Descriptive Chemistry Occurrence of the Elements on Earth Cosmic
advertisement
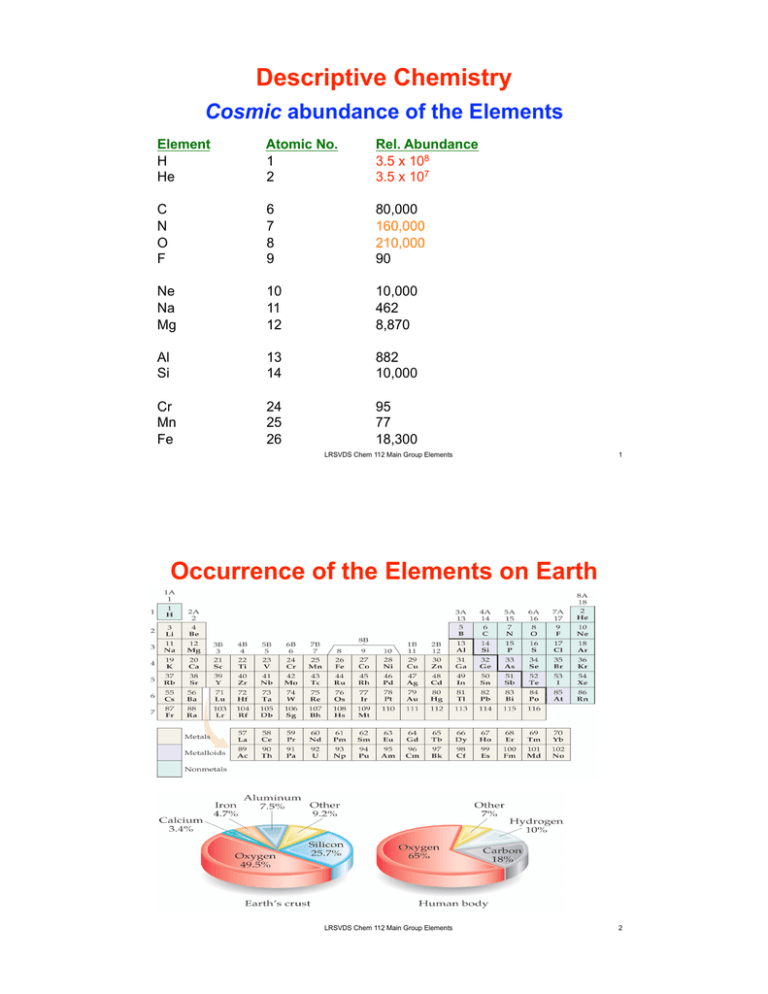
Descriptive Chemistry Cosmic abundance of the Elements Element H He Atomic No. 1 2 Rel. Abundance 3.5 x 108 3.5 x 107 C N O F 6 7 8 9 80,000 160,000 210,000 90 Ne Na Mg 10 11 12 10,000 462 8,870 Al Si 13 14 882 10,000 Cr Mn Fe 24 25 26 95 77 18,300 LRSVDS Chem 112 Main Group Elements 1 Occurrence of the Elements on Earth LRSVDS Chem 112 Main Group Elements 2 Abundance of elements Terrestrial abundance; On Earth! Element Atomic No. H 1 He 2 C 6 N 7 O 8 F 9 PPM 8,700 0.003 800 300 495,000 270 Rank 9 Na Mg Al Si 11 12 13 14 26,000 19,000 75,000 257,000 6 8 3 2 K Ca Ti Fe 19 20 22 26 24,000 34,000 5,800 47,000 7 5 10 4 1 LRSVDS Chem 112 Main Group Elements 3 Abundance of elements Terrestrial abundance O Si 27.72% 46.6% 10 9 8 % by weight 7 6 5 4 3 2 1 0 H B C N F Na Mg Al P O S Cl K Ca Ti V Cr Mn Fe Co Cu Zn LRSVDS Chem 112 Main Group Elements Si Se Rb Mo Sn I 4 Isolation of the Elements From their Natural state Sources of Gas-Phase Nonmetals: 1) HEAVY Noble Gases, O2, N2 2) Helium 3) Halogens a) b) 4) Hydrogen a) Steam Reforming CH4(g) + H2O(g) ! CO(g) + 3 H2(g) CO(g) + H2O(g) ! CO2(g) + H2(g) b) c) LRSVDS Chem 112 Main Group Elements 5 Isolation of the Elements From their Natural state Sources of Other Nonmetals: 1) Carbon 2) Sulfur 3) P 4) Se, Te 5)! Boron 6)! Metalloids LRSVDS Chem 112 Main Group Elements 6 Periodic Trends Summary n Zeff 1) Atomic Size 2) ionization energy 3) electron affinity both + and - ; halogens most negative 4) metallic character - metals lose electrons - nonmetals gain electrons increasing metallic character LRSVDS Chem 112 Main Group Elements 7 Valence in Period II N Max. of 4 bonds, 1 lone pair; usually has a valence of 3 HNO3 (4 bonds to N) NH3, NCl3, CH3NH2, HNO2 O Max. of 4 bonds, 2 lone pairs; usually has a valence of 2 H2O, OF2, H2C=O C Max. of 4 bonds, 0 lone pairs; always has a valence of 4 can gain 4 or lose 4 electrons to make an octet So carbon always makes 4 bonds CH4 (4 single bonds) O=C=O (2 double bonds) H-C!C-H (1 single + 1 triple bond) H 2N H 2N C=O (2 single + 1 double bond) (urea) LRSVDS Chem 112 Main Group Elements 8 Bonding in Period II vs. III Valence: Heavy Group V elements can gain 3 electrons or lose 5 to make an octet; " expect either 5 covalent bonds (PF5) or 3 covalent bonds (PF3) Multiple Bonds: elements past the second row are too big to allow good sidewise overlap of p-orbitals LRSVDS Chem 112 Main Group Elements 9 Difference in Bonding: Period 2 vs. 3 O2 is molecular (O=O, has a double bond) But S forms rings with single bonds (e.g., S8) N2 has a triple bond (:N!N:, very stable) But phosphorus is found in several forms (white, red, black), all of which have only single bonds. LRSVDS Chem 112 Main Group Elements 10 Chemistry of Hydrogen Forms binary hydrides (hydrogen and one other element) ionic, metallic or molecular hydrides Molecular hydrides: H bonded covalently to a non-metal Examples: HCl, HBr, CH4, H2Se, SiH4, … acidic or show no acid-base properties acid strength increases from left to right PH3 < H2S < HCl acid strength increases going down family H2O < H2S < H2Se < H2Te NH3 and other amines are basic (lone pair) LRSVDS Chem 112 Main Group Elements 11 Ionic Hydrides: Hydrogen plus a group 1 