Nuclear forces and Radioactivity
advertisement

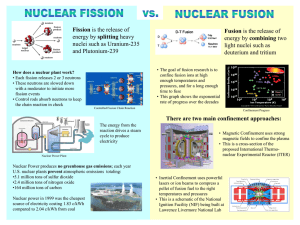
Nuclear forces and Radioactivity The nucleus is a competition between opposing forces Radioactivity is a result of imbalance between the forces Learning objectives Describe the basic forces and particles involved in nuclear structure Describe principles behind nuclear decay and radioactivity Describe the particles emitted in nuclear decay Define half-life and apply the concept to simple problems Describe the relationship between energy and matter Identify the differences between nuclear fission and fusion and their importance in generation of nuclear power Forces act in opposing directions Electrostatic repulsion: pushes protons apart Strong nuclear force: pulls protons together Nuclear force is much shorter range: protons must be close together Neutrons only experience the strong nuclear force Proton pair experiences both forces Neutrons experience only the strong nuclear force But: neutrons alone are unstable Neutrons act like nuclear glue Helium nucleus contains 2 protons and 2 neutrons – increase attractive forces Overall nucleus is stable As nuclear size increases, electrostatic repulsion builds up There are electrostatic repulsions between protons that don’t have attractive forces Long range repulsive force with no compensation from attraction More neutrons required Neutron to proton ratio increases with atomic number Upper limit of stability 4 U 234 90Th 2 He 238 92 Upper limit to nuclear stability Beyond atomic number 83, all nuclei are unstable and decay via radioactivity Radioactive decay (Transmutation) – Alpha formation of new element particle Mass number U Th He 238 92 234 90 Atomic number Atomic number decreases 4 2 emitted Stability is not achieved in one step: products also decay Atomic number (Z) increases Neutron:proton ratio decreases Th Pa e 234 90 0 1 Beta particle emission occurs with neutron-excess nuclei 234 91 Larger M/Z Alpha particle emission occurs with proton-heavy nuclei Smaller M/Z Beta particle emitted Summary of types of radiation Radioactive series are complex • The decay series from thorium-232 to lead-208 • Each intermediate nuclide is radioactive and undergoes nuclear decay • Left-pointing longer arrows (red) are alpha emissions • M and Z decrease • Right-pointing shorter arrows (blue) are beta emissions • M constant, Z increases Half-life measures rate of decay Concentration of nuclide is halved after the same time interval regardless of the initial amount – Half-life Can range from fractions of a second to billions of years Fission and fusion: Radical nuclear engineering Attempts to grow larger nuclei by bombardment with neutrons yielded smaller atoms instead. Distorting the nucleus causes the repulsive forces to overwhelm the attractive The foundation of nuclear energy and the atomic bomb Nuclear fission Nuclear fission produces nuclei with lower nucleon mass 1 0 n U Kr Ba 3 n 235 92 91 36 142 56 1 0 One neutron produces three: the basis for a chain reaction – explosive potential Neutrons must be obtained from other nuclear processes such as bombardment of aluminum with alpha particles Chain reactions require rapid multiplication of species Chain reactions have the potential for nuclear explosions Bomb requires creation of high rate of collisions in small volume How to achieve this at the desired time in a controlled manner? The importance of U-235 U-235 is less than 1 % of naturally occurring uranium, but undergoes fission with much greater efficiency than U-238 1 0 n U Kr Ba 3 n 235 92 91 36 142 56 1 0 Fission can follow many paths: over 200 different isotopes have been observed Each process produces more neutrons than it consumes 1 235 90 137 1 0 n 92 U 40 Zr 52Te 20 n Enrichment of uranium Weapons grade uranium requires a high concentration of U-235 This is achieved by isotope separation The lighter U-235 diffuses more rapidly than the heavier U-238 in the gas phase as UF6 Why is fission so energetic? Conversion of mass into energy Binding energy measures nuclear stability Higher binding energy particles have lower mass Mass converted to energy E = mc2 (Einstein’s relation) Fission: combined mass of smaller nuclei less than original nucleus AB+C MA > M B + M C Loss in mass = energy released: Comparison of nuclear and chemical energy sources Conversion of nuclear matter into energy produces masses: 1 gram 1014 J Chemical process: 1 gram fuel produces 103 J Nuclear process: 1 gram uranium at 0.08 % produces 1011 J Nuclear fusion: opposite of fission Fusion: combine small nuclei to make larger ones Nuclear mass converted to energy +E Example is the deuterium – tritium reaction About 0.7 % mass is converted into energy So why is there still hydrogen? The sun is a helium factory The sun’s energy derives from the fusion of hydrogen atoms to give helium 4 H He 2 e 2 e 0 0 1 e 1 e 2 1 1 4 2 0 1 0 1 So what’s the catch? The benefits: The problem: High energy output (10 x more output than fission) Clean products – no long-lived radioactive waste or toxic heavy metals Positive charges repel Reproduce the center of the sun in the lab Fusion is demonstrated but currently consumes more energy than it produces