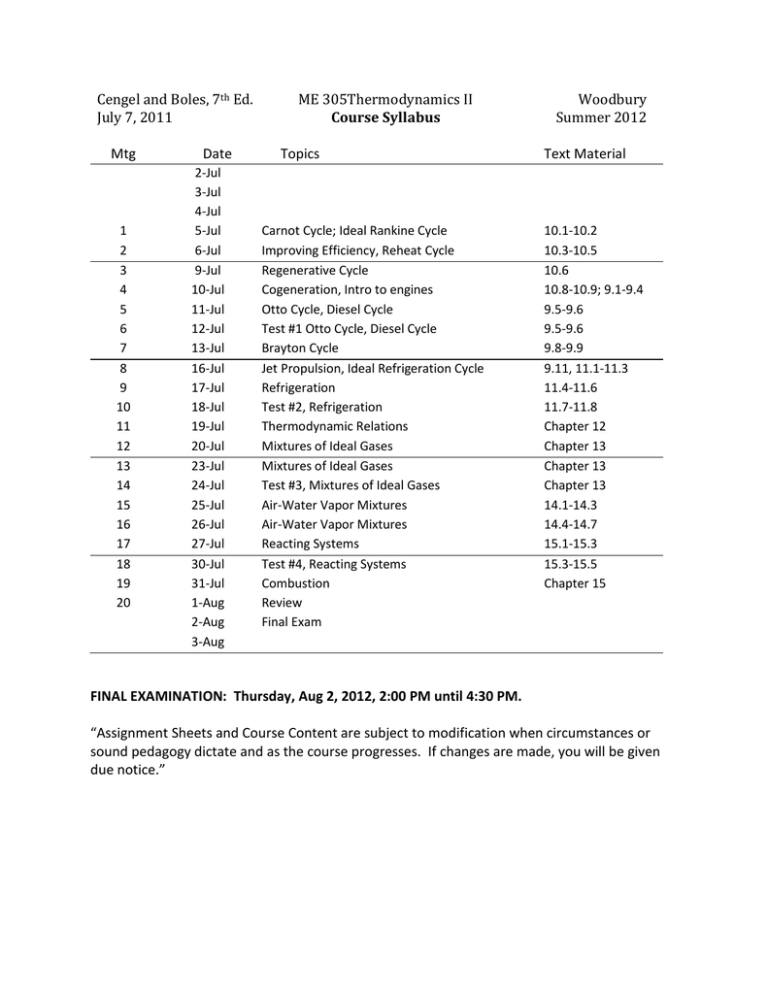
Cengel and Boles, 7th Ed. July 7, 2011 Mtg 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Date 2-Jul 3-Jul 4-Jul 5-Jul 6-Jul 9-Jul 10-Jul 11-Jul 12-Jul 13-Jul 16-Jul 17-Jul 18-Jul 19-Jul 20-Jul 23-Jul 24-Jul 25-Jul 26-Jul 27-Jul 30-Jul 31-Jul 1-Aug 2-Aug 3-Aug ME 305Thermodynamics II Course Syllabus Topics Carnot Cycle; Ideal Rankine Cycle Improving Efficiency, Reheat Cycle Regenerative Cycle Cogeneration, Intro to engines Otto Cycle, Diesel Cycle Test #1 Otto Cycle, Diesel Cycle Brayton Cycle Jet Propulsion, Ideal Refrigeration Cycle Refrigeration Test #2, Refrigeration Thermodynamic Relations Mixtures of Ideal Gases Mixtures of Ideal Gases Test #3, Mixtures of Ideal Gases Air-Water Vapor Mixtures Air-Water Vapor Mixtures Reacting Systems Test #4, Reacting Systems Combustion Review Final Exam Woodbury Summer 2012 Text Material 10.1-10.2 10.3-10.5 10.6 10.8-10.9; 9.1-9.4 9.5-9.6 9.5-9.6 9.8-9.9 9.11, 11.1-11.3 11.4-11.6 11.7-11.8 Chapter 12 Chapter 13 Chapter 13 Chapter 13 14.1-14.3 14.4-14.7 15.1-15.3 15.3-15.5 Chapter 15 FINAL EXAMINATION: Thursday, Aug 2, 2012, 2:00 PM until 4:30 PM. “Assignment Sheets and Course Content are subject to modification when circumstances or sound pedagogy dictate and as the course progresses. If changes are made, you will be given due notice.” Cengal and Boles, 7th Ed. July 3, 2012 ME 305 Thermodynamics Course Policies Woodbury Summer 2012 Prerequisites. ME 215 Thermodynamics I and MATH227 Calculus III. You are reminded that the College of Engineering requires a grade of 'C' or better for all prerequisites. Catalog Description. Thermodynamic cycle analysis; Maxwell relations and development of thermodynamic properties; thermodynamics of non-reacting and reacting mixtures and chemical equilibrium (ES3). Attendance. Your best opportunity to learn the concepts for the course is to actively participate in the class meetings. An informal record of attendance will be kept. Tests. There will be four major tests which will be administered on the days indicated on the assignment sheet. Pop Quizzes. Unannounced or “pop” quizzes may be given. These scores will be counted the same as additional homework problems. Homework. Problems will be assigned regularly and will be collected for grading. Late homework cannot be accepted for credit. Final Exam. The final exam will be administered during the University-assigned time period. For the MTWThF 12:00-1:45 PM class meeting time, the final exam will be held on Thursday, August 2, 2012, from 2:00 PM to 4:30 PM. Make-up Tests. Make-up tests will not be administered. Only in the case of a student's extreme illness or family member's death may exceptions be granted. Required Text. Thermodynamics: an engineering approach, Seventh Edition, by Cengal and Boles (McGraw Hill, 2011). Final Grade. The final grade will be computed according to the following algorithm: Homework and Quizzes Test #1 Test #2 Test #3 Test #4 Final Exam 12% 17% 17% 17% 17% 