or 2 metal Examples: LiH, LiAlH4, CaH2 these metals are much less electronegative than hydrogen; H gains electrons, produces a hydride anion H **high melting ionic solids, very basic, strong reducing agents CaH2(s) + H2O (l) # H2(g) + Ca(OH)2 (aq) Metallic hydrides: H plus a transition metal many retain their metallic characteristics If ratio of M:H is not fixed; hydrogen atoms are absorbed into the interstices of the metal lattice If M-H ratio is fixed; organometallic metal hydrides or metaldihydrogen complexes LRSVDS Chem 112 Main Group Elements Fe(H2)(H)2 (PR3) 3 12 Group III; B, Al, Ga, In, Tl Properties of Boron "!Only group 3 element that is a nonmetal "!Network covalent solid (mp=2300ºC) Sources of Boron: Rare, found in one mineral Borax: Na2B4O7.(OH2)10 Chemistry of Boron: "!Oxide B2O3 is used to make Pyrex glass (anhydride of boric acid H3BO3) "!Boranes (B plus H) are electron deficient (6 v.e.) "!Borohydrides (borane anions); source of H! ions "!Boron Nitride, BN Boron is one element to the left of carbon. Nitrogen is one element to the right of carbon. BN hardness = 9.8 LRSVDS Chem 112 Main Group Elements 13 The Inert Pair Effect: As you get closer to the bottom of the group, there is an increasing tendency for the s2 electrons not to be used in bonding. Trends in Group III; B, Ga, In, Tl •! Inert Pair Effect •! On descending the group +1 oxidation state becomes more stable than +3 •! Oxides and hydroxides become more basic Going down the group metallic character increases: B(OH)3 is a weak acid Al(OH)3 and Ga(OH)3 are amphoteric In(OH)3 is basic •! Bonding: covalent # ionic going down the group B is most electronegative (ONLY covalent bonds) Al, Ga, and In form both covalent and ionic bonds. LRSVDS Chem 112 Main Group Elements 14 Group IV: C, Si, Ge, Sn, Pb Chemistry of carbon #!Organic Chemistry (C bonded to H) #!Inorganic Chemistry (C not bonded to H) $! Oxides CO, CO2 $! Carbides 1. ionic (e.g., CaC, C with active metals ) contain C4- or C22 - (-C!C-) C4- : Be2C, Al4C3 reacts with water to form CH4 C22 - : CaC2 reacts with water to give HC!CH 2. covalent (e.g., SiC, covalent bonds with metalloids and nonmetals) SiC: does not react with water; very hard 3. interstitial (e.g., steel, C incorporated into interstitial spaces of transition metals in non-stoichiometric proportions) •!steel is an interstitial carbide: harder than pure Fe LRSVDS Chem 112 Main Group Elements 15 TRENDS IN GROUP IV Going down the periodic table in Group IV: 1)! The +2 oxidation state becomes more stable than +4 due to the “inert pair” effect. +2 is rare for C, Si, Ge. +2, +4 is common for Sn. +4 is unstable for Pb " strong oxidizing agent " prefers to be +2. 2)! Basicity of oxides and hydroxides increases CO2, SiO2, GeO2 are weakly acidic. SnO, SnO2, PbO are amphoteric. 3)! Hydrides become less stable THOUSANDS of stable hydrocarbons (compounds of C and H) SiH4 is stable but is spontaneously flammable. Ge, Sn, Pb hydrides are very unstable. LRSVDS Chem 112 Main Group Elements 16 Group 5-6: Hydrolysis of nitrogen oxides Hydrolysis = reaction with water N is a non metal: oxides are acidic. N-oxide + H2O = oxyacid N2O5 + H2O # 2HNO3 (nitric acid) 3NO2 + H2O # 2HNO3 + NO N2O3 + H2O # 2HNO2 (nitrous acid) HNO3 is colorless, corrosive; turns yellow in sunlight (photochemical decomposition) LRSVDS Chem 112 Main Group Elements 17 Trends for GROUP V ELEMENTS; N, P, As, Sb, Bi Trends Going down the periodic table: 1)! Electronegativity decreases. 