Lecture 2: General Overview Presentation from typical Occurrence Properties based on filling
advertisement

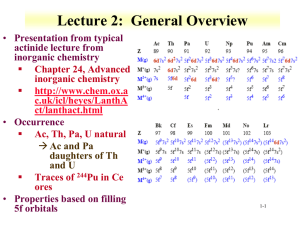
Lecture 2: General Overview • Presentation from typical actinide lecture from inorganic chemistry Chapter 24, Advanced inorganic chemistry http://www.chem.ox.a c.uk/icl/heyes/LanthA ct/lanthact.html • Occurrence Ac, Th, Pa, U natural Ac and Pa daughters of Th and U Traces of 244Pu in Ce ores • Properties based on filling 5f orbitals 1-1 Electronic structure • Electronic Configurations of Actinides are not always easy to confirm atomic spectra of heavy elements are very difficult to interpret in terms of configuration • Competition between 5fn7s2 and 5fn-16d7s2 configurations for early actinides promotion 5f 6d occurs to provide more bonding electrons much easier than corresponding 4f 5d promotion in lanthanides second half of actinide series resemble lanthanides more closely Similarities for trivalent lanthanides and actinides • 5f orbitals have greater extension with respect to 7s and 7p than do 4f relative to 6s and 6p orbitals The 5 f electrons can become involved in bonding ESR evidence for bonding contribution in UF3, but not in NdF3 * Actinide f covalent bond contribution to ionic bond * Lanthanide 4f occupy inner orbits that are not accessable • Basis for chemical differences between lanthanides and actinides 1-2 Electronic Structure • 5f / 6d / 7s / 7p orbitals are of comparable energies over a range of atomic numbers especially U - Am Bonding can include any orbitals since energetically similar Explains tendency towards variable valency • greater tendency towards (covalent) complex formation than for lanthanides Lanthanide complexes tend to be primarily ionic • Actinide complexes complexation with π-bonding ligands • Hybrid bonds involving f electrons • Since 5f / 6d / 7s / 7p orbital energies are similar orbital shifts may be on the order of chemical binding energies Electronic structure of element in given oxidation state may vary with ligand Difficult to state which orbitals are involved in bonding 1-3 Ionic Radii and trends Trends based on ionic radii Actinide contraction 1-4 Absorption Spectra and Magnetic Properties • • Electronic Spectra 5fn transitions narrow bands (compared to transition metal spectra) relatively uninfluenced by ligand field effects intensities are ca. 10x those of lanthanide bands complex to interpret Magnetic Properties hard to interpret spin-orbit coupling is large Russell-Saunders (L.S) Coupling scheme doesn't work, lower values than those calculated * LS (http://hyperphysics.phyastr.gsu.edu/hbase/atomic/lcoup. html) * Weak spin orbit coupling Sum spin and orbital angular momentum J=S+L ligand field effects are expected where 5f orbitals are involved in bonding 1-5 http://www.sciencedirect.com/science/article/pii/S002016 9300924873# Example: Pu absorbance spectrum 5 • Ability to distinguish between Pu oxidation states Pu6+(835 nm) Pu4+ (489 nm) Absorbance 4 Normal Heavy Light 3 2 1 0 400 500 600 700 800 Variation in molar absorptivity • Determine speciation of Pu by spectroscopy Wavelength (nm) f electrons and hybrid orbitals • • • Various orbital combinations similar to sp or d orbital mixing Linear: sf Tetrahedral: sf3 Square: sf2d Octahedral: d2sf3 A number of orbital sets could be energetically accessible General geometries Trivalent: octahedral Tetravalent: 8 coordination Pentavalent and hexavalent actinides have double bonded oxygens O=U=O2+ 1-6 Redox chemistry • • • • • • actinides are electropositive From 2+ to 7+ Pa - Pu show significant redox chemistry all 4 oxidation states of Pu can co-exist in appropriate conditions stability of high oxidation