20% ME 305 – Thermodynamics II Catalog Data: ME 305. Thermodynamics II. (3-0). Three hours. Thermodynamic cycle analysis; thermodynamics of non-reacting and reacting mixtures; chemical equilibrium. Prerequisites: ME 215. Corequisites: MATH 227. Textbook/references: Thermodynamics, An Engineering Approach, 7th ed., by Çengel, Y.A. and Boles, M.A., 2011, McGraw-Hill, New York, ISBN 978-0-0736674-2. Course objectives: (letters in brackets show the relationship with mechanical engineering program outcomes) • Define the assumptions associated with a basic power cycle for application in preliminary design analysis. (a2) • Define the assumptions associated with an Air Standard Power Cycle for application in preliminary design analysis. (a2) • Analyze the operation of a simple internal combustion engine through the ideal Otto and Diesel cycles and apply a first law analysis to show the effects of basic design parameters on overall system performance. (a2, e) • Analyze the operation of a simple gas turbine through the ideal Brayton cycle and apply a first law analysis to show the effects of basic design parameters on overall system performance. (a2, e) • Calculate the system thermal efficiency and net work output for an ideal Brayton cycle with regeneration, intercooling, and/or reheat and be able to explicitly show cycle improvements over the simple Brayton cycle. (a2, e) • Analyze the operation of a simple steam power plant through the ideal Rankine cycle and apply a first law analysis to show the effects of basic design parameters on overall system performance. (a2, e) • Calculate the system thermal efficiency and net work output for a Rankine cycle with reheat and/or regeneration and show cycle improvements over the simple Rankine cycle. (a2, e) • Calculate the degradation in system performance for the deviations from ideal operation conditions for the simple Rankine cycle and the variants of this cycle on temperature versus entropy diagrams. (e) • Define a refrigeration/air conditioning system and calculate the cooling capacity and coefficient of performance for an ideal vapor-compression refrigeration cycle. Calculate the degradation of system performance from system deviation from ideal cycle operation. (a2, e) • Calculate thermodynamic properties for mixtures of ideal gases. (a1, e) • Define and calculate thermodynamic properties of air-water vapor mixtures. Be able to use psychometric charts for obtaining properties of air-water vapor mixtures. (a2, e) • Define terms associated with the combustion of a hydrocarbon fuel and apply conservation of mass to balance a chemical equation. (a1, a2) • Apply the First Law of Thermodynamics to calculate the heat released during the combustion of a hydrocarbon fuel. (e) • Calculate the adiabatic flame temperature for combustion of a hydrocarbon fuel. (e) • Define the requirements for chemical equilibrium. (a1) • Calculate the equilibrium composition of a mixture of ideal gases at a specified temperature. (e)