2)! Size increases 3) Switch from non-metallic to metallic 4) Hydroxides and oxides start acidic, become more basic. 5)! Hydrides become less stable. NH3 is stable. PH3 is stable but burns in air. AsH3 decomposes easily. SbH3, BiH3 are very unstable. 7)! “Inert pair effect” becomes more pronounced: +3 becomes more stable than +5. P: +5 dominates (lower EN than N) As3+, As5+ are equally common. Sb: +3 dominates. Bi: +3 dominates. LRSVDS Chem 112 Main Group Elements 18 Group VI Elements: O, S, Se, Te (Po) O2: Most abundant element on earth (21% of air) 50% of earth’s crust is oxygen (but not O2) General Properties Most widely used oxidizing agent O-O bond in O2 is strong (495 kJ/mol), as are many X-O bonds ns2np4 configuration; can gain 2 e- or share 2 e- to fill the shell " Oxidation states -2 (O in all compounds except with F; O2F2, OF2 ) -1 (only in peroxide ion, O22-) +2, +4 +6 (NOT O, only larger members of group) Allotropes of oxygen: Light or electrical discharge 3O2 Strong oxidizing agent O3 # O2 + O 2 O3 decomposition LRSVDS Chem 112 Main Group Elements 19 Anhydrides and Dehydration Anhydride: compound formed by loss of ___________________ 2 NaOH ! H2O + Na2O base anhydride H2SO4 ! H2O + SO3 acid anhydride H3PO4 ! H2O + H4P2O7 acid anhydride Anhydrides are good dehydrating agents; They react with water (hydrolysis rxn) Sucrose + H2SO4 product: (C12H22O11) (removes H2O) carbon LRSVDS Chem 112 Main Group Elements 20 Halogens (Group 7) •! Have valence of 1 (same as H) Can be used to replace H in many compounds thus influencing the properties. •! High reactivity of halogens WHY?? •! Can also have positive oxidation states when combined with more electronegative elements (e.g. oxygen to form oxyhalides: ClO4–, IO3–, etc.) Selected uses Fluorine – prepare polymers, fluorocarbons (Teflon, CFC’s), glass etching Chlorine – chlorinated organics/plastics (PVC), bleaching agent (NaClO), water treatment (destroy bacteria) Bromine – photographic film (AgBr) Iodine – iodized salt LRSVDS Chem 112 Main Group Elements 21 INTERHALOGEN COMPOUNDS: Combine halogens; central atom is least electronegative (it has to share electrons with surrounding atoms) 1:1 ratio BrF, ClF, ICl, BrCl 1:3 ratio ClF3, BrF3, IF3 , ICl3 (the only non-F) 1:5 ratio BrF5, IF5 (ClF5 formed with difficulty) Only Br & I large enough to be central atoms with 5 surrounding atoms 1:7 ratio IF7 Only I is large enough to be a central atom with 7 surrounding atoms Central atom is always bigger in size and less electronegative Extremely Reactive: superb oxidizing agents Oxidation state of central atom decreases to 0 or -1 LRSVDS Chem 112 Main Group Elements 22 Chemistry of Noble Gases; Group 8 React only under rigorous conditions Heavier elements have lower ionization energy, can more easily share eXe Ionization E =1176 kJ O2 Ionization E = 1171kJ "!Xe forms compounds with F and O! Xe + n F2 # XeF2 OR XeF4 OR XeF6 Oxidation states of +2, +4, +6 XeF6(s) + H2O(l) # XeOF4(l) + 2HF(g) XeF6(s) + 3H2O(l) # XeO3(l) + 6HF(aq) Kr forms only one compound; KrF2 Only one known “compound” made with He; Endohedral complex of He inside a C60 cage LRSVDS Chem 112 Main Group Elements 23