states peaks at U (Np) redox potentials show strong dependence on pH (data for Ac - Cm) high oxidation states are more stable in basic conditions even at low pH hydrolysis occurs tendency to disproportionation is particularly dependent on pH at high pH 3Pu4+ + 2H2O PuO22+ + 2Pu3+ + 4H+ early actinides have a tendency to form complexes complex formation influences reduction potentials Am4+(aq) exists when complexed by fluoride (15 M NH4F(aq)) radiation-induced solvent decomposition produces H• and OH• radicals lead to reduction of higher oxidation states e.g. PuV/VI, AmIV/VI 1-7 Redox chemistry (Frost diagrams) 1-8 Stereochemistry C.N. Geometry O.N. e.g. 4 distorted +4 U(NPh2)4 5 distorted tbp +4 U2(NEt2)8 6 octahedral +3 An(H2O)63+, An(acac)3 +4 UCl62- +5 UF6-, α-UF5 +6 AnF6 +7 Li5[AnO6] (An = Np, Pu) +6 Li4UO5 , UO3 +5/+6 U5O8 +6 UO2(S2CNEt2)2(ONMe3) +4 (Et4N)4[U(NCS)8], ThO2, UO2 +5 AnF83- +4 ThI4, U(acac)4, Cs4[U(NCS)8], +5 β-UF5 distorted octahedral 8 cubic square antiprismatic 1-9 Stereochemistry dodecahedral +4 Th(ox)44-, Th(S2CNEt2)4 bicapped trigonal prismatic +3 PuBr3 hexagonal bipyramidal +6 UO2(η2-NO3)2(H2O)2 ? +6 UF82- tricapped trigonal prismatic +3 UCl3 capped square antiprismatic +4 Th(trop)4(H2O) dodecahedral +4 Th(ox)44-, Th(S2CNEt2)4 bicapped trigonal prismatic +3 PuBr3 hexagonal bipyramidal +6 UO2(η2-NO3)2(H2O)2 ? +6 UF82- 10 bicapped square antiprismatic +4 KTh(ox)4.4H2O 11? fully capped trigonal prismatic? +3 UF3 12 irregular icosahedral +4 Th(NO3)62- distorted cuboctahedral +4 An(η3-BH4)4, (Np, Pu) 8 9 14? complex +4 An(η3-BH 4)4, (Th, Pa, U) 1-10 Actinide metals • Preparation of actinide metals Reduction of AnF3 or AnF4 with vapors of Li, Mg, Ca or Ba at 1100 – 1400 °C Other redox methods are possible Thermal decomposition of iodine species Am from Am2O3 with La * Am volatility provides method of separation • Metals tend to be very dense U 19.07 g/mL Np 20.45 g/mL Am lighter at 13.7 g/mL • Some metals glow due to activity Ac, Cm, Cf 1-11 Pu metal Plutonium α β γ δ δ′ ε Symmetry monoclinic monoclinic orthorhombic fcc bc tetragonal bcc Stability < 122°C 122-207°C 207-315°C 315-457°C 457-479°C 479-640°C ρ / gcm-3 19.86 17.70 17.14 15.92 16.00 16.51 • Some controversy surrounding behavior of metal http://www.fas.org/s gp/othergov/doe/lan l/pubs/00818030.pdf 1-12 1-13 Organometallic • Organometallic chemistry of actinides is relatively recent Interest is expanding but still focused on U • Similar to lanthanides in range of cyclopentadienides / cyclooctatetraenides / alkyls • Cyclopentadienides are π-bonded to actinides 1-14 Uranocene • • • • • • Paramagnetic Pyrophoric Stable to hydrolysis Planar 'sandwich' Eclipsed D8h conformation UV-PES studies show that bonding in uranocene has 5f & 6d contributions • e2u symmetry interaction shown can only occur via f-orbitals 1-15 Overview • Radius trends for ions and metals of the actinides • General trends in actinide electronic structure • Electronic and magnetic spectroscopy Variations in the actinides • Actinide stereochemistry • Range of oxidation states for the actinides • Role of organometallic chemistry for understanding f-electrons 1-16 Questions • What is the trend in for the ionic radii of actinides? • Which electrons are more likely to be involved in bonding, 4f or 5f? Why? • What is the spectroscopic nature of 5f electrons and how is this observed? • What are examples of f electron hybridization? • What is the relationship between molecular geometry and coordination number? • Describe a method for the preparation of actinide metals? • How many phases of Pu metal exist under normal pressure? What drives the change in phases? 1-17 Pop Quiz • List 3 pentavalent actinides. 1-18